What Have We Learned from Molecularly Informed Clinical Trials on Thymomas and Thymic Carcinomas—Current Status and Future Directions?
Abstract
Simple Summary
Abstract
1. Introduction
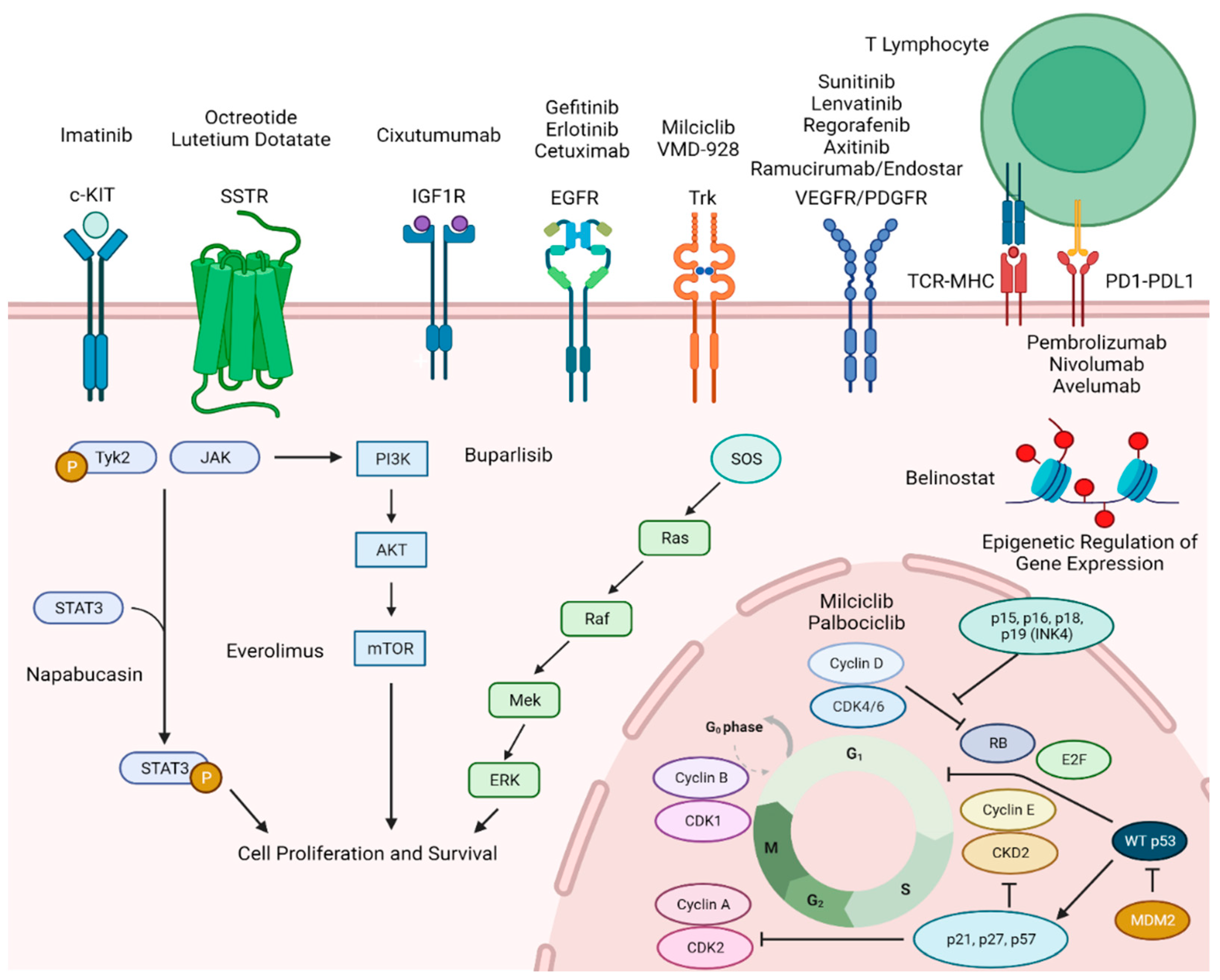
2. Somatostatin Receptor Targeting Therapies
3. EGFR and c-KIT
4. Epigenetic Alterations and Associated Therapies
5. Anti-Angiogenesis
6. IGFR-PI3K-AKT-mTOR Pathway
7. Cell Cycle Regulation/STAT 3 Pathway
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemonick, M.D.; Park, A. New hope for cancer. Time 2001, 157, 62–69. [Google Scholar] [PubMed]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, A.; Younis, T. Trastuzumab for HER2-Positive Metastatic Breast Cancer: Clinical and Economic Considerations. Clin. Med. Insights Oncol. 2012, 6, 179–187. [Google Scholar] [CrossRef]
- Singhal, S.; Hellyer, J.; Ouseph, M.M.; Wakelee, H.A.; Padda, S.K. Autoimmune Disease in Patients with Advanced Thymic Epithelial Tumors. JTO Clin. Res. Rep. 2022, 3, 100323. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.W.; Okumura, M.; Detterbeck, F.C.; Marom, E.M. Approaching the patient with an anterior mediastinal mass: A guide for radiologists. J. Thorac. Oncol. 2014, 9 (Suppl. S2), S110–S118. [Google Scholar] [CrossRef]
- Radovich, M.; Pickering, C.R.; Felau, I.; Ha, G.; Zhang, H.; Jo, H.; Hoadley, K.A.; Anur, P.; Zhang, J.; McLellan, M.; et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018, 33, 244–258.e10. [Google Scholar] [CrossRef] [PubMed]
- Padda, S.K.; Gökmen-Polar, Y.; Hellyer, J.A.; Badve, S.S.; Singh, N.K.; Vasista, S.M.; Basu, K.; Kumar, A.; Wakelee, H.A. Genomic clustering analysis identifies molecular subtypes of thymic epithelial tumors independent of World Health Organization histologic type. Oncotarget 2021, 12, 1178–1186. [Google Scholar] [CrossRef]
- Girard, N.; Basse, C.; Schrock, A.; Ramkissoon, S.; Killian, K.; Ross, J.S. Comprehensive Genomic Profiling of 274 Thymic Epithelial Tumors Unveils Oncogenic Pathways and Predictive Biomarkers. Oncologist 2022, 27, 919–929. [Google Scholar] [CrossRef]
- Ardeshir-Larijani, F.; Schneider, B.P.; Althouse, S.K.; Radovich, M.; Masood, A.; Perna, F.; Salman, H.; Loehrer, P.J. Clinicogenomic Landscape of Metastatic Thymic Epithelial Tumors. JCO Precis. Oncol. 2023, 7, e2200465. [Google Scholar] [CrossRef]
- Palmieri, G.; Montella, L.; Martignetti, A.; Muto, P.; Di Vizio, D.; De Chiara, A.; Lastoria, S. Somatostatin analogs and prednisone in advanced refractory thymic tumors. Cancer 2002, 94, 1414–1420. [Google Scholar] [CrossRef]
- Loehrer, P.J., Sr.; Wang, W.; Johnson, D.H.; Aisner, S.C.; Ettinger, D.S.; Eastern Cooperative Oncology Group Phase, I.I.T. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: An Eastern Cooperative Oncology Group Phase II Trial. J. Clin. Oncol. 2004, 22, 293–299. [Google Scholar] [CrossRef]
- Grupo Espanol de Tumores Neuroendocrinos. Efficacy and Safety of Radiotherapy Compared to Everolimus in Somatostatin Receptor Positive Neuroendocrine Tumors of the Lung and Thymus. 2028. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05918302 (accessed on 1 November 2023).
