Combined Merkel Cell Carcinoma and Squamous Cell Carcinoma: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
Inclusion and Exclusion Criteria
2.3. Search Strategy
2.4. Data Extraction
2.5. Risk of Bias
2.6. Statistical Analysis
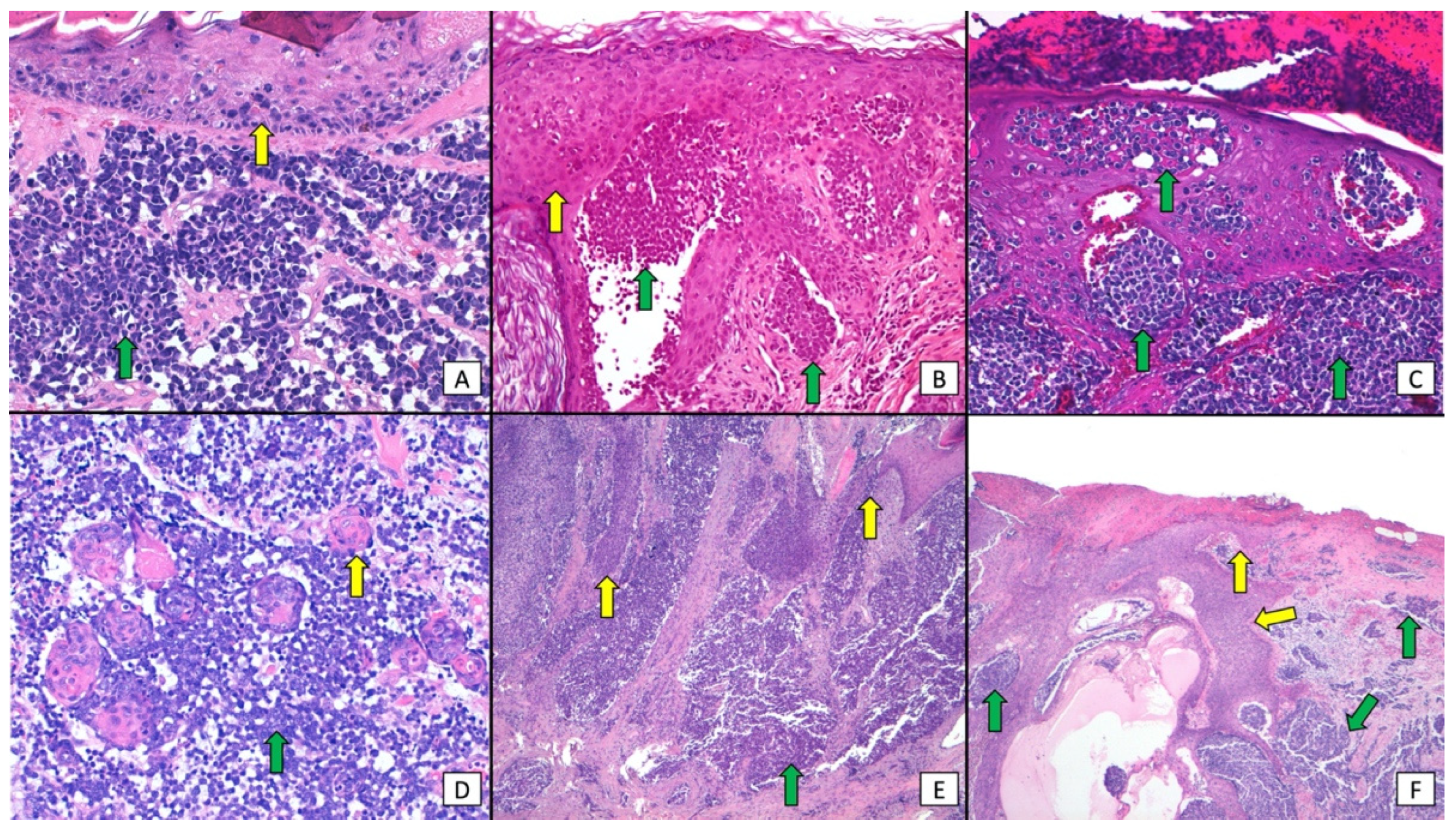
3. Results
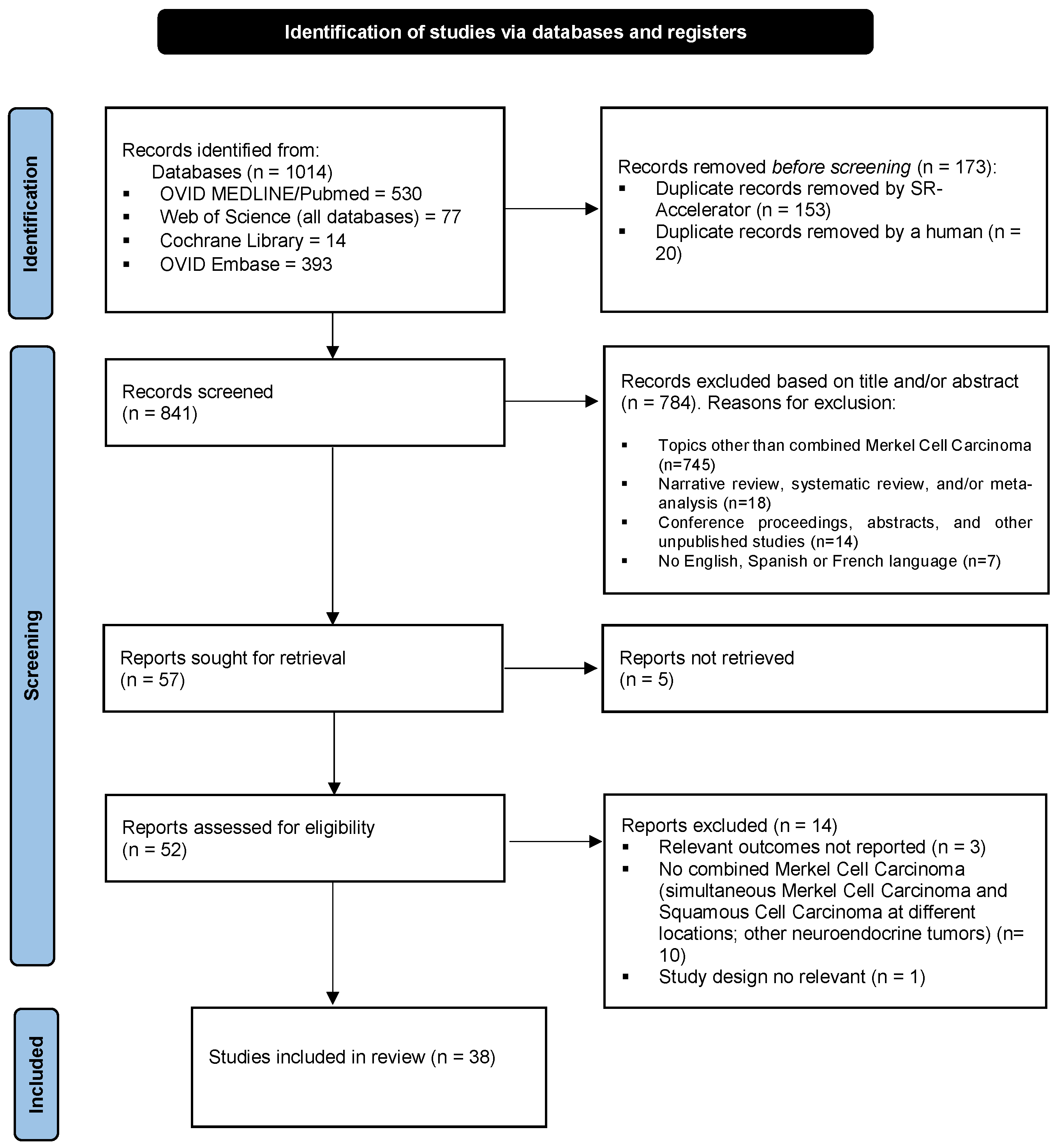
3.1. Study Selection
3.2. Study Quality and Bias Results
3.3. Study and Population Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Toker, C. Trabecular Carcinoma of the Skin. Arch. Dermatol. 1972, 105, 107–110. [Google Scholar] [CrossRef]
- Nirenberg, A.; Steinman, H.; Dixon, J.; Dixon, A. Merkel Cell Carcinoma Update: The Case for Two Tumours. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Appenzeller, S.; Samimi, M.; Sarma, B.; Sarosi, E.-M.; Berthon, P.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Blom, A.; Benethon, N.; et al. Merkel Cell Polyomavirus–Negative Merkel Cell Carcinoma Originating from In Situ Squamous Cell Carcinoma: A Keratinocytic Tumor with Neuroendocrine Differentiation. J. Investig. Dermatol. 2021, 142, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Tallet, A.; Miquelestorena-Standley, E.; Houben, R.; Schrama, D.; Gambichler, T.; Berthon, P.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Aubin, F.; et al. Morphologic and Immunophenotypical Features Distinguishing Merkel Cell Polyomavirus-Positive and Negative Merkel Cell Carcinoma. Mod. Pathol. 2019, 32, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- González-Vela, M.D.C.; Curiel-Olmo, S.; Derdak, S.; Beltran, S.; Santibañez, M.; Martínez, N.; Castillo-Trujillo, A.; Gut, M.; Sánchez-Pacheco, R.; Almaraz, C.; et al. Shared Oncogenic Pathways Implicated in Both Virus-Positive and UV-Induced Merkel Cell Carcinomas. J. Investig. Dermatol. 2017, 137, 197–206. [Google Scholar] [CrossRef]
