The Facilitators and Barriers of the Implementation of a Clinical Decision Support System for Breast Cancer Multidisciplinary Team Meetings—An Interview Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants and Procedures
2.3. CDSS Features Example
2.4. Data Analysis
3. Results
3.1. Participant and Hospital Overview
3.2. Interview Results
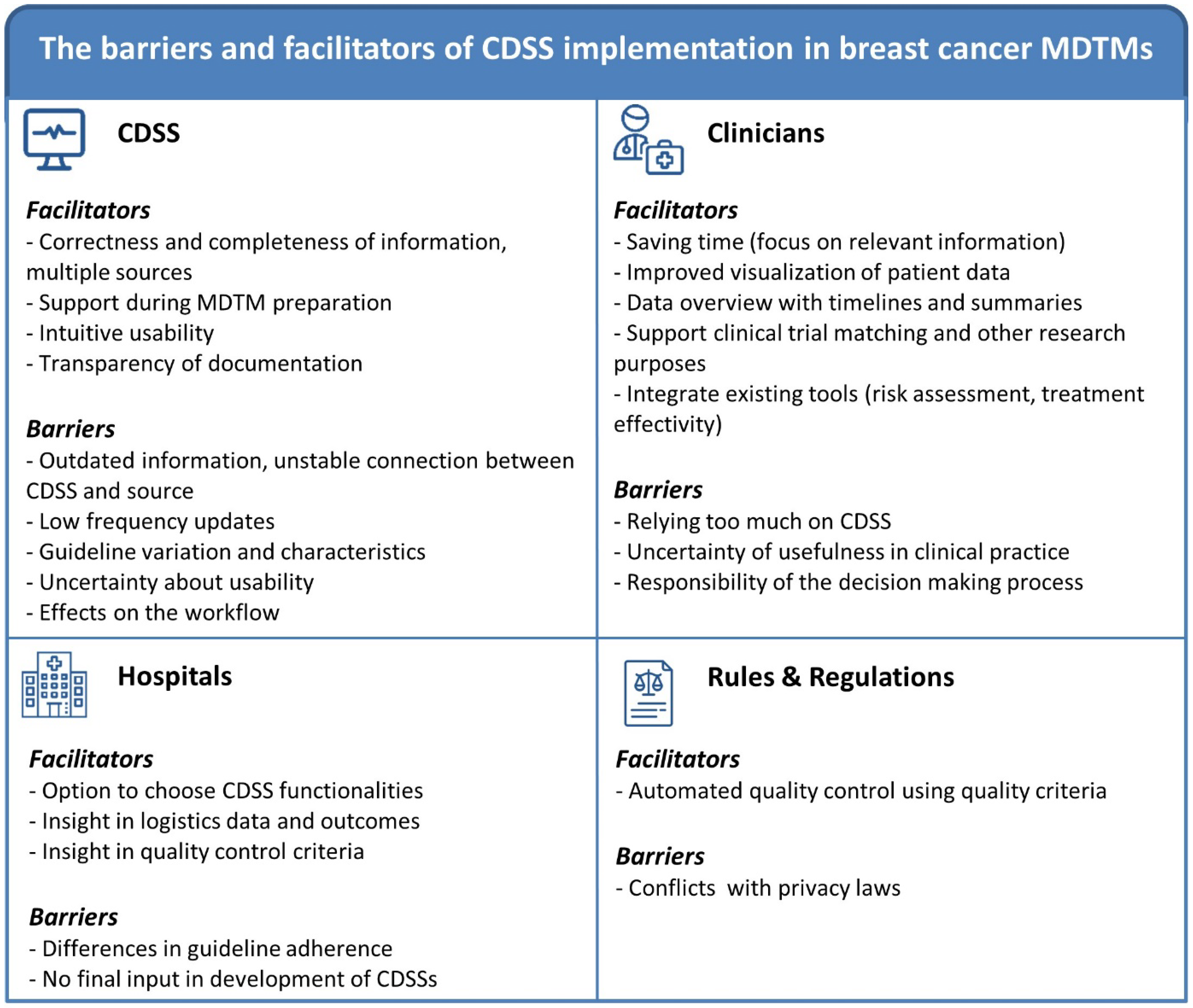
3.2.1. Results Associated with Innovation (CDSS) Use and Implementation
| Innovation: CDSSs | ||
|---|---|---|
| MIDI Determinant | Facilitators | Barriers |
| Correctness (2) |
| (1) Clinical trial matching would be useful
|
| Completeness (3) |
|
|
| Complexity (4) |
|
|
| Compatibility (5) |
|
|
| Observability (6) |
|
|
| Relevance for client (7) | (1) Clinical trial matching would be useful
|
|
Visualizing Patient Information
“Maybe you can indeed have that general overview in one screen and then show a summary or conclusion. And then have the advice summarized on another screen. So, you’re not scrolling through it. What you naturally hope for is to make it even more efficient than it is now. So that all the data is right there at one glance, without the need for searching or extra effort. That would be great.”(Breast cancer specialized nurse)
“It’s quite inconvenient not to have certain information you need readily available in the EMR, leading to a lot of searching. Everyone starts searching. That can be confusing at times. Even though that information is actually already available in another system but not shown in the EMR. Ideally you want it to be as up-to-date and accurate as possible.”(Pathologist)
Identifying Potential Clinical Trial Participants
“Currently it is our responsibility to decide whether a patient is eligible for a study. If a system could pick that up and say, ‘Hey, I see that this patient qualifies for this particular study.’ I think that would be extremely helpful because it’s a shame to forget these types of things.”(Radiotherapist)
Automatic Suggestions for Diagnostic Procedures and Treatment
“I do think it’s good to take a step back to what the standard (guideline) is, when we opt for something different. Because, when you look back at your notes, they’re very concise. If you weren’t present, you could still understand why we decided on certain recommendations. I know that when I haven’t been present myself, sometimes the notes are so concise that I wonder: which steps did they take. Yes, I believe it does make it clearer when you present it that way. And it’s also more comprehensible for those who weren’t there.”(Breast cancer specialized nurse)
“Looking at the breast cancer guidelines for oncologists, it mainly states, ‘consider this’ and ‘discuss with the patient.’ And those are actually the two most important things. So, yes, it remains a bit vague. It’s more about leaving various different options that could be considered. You might be able to include guidelines for systemic therapy in a CDSS. But I think the nuance of discussing or considering will likely remain, and it might not significantly change the policy, although it’s more convenient to have it already there on screen, so you don’t have to type it.”(Oncologist)
3.2.2. Results Associated with Users (Breast Cancer Clinicians)
| User: Breast Cancer Clinicians | ||
|---|---|---|
| MIDI Determinant | Facilitators | Barriers |