- Vastra Gotaland Region; Advanced Accelerator Applications. 177Lu-DOTA-TATE and Olaparib in Somatostatin Receptor Positive Tumours. 2024. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04375267 (accessed on 1 November 2023).
- Kurup, A.; Burns, M.; Dropcho, S.; Pao, W.; Loehrer, P.J. Phase II study of gefitinib treatment in advanced thymic malignancies. J. Clin. Oncol. 2005, 23 (Suppl. S16), 7068. [Google Scholar] [CrossRef]
- Bedano, P.M.; Perkins, S.; Burns, M.; Kessler, K.; Nelson, R.; Schneider, B.P.; Risley, L.; Dropcho, S.; Loehrer, P.J. A phase II trial of erlotinib plus bevacizumab in patients with recurrent thymoma or thymic carcinoma. J. Clin. Oncol. 2008, 26 (Suppl. S15), 19087. [Google Scholar] [CrossRef]
- Memorial Sloan Kettering Cancer Center; Eli Lilly and Company; M.D. Anderson Cancer Center; City of Hope National Medical Center. Chemotherapy Plus Cetuximab Followed by Surgical Resection in Patients with Locally Advanced or Recurrent Thymoma or Thymic Carcinoma. 2024. Available online: https://clinicaltrials.gov/study/NCT01025089 (accessed on 15 November 2023).
- Salter, J.T.; Lewis, D.; Yiannoutsos, C.; Loehrer, P.J.; Risley, L.; Chiorean, E.G. Imatinib for the treatment of thymic carcinoma. J. Clin. Oncol. 2008, 26 (Suppl. S15), 8116. [Google Scholar] [CrossRef]
- Giaccone, G.; Rajan, A.; Ruijter, R.; Smit, E.; van Groeningen, C.; Hogendoorn, P.C. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J. Thorac. Oncol. 2009, 4, 1270–1273. [Google Scholar] [CrossRef]
- Giaccone, G.; Rajan, A.; Berman, A.; Kelly, R.J.; Szabo, E.; Lopez-Chavez, A.; Trepel, J.; Lee, M.J.; Cao, L.; Espinoza-Delgado, I.; et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J. Clin. Oncol. 2011, 29, 2052–2059. [Google Scholar] [CrossRef]
- Thomas, A.; Rajan, A.; Szabo, E.; Tomita, Y.; Carter, C.A.; Scepura, B.; Lopez-Chavez, A.; Lee, M.J.; Redon, C.E.; Frosch, A.; et al. A phase I/II trial of belinostat in combination with cisplatin, doxorubicin, and cyclophosphamide in thymic epithelial tumors: A clinical and translational study. Clin. Cancer Res. 2014, 20, 5392–5402. [Google Scholar] [CrossRef]
- Thomas, A.; Rajan, A.; Berman, A.; Tomita, Y.; Brzezniak, C.; Lee, M.J.; Lee, S.; Ling, A.; Spittler, A.J.; Carter, C.A.; et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: An open-label phase 2 trial. Lancet Oncol. 2015, 16, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Satouchi, M.; Itoh, S.; Okuma, Y.; Niho, S.; Mizugaki, H.; Murakami, H.; Fujisaka, Y.; Kozuki, T.; Nakamura, K.; et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): A multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Perrino, M.; De Pas, T.; Bozzarelli, S.; Giordano, L.; De Vincenzo, F.; Conforti, F.; Digiacomo, N.; Cordua, N.; D’Antonio, F.; Borea, F.; et al. Resound Trial: A phase 2 study of regorafenib in patients with thymoma (type B2-B3) and thymic carcinoma previously treated with chemotherapy. Cancer 2022, 128, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Zucali, P.A.; Pala, L.; Catania, C.; Bagnardi, V.; Sala, I.; Della Vigna, P.; Perrino, M.; Zagami, P.; Corti, C.; et al. Avelumab plus axitinib in unresectable or metastatic type B3 thymomas and thymic carcinomas (CAVEATT): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Girard, N.; Novello, S.; de Castro, J.; Bigay-Game, L.; Bernabe, R.; Greillier, L.; Mosquera, J.; Cousin, S.; Juan, O.; et al. PECATI: A Multicentric, Open-Label, Single-Arm Phase II Study to Evaluate the Efficacy and Safety of Pembrolizumab and Lenvatinib in Pretreated B3-Thymoma and Thymic Carcinoma Patients. Clin. Lung Cancer 2022, 23, e243–e246. [Google Scholar] [CrossRef] [PubMed]
- Dwight, O.; National Cancer Institute; Ohio State University Comprehensive Cancer Center. Pembrolizumab and Sunitinib Malate in Treating Participants with Refractory Metastatic or Unresectable Thymic Cancer. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03463460 (accessed on 15 November 2023).