- Moshiri, A.S.; Doumani, R.; Yelistratova, L.; Blom, A.; Lachance, K.; Shinohara, M.M.; Delaney, M.; Chang, O.; McArdle, S.; Thomas, H.; et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J. Investig. Dermatol. 2017, 137, 819–827. [Google Scholar] [CrossRef]
- Houben, R.; Celikdemir, B.; Kervarrec, T.; Schrama, D. Merkel Cell Polyomavirus: Infection, Genome, Transcripts and Its Role in Development of Merkel Cell Carcinoma. Cancers 2023, 15, 444. [Google Scholar] [CrossRef]
- Martin, B.; Poblet, E.; Rios, J.J.; Kazakov, D.; Kutzner, H.; Brenn, T.; Calonje, E. Merkel Cell Carcinoma with Divergent Differentiation: Histopathological and Immunohistochemical Study of 15 Cases with PCR Analysis for Merkel Cell Polyomavirus. Histopathology 2013, 62, 711–722. [Google Scholar] [CrossRef]
- Pulitzer, M.P.; Brannon, A.R.; Berger, M.F.; Louis, P.; Scott, S.N.; Jungbluth, A.A.; Coit, D.G.; Brownell, I.; Busam, K.J. Cutaneous Squamous and Neuroendocrine Carcinoma: Genetically and Immunohistochemically Different from Merkel Cell Carcinoma. Mod. Pathol. 2015, 28, 1023–1032. [Google Scholar] [CrossRef]
- Mendoza, M.-D.; Santonja, C.; Gonzalez-Vela, C.; Concha, A.; Iglesias Pena, N.; Andrés-Esteban, E.-M.; Vaque, J.P.; Cereceda, L.; Pajares, R.; Kutzner, H.; et al. The Presence of Merkel Cell Carcinoma Polyomavirus Is Associated with a Distinct Phenotype in Neoplastic Merkel Cell Carcinoma Cells and Their Tissue Microenvironment. PLoS ONE 2020, 15, e0232517. [Google Scholar] [CrossRef]