| Personal benefits and disadvantage (8) | Time-saving functionalities of CDSS according to clinicians:
|
|
| Outcome expectation (9) |
|
|
| Complexity (4) |
| |
“Maybe someday you would want an MDTM report to be automatically generated. Where you can specify input that really matters, and then you already have a sort of pre-generated report to work in. Because then you only need to type the advice. And what you notice in this EMR system is that some people do a complete copy-paste from the initial consultation and keep repeating it continuously in other reports. I want to know what it’s about, without very vague language. I just want to know relevant information for that report, briefly, the characteristics.”(Surgeon)
“Sometimes people end up repeating things during the MDTM. So sometimes, all these ideas are put on the table, and then they say we should do this now, we should do that now. And then it’s discussed for a bit, and people start suggesting doing this or that again. And then no decision is made. So, I think having some form of clinical decision-making tool would be quite helpful.”(Radiologist)
“Automatic suggestions could also be helpful to check, but you might have to do it in a certain order. First making your own recommendation, and then seeing what the CDSS suggests based on guidelines, and then discussing that. But if you let only the computer, make a suggestion first and then the clinician, the clinician might get lazy because after ten times, they’ll just agree with the computer.”(Pathologist)
“I find it hard to have an expectation about the CDSS now. What you naturally hope for is to make it even more efficient than it is now. And how that will actually look, well, I can’t really say for sure right now.”(Breast cancer specialized nurse)
“If there’s a pattern in the data that we’re not picking up ourselves, then I think it would be fantastic if we could use deep learning techniques to extract new information to advance our knowledge. I don’t necessarily see this as support for an MDTM, but more in the general development of our healthcare and knowledge. We make estimations based on statistics as best as we can to determine who needs adjuvant treatment or not, but it’s still very rough. If there’s an artificial intelligence or deep learning system that can do it better, I’m all for it. But again, that’s more of a research setting than an CDSS or MDTM setting.”(Oncologist)
3.2.3. Results Associated with Organization (Hospitals) and Socio-Political Context
| 4.1 Organization: Hospitals | ||
| MIDI Determinant | Facilitators | Barriers |
| Material resources and facilities (24) |
| |
| Performance feedback (28) |
|
|
| 4.2 Socio-Political Context: Quality of Care Indicators | ||
| MIDI Determinant | Facilitators | Barriers |
| Legislation and regulations (29) |
| |
“Having unwanted pop-ups from the national guideline saying, ‘do this or that’, no. Being able to select the functionality that you want to use is better. As soon as there are functions that only give you trouble, making you have to close them, then it works against you because it becomes a nuisance. That’s a shame considering the other functions that might actually be beneficial.”(Radiotherapist)
4. Discussion
4.1. Summary of Evidence
4.2. General Status of CDSS Implementation in Clinical Practice
4.3. Barriers and Facilitators: CDSS and Processes
4.4. Barriers and Facilitators: Clinicians