- Phase I/II Eval Safety & Prelim Activity Nivolumab Comb W/Vorolanib Pts W/Refractory Thoracic Tumors. 2023. Available online: https://clinicaltrials.gov/study/NCT03583086 (accessed on 15 November 2023).
- Carboplatin and Paclitaxel with or Without Ramucirumab in Treating Patients with Locally Advanced, Recurrent, or Metastatic Thymic Cancer That Cannot Be Removed by Surgery. 2024. Available online: https://clinicaltrials.gov/study/NCT03694002 (accessed on 15 November 2023).
- Rajan, A.; Carter, C.A.; Berman, A.; Cao, L.; Kelly, R.J.; Thomas, A.; Khozin, S.; Chavez, A.L.; Bergagnini, I.; Scepura, B.; et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: A multicentre, open-label, phase 2 trial. Lancet Oncol. 2014, 15, 191–200. [Google Scholar] [CrossRef]
- Zucali, P.A.; De Pas, T.; Palmieri, G.; Favaretto, A.; Chella, A.; Tiseo, M.; Caruso, M.; Simonelli, M.; Perrino, M.; De Vincenzo, F.; et al. Phase II Study of Everolimus in Patients with Thymoma and Thymic Carcinoma Previously Treated with Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2018, 36, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Abu Zaid, M.I.; Radovich, M.; Althouse, S.; Liu, H.; Spittler, A.J.; Solzak, J.; Badve, S.; Loehrer, P.J. A phase II study of buparlisib in relapsed or refractory thymomas. Front. Oncol. 2022, 12, 891383. [Google Scholar] [CrossRef]
- Besse, B.; Garassino, M.C.; Rajan, A.; Novello, S.; Mazieres, J.; Weiss, G.J.; Kocs, D.M.; Barnett, J.M.; Davite, C.; Crivori, P.; et al. Efficacy of milciclib (PHA-848125AC), a pan-cyclin d-dependent kinase inhibitor, in two phase II studies with thymic carcinoma (TC) and B3 thymoma (B3T) patients. J. Clin. Oncol. 2018, 36 (Suppl. S15), 8519. [Google Scholar] [CrossRef]
- Kalra, M.; Cote, G.M.; Heist, R.S.; Spittler, A.J.; Yu, S.; Hitron, M.; Loehrer, P.J. A phase 1b study of napabucasin (NAPA) + weekly paclitaxel (PTX) in patients (pts) with advanced thymoma and thymic carcinoma. J. Clin. Oncol. 2018, 36 (Suppl. S15), e20578. [Google Scholar] [CrossRef]
- Jung, H.A.; Kim, M.; Kim, H.S.; Kim, J.H.; Choi, Y.H.; Cho, J.; Park, J.H.; Park, K.U.; Ku, B.M.; Park, S.; et al. A Phase 2 Study of Palbociclib for Recurrent or Refractory Advanced Thymic Epithelial Tumors (KCSG LU17-21). J. Thorac. Oncol. 2023, 18, 223–231. [Google Scholar] [CrossRef]
- VM Oncology, LLC. Selective TrkA Inhibitor VMD-928 to Treat TrkA Overexpression Driven Solid Tumors or Lymphoma. 2024. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03556228 (accessed on 15 November 2023).
- Priyadarshini, S.; Allison, D.B.; Chauhan, A. Comprehensive Assessment of Somatostatin Receptors in Various Neoplasms: A Systematic Review. Pharmaceutics 2022, 14, 1394. [Google Scholar] [CrossRef]
- Ferone, D.; van Hagen, M.P.; Kwekkeboom, D.J.; van Koetsveld, P.M.; Mooy, D.M.; Lichtenauer-Kaligis, E.; Schonbrunn, A.; Colao, A.; Lamberts, S.W.; Hofland, L.J. Somatostatin receptor subtypes in human thymoma and inhibition of cell proliferation by octreotide in vitro. J. Clin. Endocrinol. Metab. 2000, 85, 1719–1726. [Google Scholar] [CrossRef]
- Lastoria, S.; Vergara, E.; Palmieri, G.; Acampa, W.; Varrella, P.; Caracò, C.; Bianco, R.A.; Muto, P.; Salvatore, M. In vivo detection of malignant thymic masses by indium-111-DTPA-D-Phe1-octreotide scintigraphy. J. Nucl. Med. 1998, 39, 634–639. [Google Scholar] [PubMed]
- Palmieri, G.; Lastoria, S.; Colao, A.; Vergara, E.; Varrella, P.; Biondi, E.; Selleri, C.; Catalano, L.; Lombardi, G.; Bianco, A.R.; et al. Successful treatment of a patient with a thymoma and pure red-cell aplasia with octreotide and prednisone. N. Engl. J. Med. 1997, 336, 263–265. [Google Scholar] [CrossRef]
- Kirzinger, L.; Boy, S.; Marienhagen, J.; Schuierer, G.; Neu, R.; Ried, M.; Hofmann, H.S.; Wiebe, K.; Strobel, P.; May, C.; et al. Octreotide LAR and Prednisone as Neoadjuvant Treatment in Patients with Primary or Locally Recurrent Unresectable Thymic Tumors: A Phase II Study. PLoS ONE 2016, 11, e0168215. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Makis, W.; McCann, K.; McEwan, A.J. Thymoma treated with 177Lu DOTATATE induction and maintenance PRRT. Clin. Nucl. Med. 2015, 40, e278–e281. [Google Scholar] [CrossRef]
- Ottaviano, M.; Damiano, V.; Tortora, M.; Capuano, M.; Perrone, P.; Forino, C.; Matano, E.; Palmieri, G. P1.17-015 Long Acting Octreotide plus Prednisone in Advanced Thymic Epithelial Tumors: A Real Life Clinical Experience. J. Thorac. Oncol. 2017, 12, S2066. [Google Scholar] [CrossRef]
- Sorejs, O.; Pesek, M.; Finek, J.; Fiala, O. Octreotide in the treatment of malignant thymoma–Case report. Rep. Pract. Oncol. Radiother. 2020, 25, 882–885. [Google Scholar] [CrossRef]