- Ferrándiz-Pulido, C.; Gómez-Tomás, A.; Llombart, B.; Mendoza, D.; Marcoval, J.; Piaserico, S.; Baykal, C.; Bouwes-Bavinck, J.N.; Rácz, E.; Kanitakis, J.; et al. Clinicopathological Features, MCPyV Status and Outcomes of Merkel Cell Carcinoma in Solid-Organ Transplant Recipients: A Retrospective, Multicentre Cohort Study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1991–2001. [Google Scholar] [CrossRef]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Clinical Factors Associated with Merkel Cell Polyomavirus Infection in Merkel Cell Carcinoma. JNCI J. Natl. Cancer Inst. 2009, 101, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Naseri, S.; Steiniche, T.; Georgsen, J.B.; Thomsen, R.; Ladekarl, M.; Heje, M.; Damsgaard, T.E.; Bønnelykke-Behrndtz, M.L. Tumor Ulceration, Reduced Infiltration of CD8-Lymphocytes, High Neutrophil-to-CD8-Lymphocyte Ratio and Absence of MC Virus Are Negative Prognostic Markers for Patients with Merkel Cell Carcinoma. Cancers 2020, 12, 888. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-Step Guide on How to Design, Conduct, and Successfully Publish a Systematic Review and Meta-Analysis in Medical Research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Siddaway, A.P.; Wood, A.M.; Hedges, L.V. How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-Analyses, and Meta-Syntheses. Annu. Rev. Psychol. 2019, 70, 747–770. [Google Scholar] [CrossRef]
- Samimi, M.; Kervarrec, T.; Touze, A. Immunobiology of Merkel Cell Carcinoma. Curr. Opin. Oncol. 2020, 32, 114–121. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Martín-Sanz, R.; García-Piqueras, J.; Cobo, R.; Muñoz-Bravo, S.; García-Suárez, O.; Martín-Biedma, B.; Vega, J.A.; Feito, J. Merkel Cell Carcinoma Display PIEZO2 Immunoreactivity. J. Pers. Med. 2022, 12, 894. [Google Scholar] [CrossRef]