4.5. Rules and Regulations
4.6. Patient Perspectives
4.7. Strengths and Limitations of This Study
4.8. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Saghir, N.S.; Keating, N.L.; Carlson, R.W.; Khoury, K.E.; Fallowfield, L. Tumor boards: Optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e461–e466. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Sainsbury, R. Breast cancer: Multidisciplinary care and clinical outcomes. Eur. J. Cancer 2006, 42, 2480–2491. [Google Scholar] [CrossRef]
- Li, J.; Robertson, T.; Hansen, S.; Mansfield, T.; Kjeldskov, J. Multidisciplinary medical team meetings: A field study of collaboration in health care. In Proceedings of the 20th Australasian Conference on Computer-Human Interaction: Designing for Habitus and Habitat, Cairns, Australia, 8–12 December 2008; pp. 73–80. [Google Scholar]
- Chinai, N.; Bintcliffe, F.; Armstrong, E.M.; Teape, J.; Jones, B.M.; Hosie, K.B. Does every patient need to be discussed at a multidisciplinary team meeting? Clin. Radiol. 2013, 68, 780–784. [Google Scholar] [CrossRef] [PubMed]
- De Ieso, P.B.; Coward, J.I.; Letsa, I.; Schick, U.; Nandhabalan, M.; Frentzas, S.; Gore, M.E. A study of the decision outcomes and financial costs of multidisciplinary team meetings (MDMs) in oncology. Br. J. Cancer 2013, 109, 2295–2300. [Google Scholar] [CrossRef]
- Vondeling, G.; Menezes, G.; Dvortsin, E.; Jansman, F.; Konings, I.; Postma, M.; Rozenbaum, M. Burden of early, advanced and metastatic breast cancer in The Netherlands. BMC Cancer 2018, 18, 262. [Google Scholar] [CrossRef] [PubMed]
- Hoinville, L.; Taylor, C.; Zasada, M.; Warner, R.; Pottle, E.; Green, J. Improving the effectiveness of cancer multidisciplinary team meetings: Analysis of a national survey of MDT members’ opinions about streamlining patient discussions. BMJ Open Qual. 2019, 8, e000631. [Google Scholar] [CrossRef]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An overview of clinical decision support systems: Benefits, risks, and strategies for success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Patkar, V.; Acosta, D.; Davidson, T.; Jones, A.; Fox, J.; Keshtgar, M. Cancer Multidisciplinary Team Meetings: Evidence, Challenges, and the Role of Clinical Decision Support Technology. Int. J. Breast Cancer 2011, 2011, 831605. [Google Scholar] [CrossRef] [PubMed]
- Mazo, C.; Kearns, C.; Mooney, C.; Gallagher, W.M. Clinical Decision Support Systems in Breast Cancer: A Systematic Review. Cancers 2020, 12, 369. [Google Scholar] [CrossRef]
- Hameed, B.Z.; Naik, N.; Ibrahim, S.; Tatkar, N.S.; Shah, M.J.; Prasad, D.; Hegde, P.; Chlosta, P.; Rai, B.P.; Somani, B.K. Breaking Barriers: Unveiling Factors Influencing the Adoption of Artificial Intelligence by Healthcare Providers. Big Data Cogn. Comput. 2023, 7, 105. [Google Scholar] [CrossRef]
- Naik, N.; Hameed, B.; Shetty, D.K.; Swain, D.; Shah, M.; Paul, R.; Aggarwal, K.; Ibrahim, S.; Patil, V.; Smriti, K. Legal and ethical consideration in artificial intelligence in healthcare: Who takes responsibility? Front. Surg. 2022, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Kouri, A.; Yamada, J.; Lam Shin Cheung, J.; Van de Velde, S.; Gupta, S. Do providers use computerized clinical decision support systems? A systematic review and meta-regression of clinical decision support uptake. Implement. Sci. 2022, 17, 21. [Google Scholar] [CrossRef]