- Halperin, R.; Urban, D.; Tirosh, A. A Case of Metastatic Thymoma Responsive to Treatment with 177 Lu-DOTATATE. Clin. Nucl. Med. 2023, 48, e190–e192. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, B.; Sun, Z. Spectrum of EGFR aberrations and potential clinical implications: Insights from integrative pan-cancer analysis. Cancer Commun. 2020, 40, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Kalyankrishna, S.; Grandis, J.R. Epidermal Growth Factor Receptor Biology in Head and Neck Cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shi, W.; Zhang, Y.; Sun, M.; Liang, X.; Zheng, S. Epidermal growth factor receptor and B7-H3 expression in esophageal squamous tissues correlate to patient prognosis. Onco Targets Ther. 2016, 9, 6257–6263. [Google Scholar] [CrossRef] [PubMed]
- Viale, G.; Rotmensz, N.; Maisonneuve, P.; Bottiglieri, L.; Montagna, E.; Luini, A.; Veronesi, P.; Intra, M.; Torrisi, R.; Cardillo, A.; et al. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: Prognostic implications of EGFR immunoreactivity. Breast Cancer Res. Treat. 2009, 116, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Jones, M.; Turley, H.; Gatter, K.C.; Nagvekar, N.; Newsom-Davis, J.; Willcox, N. Oncogene proteins and proliferation antigens in thymomas: Increased expression of epidermal growth factor receptor and Ki67 antigen. J. Clin. Pathol. 1995, 48, 447–455. [Google Scholar] [CrossRef]
- Hayashi, Y.; Ishii, N.; Obayashi, C.; Jinnai, K.; Hanioka, K.; Imai, Y.; Itoh, H. Thymoma: Tumour type related to expression of epidermal growth factor (EGF), EGF-receptor, p53, v-erb B and ras p21. Virchows Arch. 1995, 426, 43–50. [Google Scholar] [CrossRef]
- Pescarmona, E.; Pisacane, A.; Pignatelli, E.; Baroni, C.D. Expression of epidermal and nerve growth factor receptors in human thymus and thymomas. Histopathology 1993, 23, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Henley, J.D.; Koukoulis, G.K.; Loehrer, P.J., Sr. Epidermal growth factor receptor expression in invasive thymoma. J. Cancer Res. Clin. Oncol. 2002, 128, 167–170. [Google Scholar] [CrossRef]
- Shah, R.; Lester, J.F. Tyrosine Kinase Inhibitors for the Treatment of EGFR Mutation-Positive Non-Small-Cell Lung Cancer: A Clash of the Generations. Clin. Lung Cancer 2020, 21, e216–e228. [Google Scholar] [CrossRef]
- Ionescu, D.N.; Sasatomi, E.; Cieply, K.; Nola, M.; Dacic, S. Protein expression and gene amplification of epidermal growth factor receptor in thymomas. Cancer 2005, 103, 630–636. [Google Scholar] [CrossRef]
- Palmieri, G.; Marino, M.; Salvatore, M.; Budillon, A.; Meo, G.; Caraglia, M.; Montella, L. Cetuximab is an active treatment of metastatic and chemorefractory thymoma (391 views). Front. Biosci. 2007, 12, 757–761. [Google Scholar] [CrossRef]
- Farina, G.; Garassino, M.C.; Gambacorta, M.; La Verde, N.; Gherardi, G.; Scanni, A. Response of thymoma to cetuximab. Lancet Oncol. 2007, 8, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Godesi, S.; Lee, J.; Nada, H.; Quan, G.; Elkamhawy, A.; Choi, Y.; Lee, K. Small Molecule c-KIT Inhibitors for the Treatment of Gastrointestinal Stromal Tumors: A Review on Synthesis, Design Strategies, and Structure—Activity Relationship (SAR). Int. J. Mol. Sci. 2023, 24, 9450. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.; Linger, R.; Pugh, J.; Dudakia, D.; Sokal, M.; Easton, D.F.; Timothy Bishop, D.; Stratton, M.; Huddart, R.; Rapley, E.A. Somatic KIT mutations occur predominantly in seminoma germ cell tumors and are not predictive of bilateral disease: Report of 220 tumors and review of literature. Genes. Chromosomes Cancer 2008, 47, 34–42. [Google Scholar] [CrossRef]
- Garrido, M.C.; Bastian, B.C. KIT as a Therapeutic Target in Melanoma. J. Investig. Dermatol. 2010, 130, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef]
- Pan, C.C.; Chen, P.C.; Chiang, H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J. Pathol. 2004, 202, 375–381. [Google Scholar] [CrossRef]
- Nakagawa, K.; Matsuno, Y.; Kunitoh, H.; Maeshima, A.; Asamura, H.; Tsuchiya, R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest 2005, 128, 140–144. [Google Scholar] [CrossRef]
- Petrini, I.; Zucali, P.A.; Lee, H.S.; Pineda, M.A.; Meltzer, P.S.; Walter-Rodriguez, B.; Roncalli, M.; Santoro, A.; Wang, Y.; Giaccone, G. Expression and mutational status of c-kit in thymic epithelial tumors. J. Thorac. Oncol. 2010, 5, 1447–1453. [Google Scholar] [CrossRef]
- Yoh, K.; Nishiwaki, Y.; Ishii, G.; Goto, K.; Kubota, K.; Ohmatsu, H.; Niho, S.; Nagai, K.; Saijo, N. Mutational status of EGFR and KIT in thymoma and thymic carcinoma. Lung Cancer 2008, 62, 316–320. [Google Scholar] [CrossRef]