- DeCoste, R.C.; Carter, M.D.; Ly, T.Y.; Gruchy, J.R.; Nicolela, A.P.; Pasternak, S. Merkel Cell Carcinoma: An Update. Hum. Pathol. 2023, 140, 39–52. [Google Scholar] [CrossRef]
- Brazel, D.; Kumar, P.; Doan, H.; Pan, T.; Shen, W.; Gao, L.; Moyers, J.T. Genomic Alterations and Tumor Mutation Burden in Merkel Cell Carcinoma. JAMA Netw. Open 2023, 6, e2249674. [Google Scholar] [CrossRef]
- Narisawa, Y.; Koba, S.; Inoue, T.; Nagase, K. Histogenesis of Pure and Combined Merkel Cell Carcinomas: An Immunohistochemical Study of 14 Cases. J. Dermatol. 2015, 42, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Kuwamoto, S.; Higaki, H.; Kanai, K.; Iwasaki, T.; Sano, H.; Nagata, K.; Kato, K.; Kato, M.; Murakami, I.; Horie, Y.; et al. Association of Merkel Cell Polyomavirus Infection with Morphologic Differences in Merkel Cell Carcinoma. Hum. Pathol. 2011, 42, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Verhaegen, M.E.; Hu, K.; Hrycaj, S.M.; Chan, M.P.; Liu, C.-J.; Grachtchouk, M.; Patel, R.M.; Udager, A.M.; Dlugosz, A.A. Genomic Evidence Suggests That Cutaneous Neuroendocrine Carcinomas Can Arise from Squamous Dysplastic Precursors. Mod. Pathol. 2022, 35, 506–514. [Google Scholar] [CrossRef]
- Kervarrec, T.; Samimi, M.; Gaboriaud, P.; Gheit, T.; Beby-Defaux, A.; Houben, R.; Schrama, D.; Fromont, G.; Tommasino, M.; Le Corre, Y.; et al. Detection of the Merkel Cell Polyomavirus in the Neuroendocrine Component of Combined Merkel Cell Carcinoma. Virchows Arch. 2018, 472, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Gruchy, J.R.; Pasternak, S.; Ly, T.Y.; DeCoste, R.C.; Fleming, K.E.; Moss, P.M.; Carter, M.D.; Walsh, N.M. Morphological Patterns of Metastases from Combined Merkel Cell Carcinomas: Study of an Eastern Canadian Cohort of Cases. Hum. Pathol. 2022, 129, 47–55. [Google Scholar] [CrossRef] [PubMed]