- Devaraj, S.; Sharma, S.K.; Fausto, D.J.; Viernes, S.; Kharrazi, H. Barriers and facilitators to clinical decision support systems adoption: A systematic review. J. Bus. Adm. Res. 2014, 3, 36. [Google Scholar] [CrossRef]
- Liberati, E.G.; Ruggiero, F.; Galuppo, L.; Gorli, M.; González-Lorenzo, M.; Maraldi, M.; Ruggieri, P.; Polo Friz, H.; Scaratti, G.; Kwag, K.H. What hinders the uptake of computerized decision support systems in hospitals? A qualitative study and framework for implementation. Implement. Sci. 2017, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Kilsdonk, E.; Peute, L.W.; Jaspers, M.W.M. Factors influencing implementation success of guideline-based clinical decision support systems: A systematic review and gaps analysis. Int. J. Med. Inform. 2017, 98, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kočo, L.; Siebers, C.C.; Schlooz, M.; Meeuwis, C.; Oldenburg, H.S.; Prokop, M.; Mann, R.M. Mapping Current Organizational Structure and Improvement Points of Breast Cancer Multidisciplinary Team Meetings–An Interview Study. J. Multidiscip. Healthc. 2022, 15, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Siemens Healthineers: AI-Pathway Companion. Available online: https://www.siemens-healthineers.com/digital-health-solutions/digital-solutions-overview/clinical-decision-support/ai-pathway-companion (accessed on 16 September 2023).
- Gale, N.K.; Heath, G.; Cameron, E.; Rashid, S.; Redwood, S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, M.A.H.; Paulussen, T.G.W.M.; Van Dommelen, P.; Van Buuren, S. Towards a measurement instrument for determinants of innovations. Int. J. Qual. Health Care 2014, 26, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Middleton, B.; Sittig, D.; Wright, A. Clinical decision support: A 25 year retrospective and a 25 year vision. Yearb. Med. Inform. 2016, 25, S103–S116. [Google Scholar]
- Magrabi, F.; Ammenwerth, E.; McNair, J.B.; De Keizer, N.F.; Hyppönen, H.; Nykänen, P.; Rigby, M.; Scott, P.J.; Vehko, T.; Wong, Z.S.-Y. Artificial intelligence in clinical decision support: Challenges for evaluating AI and practical implications. Yearb. Med. Inform. 2019, 28, 128–134. [Google Scholar] [CrossRef]
- Moja, L.; Kwag, K.H.; Lytras, T.; Bertizzolo, L.; Brandt, L.; Pecoraro, V.; Rigon, G.; Vaona, A.; Ruggiero, F.; Mangia, M. Effectiveness of computerized decision support systems linked to electronic health records: A systematic review and meta-analysis. Am. J. Public Health 2014, 104, e12–e22. [Google Scholar] [CrossRef] [PubMed]
- Pawloski, P.A.; Brooks, G.A.; Nielsen, M.E.; Olson-Bullis, B.A. A systematic review of clinical decision support systems for clinical oncology practice. J. Natl. Compr. Cancer Netw. 2019, 17, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Varonen, H.; Kortteisto, T.; Kaila, M.; EBMeDS Study Group. What may help or hinder the implementation of computerized decision support systems (CDSSs): A focus group study with physicians. Fam. Pract. 2008, 25, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Soo, K.C.; Al Jajeh, I.; Quah, R.; Seah, H.K.B.; Soon, S.; Walker, E. Virtual Multidisciplinary Review of a Complex Case Using a Digital Clinical Decision Support Tool to Improve Workflow Efficiency. J. Multidiscip. Healthc. 2021, 14, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Keikes, L.; Medlock, S.; van de Berg, D.J.; Zhang, S.; Guicherit, O.R.; Punt, C.J.A.; van Oijen, M.G.H. The first steps in the evaluation of a “black-box” decision support tool: A protocol and feasibility study for the evaluation of Watson for Oncology. J. Clin. Transl. Res. 2018, 3, 411–423. [Google Scholar] [PubMed]