- Strobel, P.; Hartmann, M.; Jakob, A.; Mikesch, K.; Brink, I.; Dirnhofer, S.; Marx, A. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N. Engl. J. Med. 2004, 350, 2625–2626. [Google Scholar] [CrossRef]
- Tsuchida, M.; Umezu, H.; Hashimoto, T.; Shinohara, H.; Koike, T.; Hosaka, Y.; Eimoto, T.; Hayashi, J.I. Absence of gene mutations in KIT-positive thymic epithelial tumors. Lung Cancer 2008, 62, 321–325. [Google Scholar] [CrossRef]
- Waddington, C.H. The epigenotype. Endeavour 1942, 1, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Pergaris, A.; Vrettou, K.; Theocharis, S.; Troungos, C. Thymic Epithelial Neoplasms: Focusing on the Epigenetic Alterations. Int. J. Mol. Sci. 2022, 23, 4045. [Google Scholar] [CrossRef] [PubMed]
- Prays, J.; Ortiz-Villalón, C. Molecular landscape of thymic epithelial tumors. Semin. Diagn. Pathol. 2022, 39, 131–136. [Google Scholar] [CrossRef]
- Wang, Y.; Thomas, A.; Lau, C.; Rajan, A.; Zhu, Y.; Killian, J.K.; Petrini, I.; Pham, T.; Morrow, B.; Zhong, X.; et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci. Rep. 2014, 4, 7336. [Google Scholar] [CrossRef] [PubMed]
- Sawas, A.; Radeski, D.; O’Connor, O.A. Belinostat in patients with refractory or relapsed peripheral T-cell lymphoma: A perspective review. Ther. Adv. Hematol. 2015, 6, 202–208. [Google Scholar] [CrossRef]
- Kim, M.S.; Blake, M.; Baek, J.H.; Kohlhagen, G.; Pommier, Y.; Carrier, F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003, 63, 7291–7300. [Google Scholar]
- Ganci, F.; Vico, C.; Korita, E.; Sacconi, A.; Gallo, E.; Mori, F.; Cambria, A.; Russo, E.; Anile, M.; Vitolo, D.; et al. MicroRNA expression profiling of thymic epithelial tumors. Lung Cancer 2014, 85, 197–204. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Xu, S.; Yang, Y.; Wei, D.; Wang, W.; Huang, X. Aberrant decrease of microRNA19b regulates TSLP expression and contributes to Th17 cells development in myasthenia gravis related thymomas. J. Neuroimmunol. 2015, 288, 34–39. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Chen, Y.; Wei, D. T Helper Type 17 Cells Expand in Patients with Myasthenia-Associated Thymoma. Scand. J. Immunol. 2012, 76, 54–61. [Google Scholar] [CrossRef]
- Cebi, M.; Cakar, A.; Erdogdu, E.; Durmus-Tekce, H.; Yegen, G.; Ozkan, B.; Parman, Y.; Saruhan-Direskeneli, G. Thymoma patients with or without myasthenia gravis have increased Th17 cells, IL-17 production and ICOS expression. J. Neuroimmunol. 2023, 381, 578129. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Matsuzaki, Y.; Edagawa, M.; Maeda, M.; Shimizu, T.; Hara, M.; Onitsuka, T. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J. Thorac. Cardiovasc. Surg. 2002, 124, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Takahashi, T.; Abe, M.; Akamatsu, H.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Murakami, H.; Tsuya, A.; Nakamura, Y.; et al. CD98 expression is associated with the grade of malignancy in thymic epithelial tumors. Oncol. Rep. 2010, 24, 861–867. [Google Scholar] [CrossRef]
- Cimpean, A.M.; Raica, M.; Encica, S.; Cornea, R.; Bocan, V. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann. Anat. 2008, 190, 238–245. [Google Scholar] [CrossRef]
- Lattanzio, R.; La Sorda, R.; Facciolo, F.; Sioletic, S.; Lauriola, L.; Martucci, R.; Gallo, E.; Palmieri, G.; Evoli, A.; Alessandrini, G.; et al. Thymic epithelial tumors express vascular endothelial growth factors and their receptors as potential targets of antiangiogenic therapy: A tissue micro array-based multicenter study. Lung Cancer 2014, 85, 191–196. [Google Scholar] [CrossRef]
- Raica, M.; Mogoantă, L.; Kondylis, A.; Cîmpean, A.M. Angiogenesis in the human thymoma assessed by subclassification of tumor-associated blood vessels and endothelial cells proliferation. Rom. J. Morphol. Embryol. 2010, 51, 627–631. [Google Scholar]
- Cimpean, A.M.; Ceauşu, R.; Encică, S.; Gaje, P.N.; Ribatti, D.; Raica, M. Platelet-derived growth factor and platelet-derived growth factor receptor-α expression in the normal human thymus and thymoma. Int. J. Exp. Pathol. 2011, 92, 340–344. [Google Scholar] [CrossRef]
- Bisagni, G.; Rossi, G.; Cavazza, A.; Sartori, G.; Gardini, G.; Boni, C. Long Lasting Response to the Multikinase Inhibitor Bay 43-9006 (Sorafenib) in a Heavily Pretreated Metastatic Thymic Carcinoma. J. Thorac. Oncol. 2009, 4, 773–775. [Google Scholar] [CrossRef]
- Ströbel, P.; Bargou, R.; Wolff, A.; Spitzer, D.; Manegold, C.; Dimitrakopoulou-Strauss, A.; Strauss, L.; Sauer, C.; Mayer, F.; Hohenberger, P.; et al. Sunitinib in metastatic thymic carcinomas: Laboratory findings and initial clinical experience. Br. J. Cancer 2010, 103, 196–200. [Google Scholar] [CrossRef]