- DeCoste, R.C.; Carter, M.D.; Pasternak, S.; Fleming, K.E.; Gaston, D.; Legge, A.; Ly, T.Y.; Walsh, N.M. Relationship between P63 and P53 Expression in Merkel Cell Carcinoma and Corresponding Abnormalities in TP63 and TP53: A Study and a Proposal. Hum. Pathol. 2021, 117, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.L.; Zhao, L.; Johnson, B.; Wang, X.; Carskadon, S.; Palanisamy, N.; Rhodes, D.R.; Mannan, R.; Vo, J.N.; Choi, J.E.; et al. Virus-Positive Merkel Cell Carcinoma Is an Independent Prognostic Group with Distinct Predictive Biomarkers. Clin. Cancer Res. 2021, 27, 2494–2504. [Google Scholar] [CrossRef]
- Suárez, A.L.; Louis, P.; Kitts, J.; Busam, K.; Myskowski, P.L.; Wong, R.J.; Chen, C.-S.J.; Spencer, P.; Lacouture, M.; Pulitzer, M.P. Clinical and Dermoscopic Features of Combined Cutaneous Squamous Cell Carcinoma (SCC)/Neuroendocrine [Merkel Cell] Carcinoma (MCC). J. Am. Acad. Dermatol. 2015, 73, 968–975. [Google Scholar] [CrossRef]
- DeCoste, R.C.; Walsh, N.M.; Gaston, D.; Ly, T.Y.; Pasternak, S.; Cutler, S.; Nightingale, M.; Carter, M.D. RB1-Deficient Squamous Cell Carcinoma: The Proposed Source of Combined Merkel Cell Carcinoma. Mod. Pathol. 2022, 35, 1829–1836. [Google Scholar] [CrossRef]
- Ríos-Viñuela, E.; Traves, V.; Cruz, J.; Machado, I.; López-Guerrero, J.A.; Requena, C.; Llombart, B. Combined Merkel Cell Carcinoma and Cutaneous Squamous Cell Carcinoma with Lymph Node Metastases: Report of Two Cases. J. Cutan. Pathol. 2023, 50, 230–237. [Google Scholar] [CrossRef]