- Lucidarme, O.; Wagner, M.; Gillard, P.; Kim, S.; Bachet, J.B.; Rousseau, B.; Mazard, T.; Louvet, C.; Chibaudel, B.; Cohen, R.; et al. RECIST and CHOI criteria in the evaluation of tumor response in patients with metastatic colorectal cancer treated with regorafenib, a prospective multicenter study. Cancer Imaging 2019, 19, 85. [Google Scholar] [CrossRef]
- Solis-Hernandez, M.P.; Fernandez Del Valle, A.; Carmona-Bayonas, A.; Garcia-Carbonero, R.; Custodio, A.; Benavent, M.; Alonso Gordoa, T.; Nunez-Valdovino, B.; Sanchez Canovas, M.; Matos, I.; et al. Evaluating radiological response in pancreatic neuroendocrine tumours treated with sunitinib: Comparison of Choi versus RECIST criteria (CRIPNET_ GETNE1504 study). Br. J. Cancer 2019, 121, 537–544. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Verderio, P.; Messina, A.; Morosi, C.; Collini, P.; Llombart-Bosch, A.; Martin, J.; Comandone, A.; Cruz, J.; Ferraro, A.; et al. Tumor response assessment by modified Choi criteria in localized high-risk soft tissue sarcoma treated with chemotherapy. Cancer 2012, 118, 5857–5866. [Google Scholar] [CrossRef]
- Ma, J.; Waxman, D.J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 2008, 7, 3670–3684. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef]
- Ansari, M.J.; Bokov, D.; Markov, A.; Jalil, A.T.; Shalaby, M.N.; Suksatan, W.; Chupradit, S.; Al-Ghamdi, H.S.; Shomali, N.; Zamani, A.; et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun. Signal 2022, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 2006–2012. [Google Scholar] [CrossRef]
- Johnson, D.H.; Fehrenbacher, L.; Novotny, W.F.; Herbst, R.S.; Nemunaitis, J.J.; Jablons, D.M.; Langer, C.J.; DeVore, R.F., 3rd; Gaudreault, J.; Damico, L.A.; et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004, 22, 2184–2191. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Dai, L.; Hu, W.; Chen, X.; Han, J.; Ma, X.; Tian, G.; Han, S.; Long, J.; et al. Efficacy and toxicities of gemcitabine and cisplatin combined with endostar in advanced thymoma and thymic carcinoma. Thorac. Cancer 2019, 10, 17–23. [Google Scholar] [CrossRef]
- Imbimbo, M.; Vitali, M.; Fabbri, A.; Ottaviano, M.; Pasello, G.; Petrini, I.; Palmieri, G.; Berardi, R.; Zucali, P.; Ganzinelli, M.; et al. RELEVENT Trial: Phase II Trial of Ramucirumab, Carboplatin, and Paclitaxel in Previously Untreated Thymic Carcinoma/B3 Thymoma with Area of Carcinoma. Clin. Lung Cancer 2018, 19, e811–e814. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Jabouille, A.; Rivera, L.B.; Lodewijckx, I.; Missiaen, R.; Steri, V.; Feyen, K.; Tawney, J.; Hanahan, D.; Michael, I.P.; et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 2017, 9, eaak9679. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Catania, C.; Zucali, P.A.; Della Vigna, P.; Pirola, S.; Stucchi, S.; Pennacchioli, E.; Queirolo, P.; Giaccone, G.; et al. Safety and activity of Combined AVElumab with Axitinib in unresectable or metastatic Thymomas B3 and Thymic carcinomas: The CAVEATT study. J. Clin. Oncol. 2020, 38 (Suppl. S15), e21114. [Google Scholar] [CrossRef]
- Wong, S.K.; Whisenant, J.G.; Bestvina, C.M.; Berry, L.D.; Owonikoko, T.K.; Sanborn, R.E.; Lammers, P.E.; El Osta, B.E.; Ramalingam, S.S.; Carlisle, J.W.; et al. Phase I/II study of nivolumab plus vorolanib in patients with thoracic malignancies: Interim efficacy of the SCLC and primary refractory NSCLC cohorts, and safety data across all cohorts. J. Clin. Oncol. 2021, 39 (Suppl. S15), 2578. [Google Scholar] [CrossRef]
- Iams, W.T.; Lovly, C.M. Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade. Clin. Cancer Res. 2015, 21, 4270–4277. [Google Scholar] [CrossRef] [PubMed]
- Zucali, P.A.; Petrini, I.; Lorenzi, E.; Merino, M.; Cao, L.; Di Tommaso, L.; Lee, H.S.; Incarbone, M.; Walter, B.A.; Simonelli, M.; et al. Insulin-like growth factor-1 receptor and phosphorylated AKT-serine 473 expression in 132 resected thymomas and thymic carcinomas. Cancer 2010, 116, 4686–4695. [Google Scholar] [CrossRef]
- Girard, N.; Teruya-Feldstein, J.; Payabyab, E.C.; Riely, G.J.; Rusch, V.W.; Kris, M.G.; Zakowski, M.F. Insulin-Like Growth Factor-1 Receptor Expression in Thymic Malignancies. J. Thorac. Oncol. 2010, 5, 1439–1446. [Google Scholar] [CrossRef]
- Haluska, P.; Shaw, H.M.; Batzel, G.N.; Yin, D.; Molina, J.R.; Molife, L.R.; Yap, T.A.; Roberts, M.L.; Sharma, A.; Gualberto, A.; et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin. Cancer Res. 2007, 13, 5834–5840. [Google Scholar] [CrossRef]
- McKian, K.P.; Haluska, P. Cixutumumab. Expert. Opin. Investig. Drugs 2009, 18, 1025–1033. [Google Scholar] [CrossRef]
- Radovich, M.; Solzak, J.P.; Hancock, B.A.; Conces, M.L.; Atale, R.; Porter, R.F.; Zhu, J.; Glasscock, J.; Kesler, K.A.; Badve, S.S.; et al. A large microRNA cluster on chromosome 19 is a transcriptional hallmark of WHO type A and AB thymomas. Br. J. Cancer 2016, 114, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Alberobello, A.T.; Wang, Y.; Beerkens, F.J.; Conforti, F.; McCutcheon, J.N.; Rao, G.; Raffeld, M.; Liu, J.; Rahhal, R.; Zhang, Y.W.; et al. PI3K as a Potential Therapeutic Target in Thymic Epithelial Tumors. J. Thorac. Oncol. 2016, 11, 1345–1356. [Google Scholar] [CrossRef]
- Maury, J.-M.; Merveilleux du Vignaux, C.; Drevet, G.; Zarza, V.; Chalabreysse, L.; Maisse, C.; Gineys, B.; Dolmazon, C.; Tronc, F.; Girard, N.; et al. Activation of the mTOR/ Akt pathway in thymic epithelial cells derived from thymomas. PLoS ONE 2019, 14, e0197655. [Google Scholar] [CrossRef]
- Girard, N.; Ostrovnaya, I.; Lau, C.; Park, B.; Ladanyi, M.; Finley, D.; Deshpande, C.; Rusch, V.; Orlow, I.; Travis, W.D.; et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin. Cancer Res. 2009, 15, 5184–5190. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Girard, N.; Marx, A. State of the art of genetic alterations in thymic epithelial tumors. J. Thorac. Oncol. 2014, 9 (Suppl. S2), S131–S136. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Marx, A.; Zettl, A.; Ströbel, P.; Müller-Hermelink, H.K.; Starostik, P. Chromosome 6 suffers frequent and multiple aberrations in thymoma. Am. J. Pathol. 2002, 161, 1507–1513. [Google Scholar] [CrossRef]
- Girard, N.; Shen, R.; Guo, T.; Zakowski, M.F.; Heguy, A.; Riely, G.J.; Huang, J.; Lau, C.; Lash, A.E.; Ladanyi, M.; et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin. Cancer Res. 2009, 15, 6790–6799. [Google Scholar] [CrossRef]
- Petrini, I.; Wang, Y.; Zucali, P.A.; Lee, H.S.; Pham, T.; Voeller, D.; Meltzer, P.S.; Giaccone, G. Copy Number Aberrations of Genes Regulating Normal Thymus Development in Thymic Epithelial Tumors. Clin. Cancer Res. 2013, 19, 1960–1971. [Google Scholar] [CrossRef]
- Noviello, C.; Courjal, F.; Theillet, C. Loss of heterozygosity on the long arm of chromosome 6 in breast cancer: Possibly four regions of deletion. Clin. Cancer Res. 1996, 2, 1601–1606. [Google Scholar]
- Theile, M.; Seitz, S.; Arnold, W.; Jandrig, B.; Frege, R.; Schlag, P.M.; Haensch, W.; Guski, H.; Winzer, K.J.; Barrett, J.C.; et al. A defined chromosome 6q fragment (at D6S310) harbors a putative tumor suppressor gene for breast cancer. Oncogene 1996, 13, 677–685. [Google Scholar]
- Bastian, B.C.; LeBoit, P.E.; Hamm, H.; Bröcker, E.B.; Pinkel, D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998, 58, 2170–2175. [Google Scholar] [PubMed]
- Aalto, Y.; Eriksson, L.; Seregard, S.; Larsson, O.; Knuutila, S. Concomitant loss of chromosome 3 and whole arm losses and gains of chromosome 1, 6, or 8 in metastasizing primary uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 313–317. [Google Scholar] [PubMed]
- Hirabayashi, H.; Fujii, Y.; Sakaguchi, M.; Tanaka, H.; Yoon, H.E.; Komoto, Y.; Inoue, M.; Miyoshi, S.; Matsuda, H. p16INK4, pRB, p53 and cyclin D1 expression and hypermethylation of CDKN2 gene in thymoma and thymic carcinoma. Int. J. Cancer 1997, 73, 639–644. [Google Scholar] [CrossRef]
- Petrini, I.; Meltzer, P.S.; Zucali, P.A.; Luo, J.; Lee, C.; Santoro, A.; Lee, H.S.; Killian, K.J.; Wang, Y.; Tsokos, M.; et al. Copy number aberrations of BCL2 and CDKN2A/B identified by array-CGH in thymic epithelial tumors. Cell Death Dis. 2012, 3, e351. [Google Scholar] [CrossRef]
- Aesif, S.W.; Aubry, M.C.; Yi, E.S.; Kloft-Nelson, S.M.; Jenkins, S.M.; Spears, G.M.; Greipp, P.T.; Sukov, W.R.; Roden, A.C. Loss of p16(INK4A) Expression and Homozygous CDKN2A Deletion Are Associated with Worse Outcome and Younger Age in Thymic Carcinomas. J. Thorac. Oncol. 2017, 12, 860–871. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.G.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef]
- Kim, D.J.; Yang, W.I.; Kim, S.H.; Park, I.K.; Chung, K.Y. Expression of neurotrophin receptors in surgically resected thymic epithelial tumors. Eur. J. Cardiothorac. Surg. 2005, 28, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Ozono, K.; Onishi, H.; Iwamoto, N.; Nakamura, K.; Miyoshi, K.; Nakamura, M.; Oda, Y. Tropomyosin-related Kinase B Is Potentially a Biomarker of Prognosis and Therapeutic Target for Malignant Thymic Epithelial Tumors. Anticancer. Res. 2022, 42, 3779–3787. [Google Scholar] [CrossRef]
- Weiss, G.J.; Hidalgo, M.; Borad, M.J.; Laheru, D.; Tibes, R.; Ramanathan, R.K.; Blaydorn, L.; Jameson, G.; Jimeno, A.; Isaacs, J.D.; et al. Phase I study of the safety, tolerability and pharmacokinetics of PHA-848125AC, a dual tropomyosin receptor kinase A and cyclin-dependent kinase inhibitor, in patients with advanced solid malignancies. Investig. New Drugs 2012, 30, 2334–2343. [Google Scholar] [CrossRef]