- Ogawa, T.; Donizy, P.; Wu, C.-L.; Cornejo, K.M.; Ryś, J.; Hoang, M.P. Morphologic Diversity of Merkel Cell Carcinoma. Am. J. Dermatopathol. 2020, 42, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.M. Primary Neuroendocrine (Merkel Cell) Carcinoma of the Skin: Morphologic Diversity and Implications Thereof. Hum. Pathol. 2001, 32, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Mitteldorf, C.; Mertz, K.D.; Fernández-Figueras, M.T.; Schmid, M.; Tronnier, M.; Kempf, W. Detection of Merkel Cell Polyomavirus and Human Papillomaviruses in Merkel Cell Carcinoma Combined with Squamous Cell Carcinoma in Immunocompetent European Patients. Am. J. Dermatopathol. 2012, 34, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Saeb-Lima, M.; Montante-Montes de Oca, D.; Albores-Saavedra, J. Merkel Cell Carcinoma with Eccrine Differentiation: A Clinicopathologic Study of 7 Cases. Ann. Diagn. Pathol. 2008, 12, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, J.; Gugelmeier, N.; Mazzei, M.E.; González, S.; Barcia, J.J.; Magliano, J. Lymph Node Metastasis With Both Components of Combined Cutaneous Squamous Cell Carcinoma/Merkel Cell (Neuroendocrine) Carcinoma. Am. J. Dermatopathol. 2018, 40, 626–628. [Google Scholar]
- Suaiti, L.; Onajin, O.; Sangueza, O. Concurrent Metastatic Merkel Cell Carcinoma and Cutaneous Squamous Cell Carcinoma in the Same Lymph Node. Am. J. Dermatopathol. 2019, 41, e61–e63. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Kang, N.-W.; Takeuchi, K.; Chuang, S.-S. Combined Merkel Cell Carcinoma with Nodal Presentation: Report of a Case Diagnosed with Excisional but Not Incisional Biopsy and Literature Review. Diagnostics 2023, 13, 449. [Google Scholar] [CrossRef]
- Carter, M.D.; Gaston, D.; Huang, W.-Y.; Greer, W.L.; Pasternak, S.; Ly, T.Y.; Walsh, N.M. Genetic Profiles of Different Subsets of Merkel Cell Carcinoma Show Links between Combined and Pure MCPyV-Negative Tumors. Hum. Pathol. 2018, 71, 117–125. [Google Scholar] [CrossRef]
| Main Clinical Characteristics | |||||||
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | Female | NA | Total | ||||
| 89/152 (59%) | 63/152 (41%) | 0/152 | 152 | ||||
| Primary Tumor Location | |||||||
| Head and Neck | Extremities | Trunk | NA | Total | |||
| 85/152 (56%) | 32/152 (21%) | 20/152 (13%) | 15/152 (10%) | 152 | |||
| Previous Skin Cancer | |||||||
| NMSC | MM | NMSC + MM | None | NA | Total | ||
| 37/152 (24%) | 1/152 (<1%) | 3/152 (2%) | 5/152(3%) | 106/152 (70%) | 152 | ||
| Immunosuppression | |||||||
| Yes | No | NA | Total | ||||
| 20/152 (13%) | 21/152 (14%) | 111/152 (73%) | 152 | ||||
| Tumor Stage at Diagnosis | |||||||
| Local | Nodal | Nodal or Visceral | NA | Total | |||
| 40/152 (26%) | 14/152 (9%) | 14/152 (9%) | 84/152 (55%) | 152 | |||
| Progression to Metastatic Disease (at Least Nodal) | |||||||
| Yes | No | NA | Total | ||||
| 67/152 (44%) | 27/152 (18%) | 58/152 (38%) | 152 | ||||
| Main Histopathological Characteristics | |||||||
|---|---|---|---|---|---|---|---|
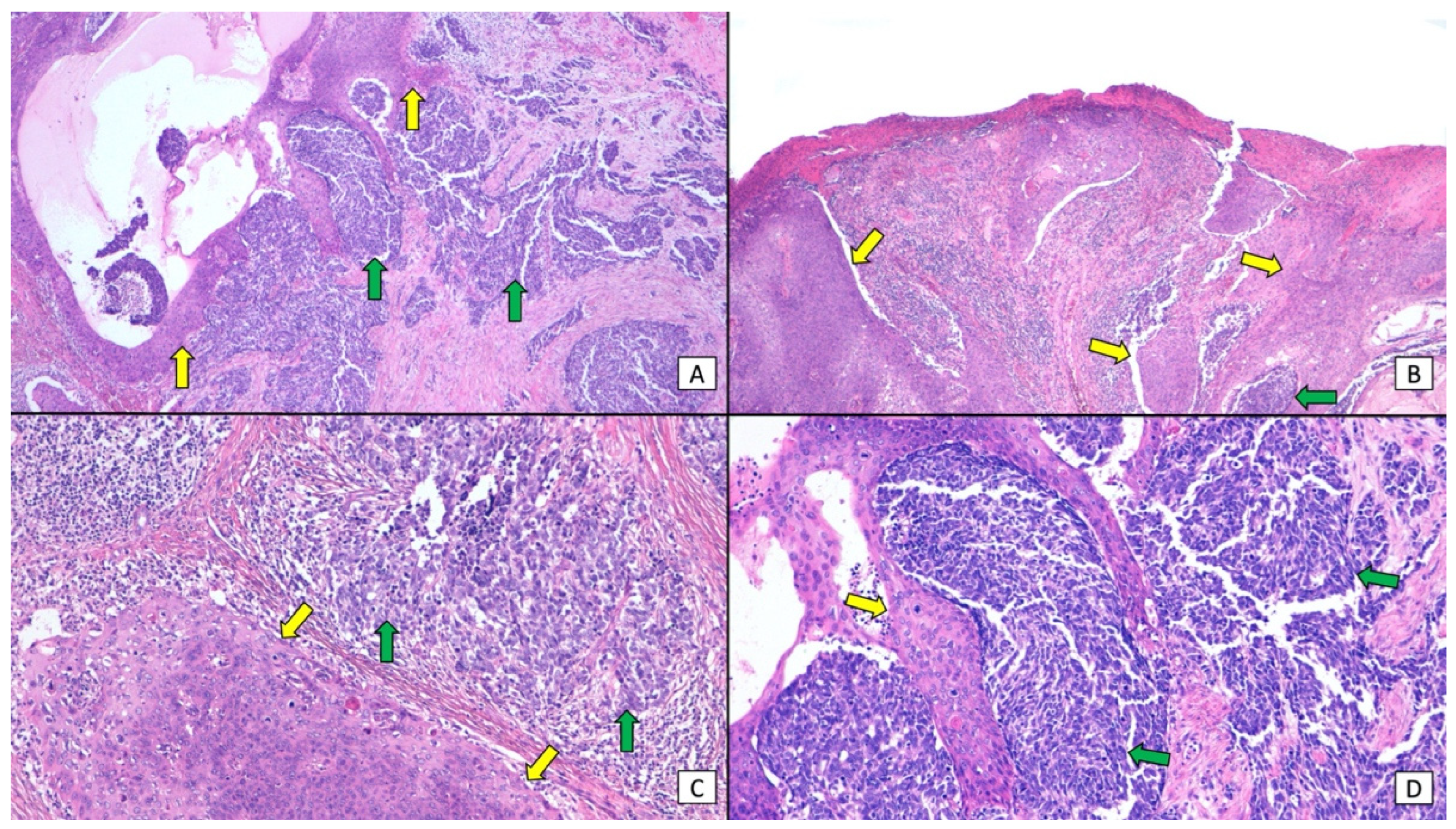
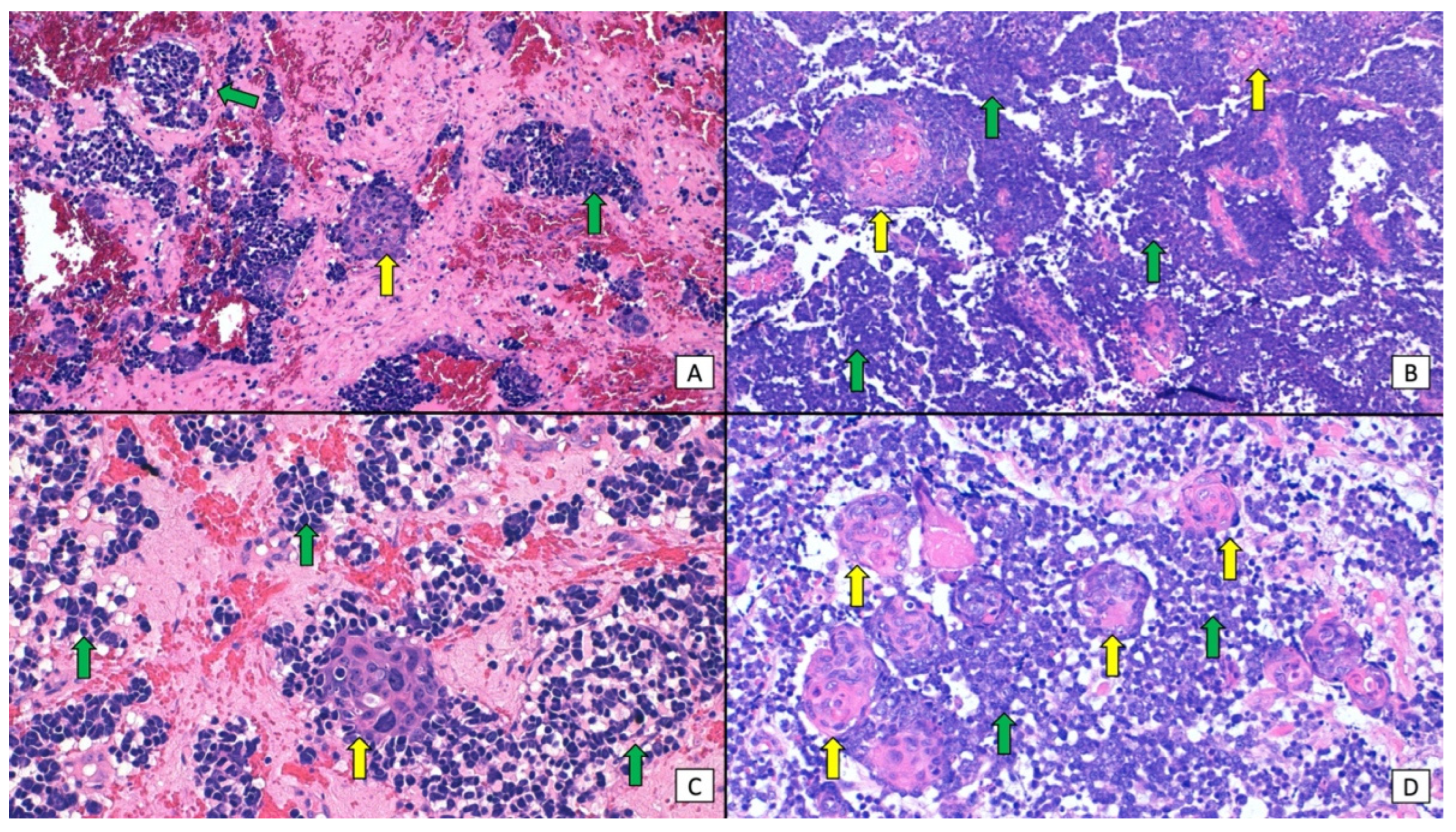
| Histopathological Anatomical Distribution of SCC and MCC | |||||||
| SCC Epidermis/MCC Dermis | SCC Epidermis + Dermis/MCC Dermis | SCC Epidermis/MCC Epidermis | SCC Epidermis/MCC Epidermis + Dermis | SCC Epidermis + Dermis/MCC Epidermis + Dermis | SCC Dermis/MCC Dermis | NA | Total |
| 54/152 (36%) | 31/152 (21%) | 4/152 (3%) | 3/152(2%) | 2/152(1%) | 16/152 (11%) | 42/152 (28%) | 152 |
| Histopathological distribution of SCC and MCC as independent (collision) tumors or tumors with divergent differentiation | |||||||