- Conforti, F.; Zhang, X.; Rao, G.; De Pas, T.; Yonemori, Y.; Rodriguez, J.A.; McCutcheon, J.N.; Rahhal, R.; Alberobello, A.T.; Wang, Y.; et al. Therapeutic Effects of XPO1 Inhibition in Thymic Epithelial Tumors. Cancer Res. 2017, 77, 5614–5627. [Google Scholar] [CrossRef]
- Abdul Razak, A.R.; Mau-Soerensen, M.; Gabrail, N.Y.; Gerecitano, J.F.; Shields, A.F.; Unger, T.J.; Saint-Martin, J.R.; Carlson, R.; Landesman, Y.; McCauley, D.; et al. First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2016, 34, 4142–4150. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Liu, Y.; Wang, P.; Zhang, R. STAT3 expression correlates with prognosis of thymic epithelial tumors. J. Cardiothorac. Surg. 2013, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Okabe, N.; Fujiwara, M.; Tachibana, K.; Tanaka, R.; Kondo, H.; Kamma, H. STAT3 activation in thymic epithelial tumors: Correlation with cyclin D1, JAK3, and clinical behavior. Gen. General. Thorac. Cardiovasc. Surg. 2021, 69, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
| Reference/Study | Treatment | N | ORR (%) | DCR (%) | Median PFS (Months) | Median OS (Months) |
|---|---|---|---|---|---|---|
| SSTR Targeting Agents Palmieri et al. (2002) [10] Loehrer et al. (2004) [11] NCT05918302 [12] NCT04375267 [13] | Octreotide + Prednisone Octreotide + Prednisone Everolimus + PRRT Everolimus Olaparib + PRRT | 16 (10 T, 3 TC, 3 NET) 38 (32 T, 5 TC, 1 NET) 120 (solid tumor) 18 (solid tumor) | 38 32 (38 T, 0 TC/NET) | 75 68 | 14 9.2 | 15 NR |
| EGFR Targeting Agents Kurup et al. (2005) [14] Bedano et al. (2008) [15] NCT01025089 [16] | Gefitinib Erlotinib + Bevacizumab Cetuximab + PAC | 26 (19 T, 7 TC) 18 (11 T, 7 TC) 18 (T, TC) | 4 0 | 58 60 | - - | - - |
| c-KIT Targeting Agents Salter et al. (2008) [17] Giaccone et al. (2009) [18] | Imatinib Imatinib | 11 (TC) 7 (2 T, 5 TC) | 0 0 (100 T, 0 TC) | 27 29 | - 2 | - 4 |
| Epigenetic Targeting Agents Giaccone et al. (2011) [19] Thomas et al. (2014) [20] | Belinostat Belinostat + PAC | 41 (25 T, 16 TC) 25 (11 T, 14 TC) | 5 (8 T, 0 TC) 40 (64 T, 21 TC) | 68 (79 T, 50 TC) 100 (93 T, 96 TC) | 5.8 (11.4 T, 2.7 TC) 9.0 (NR T, 7.2 TC) | 19.2 (NR T, 12.4 TC) 28.5 (NR T, 21.4 TC) |
| Anti-Angiogenic Agents Thomas et al. (2015) [21] Sato et al. (2020) [22] Perrino et al. (2022) * [23] Conforti et al. (2022) [24] NCT04710628 [25] NCT03463460 [26] NCT03583086 [27] NCT03694002 [28] | Sunitinib Lenvatinib Regorafenib Axitinib + Avelumab Lenvatinib + Pembrolizumab Sunitinib + Pembrolizumab Vorolanib + Nivolumab CT + Ramucirumab | 41 (16 T, 25 TC) 42 (TC) 19 (11 T, 8 TC) 32 (5 T, 27 TC) 43 (T, TC) 30 (TC) 88 (solid tumor) 66 | 6 T, 26 TC 38 37 (10 T, 0 TC) 34 (40 T, 33 TC) | 81 T, 91 TC 95 79 (90 T, 86 TC) 91 (100 T, 89 TC) | 8.5 T, 7.2 TC 9.3 9.6 (9.6 T, 9.2 TC) 7.5 | 15.5 T, NR TC NR 33.8 (NR T, 20.1 TC) 26.6 |
| IGFR-PI3K-AKT Agents Rajan et al. (2014) [29] Zucali et al. (2018) [30] Abu Zaid et al. (2022) [31] | Cixutumumab Everolimus Buparlisib | 49 (37 T, 12 TC) 50 (32 T, 18 TC) 14 (T) | 10 (14 T, 0 TC) 12 (9 T, 17 TC) 7 | 78 (89 T, 42 TC) 88 (94 T, 78 TC) 50 | 8.2 (9.9 T, 1.7 TC) 10.1 (16.6 T, 5.6 TC) 11.1 | 16.2 (27.5 T, 8.4 TC) 25.7 (NR T, 14.7 TC) 40.0 |
| Cell Cycle/STAT3 Agents Besse et al. (2018) [32] Besse et al. (2018) [32] Kalra et al. (2018) [33] Jung et al. (2023) [34] NCT03556228 [35] | Milciclib Milciclib Napabucasin Palbociclib VMD-928 | 72 (20 T, 52 TC) 30 (17 T, 13 TC) 16 (10 T, 6 TC) 48 (24 T, 23 TC, 1 UNK) 74 (solid tumor or lymphoma) | 4.2 3.3 25 T, 67 TC 13 (17 T, 9 TC) | 72 70 63 T, 83 TC 79 (79 T, 78 TC) | 5.8 5.7 - 11.0 (13 T, 9.2 TC) | 24.4 21.0 - 26.4 (26.4 T, 25.6 TC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniar, R.; Loehrer, P.J., Sr. What Have We Learned from Molecularly Informed Clinical Trials on Thymomas and Thymic Carcinomas—Current Status and Future Directions? Cancers 2024, 16, 416. https://doi.org/10.3390/cancers16020416
Maniar R, Loehrer PJ Sr. What Have We Learned from Molecularly Informed Clinical Trials on Thymomas and Thymic Carcinomas—Current Status and Future Directions? Cancers. 2024; 16(2):416. https://doi.org/10.3390/cancers16020416
Chicago/Turabian StyleManiar, Rohan, and Patrick J. Loehrer, Sr. 2024. "What Have We Learned from Molecularly Informed Clinical Trials on Thymomas and Thymic Carcinomas—Current Status and Future Directions?" Cancers 16, no. 2: 416. https://doi.org/10.3390/cancers16020416
APA StyleManiar, R., & Loehrer, P. J., Sr. (2024). What Have We Learned from Molecularly Informed Clinical Trials on Thymomas and Thymic Carcinomas—Current Status and Future Directions? Cancers, 16(2), 416. https://doi.org/10.3390/cancers16020416