| Independent (collision) tumors | Tumors with divergent differentiation | Tumors showing both patterns | NA | Total | |||
| 71/152 (47%) | 14/152 (9%) | 12/152 (8%) | 55/152 (36%) | 152 | |||
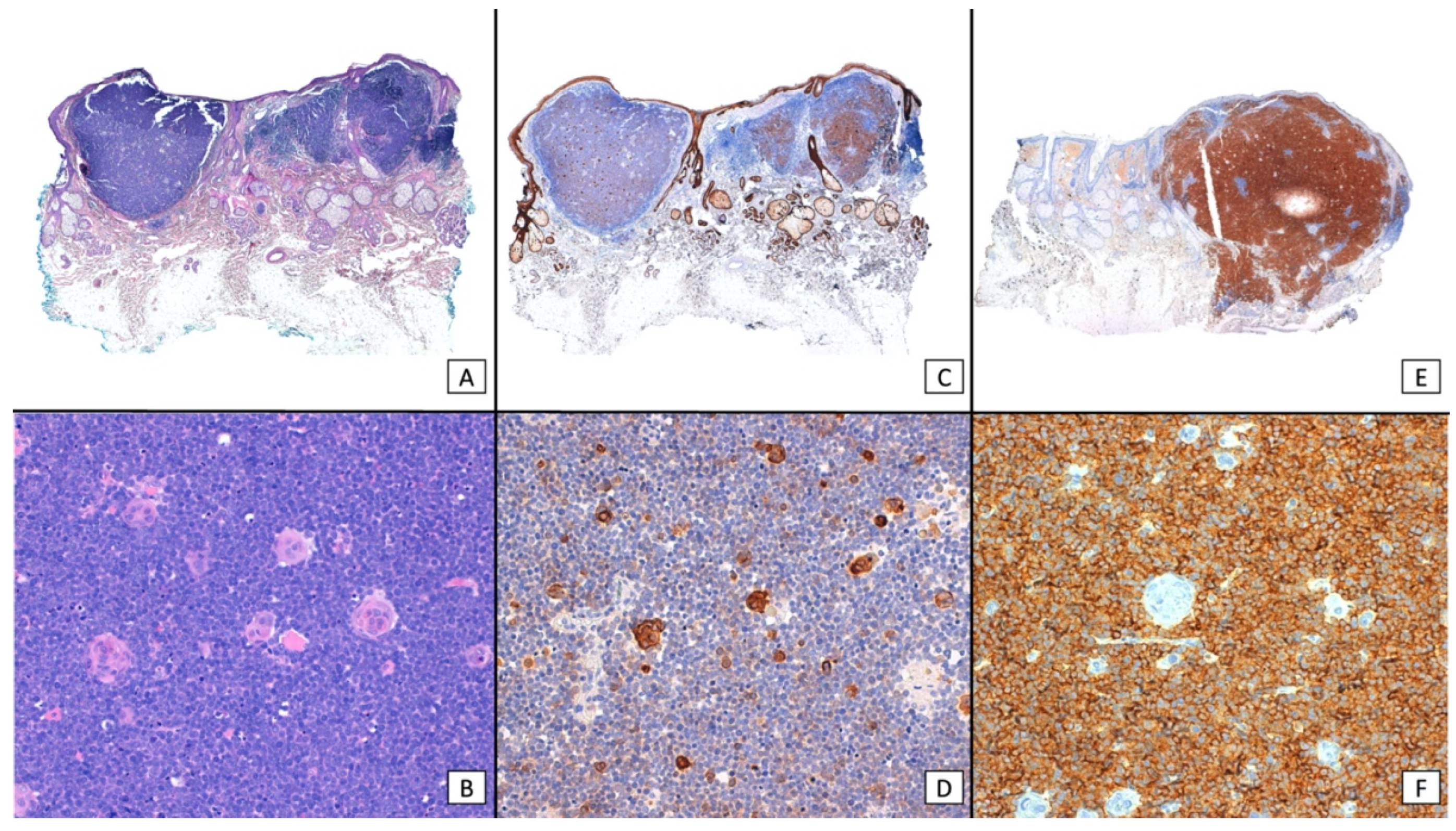
| MCPyV status | |||||||
| Negative IHC (CM2B4 large T antigen) | Negative PCR | Negative IHC and PCR | Positive IHC (CM2B4 large T antigen) | Positive PCR | Positive IHC and PCR | NA | Total |
| 56/152 (37%) | 6/152 (4%) | 54/152 (36%) | 3/152 (2%) | 1/152 (<1%) | 2/152 (1%) | 30/152 (20%) | 152 |
| CK20 (IHC) | |||||||
| CK20+ | CK20− | NA | Total | ||||
| 71/152 (47%) | 13/152 (9%) | 68/152 (45%) | 152 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Viñuela, E.; Mayo-Martínez, F.; Nagore, E.; Millan-Esteban, D.; Requena, C.; Sanmartín, O.; Llombart, B. Combined Merkel Cell Carcinoma and Squamous Cell Carcinoma: A Systematic Review. Cancers 2024, 16, 411. https://doi.org/10.3390/cancers16020411
Ríos-Viñuela E, Mayo-Martínez F, Nagore E, Millan-Esteban D, Requena C, Sanmartín O, Llombart B. Combined Merkel Cell Carcinoma and Squamous Cell Carcinoma: A Systematic Review. Cancers. 2024; 16(2):411. https://doi.org/10.3390/cancers16020411
Chicago/Turabian StyleRíos-Viñuela, Elisa, Fatima Mayo-Martínez, Eduardo Nagore, David Millan-Esteban, Celia Requena, Onofre Sanmartín, and Beatriz Llombart. 2024. "Combined Merkel Cell Carcinoma and Squamous Cell Carcinoma: A Systematic Review" Cancers 16, no. 2: 411. https://doi.org/10.3390/cancers16020411
APA StyleRíos-Viñuela, E., Mayo-Martínez, F., Nagore, E., Millan-Esteban, D., Requena, C., Sanmartín, O., & Llombart, B. (2024). Combined Merkel Cell Carcinoma and Squamous Cell Carcinoma: A Systematic Review. Cancers, 16(2), 411. https://doi.org/10.3390/cancers16020411