- Suwanvecho, S.; Suwanrusme, H.; Jirakulaporn, T.; Issarachai, S.; Taechakraichana, N.; Lungchukiet, P.; Decha, W.; Boonpakdee, W.; Thanakarn, N.; Wongrattananon, P. Comparison of an oncology clinical decision-support system’s recommendations with actual treatment decisions. J. Am. Med. Inform. Assoc. 2021, 28, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Carlfjord, S.; Lindberg, M.; Bendtsen, P.; Nilsen, P.; Andersson, A. Key factors influencing adoption of an innovation in primary health care: A qualitative study based on implementation theory. BMC Fam. Pract. 2010, 11, 60. [Google Scholar] [CrossRef]
- Carroll, C.; Marsden, P.; Soden, P.; Naylor, E.; New, J.; Dornan, T. Involving users in the design and usability evaluation of a clinical decision support system. Comput. Methods Programs Biomed. 2002, 69, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.M.; Moy, A.J.; Rossetti, S.C.; Elhadad, N.; Cato, K.D. Clinician involvement in research on machine learning–based predictive clinical decision support for the hospital setting: A scoping review. J. Am. Med. Inform. Assoc. 2021, 28, 653–663. [Google Scholar] [CrossRef]
- Mitchell, C.; Ploem, C. Legal challenges for the implementation of advanced clinical digital decision support systems in Europe. J. Clin. Transl. Res. 2018, 3, 424. [Google Scholar] [PubMed]
- Teufel, A.; Binder, H. Clinical Decision Support Systems. Visc. Med. 2021, 37, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Osterman, T.J.; Terry, M.; Miller, R.S. Improving cancer data interoperability: The promise of the Minimal Common Oncology Data Elements (mCODE) initiative. JCO Clin. Cancer Inform. 2020, 4, 993–1001. [Google Scholar] [CrossRef]
- Patt, D.; Stella, P.; Bosserman, L. Clinical challenges and opportunities with current electronic health records: Practicing oncologists’ perspective. J. Oncol. Pract. 2018, 14, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jackson, G.P.; Foreman, M.A.; Gruen, D.; Hu, J.; Das, A.K. Evaluating artificial intelligence in medicine: Phases of clinical research. JAMIA Open 2020, 3, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sepúlveda, M.-J.; Jiang, Z.; Wang, H.; Li, J.; Liu, Z.; Yin, Y.; Roebuck, M.C.; Shortliffe, E.H.; Yan, M. Effect of an artificial intelligence clinical decision support system on treatment decisions for complex breast cancer. JCO Clin. Cancer Inform. 2020, 4, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, W. Kwaliteitscriteria Multidisciplinair Overleg (mdo). Available online: https://iknl.nl/nkr/evaluatie-met-nkr-data/multidisciplinair-overleg (accessed on 7 July 2023).
| General Hospital | Academic Hospital | Cancer Institute | |
|---|---|---|---|
| Participant specialism | 1 Surgery resident 1 Surgeon 1 Pathologist 1 Radiologist 1 Radiotherapist 1 Nurse specialist (oncology) | 2 Surgeons 1 Nurse specialist (surgery) 1 Oncologist 1 Nurse specialist (oncology) 1 Pathologist 1 Nurse specialist (plastic surgery) 1 Radiotherapist 1 Radiologist | 1 Surgeon 1 Surgery resident 1 Surgery fellow 1 Oncologist 1 Pathologist 1 Radiologist 1 Radiotherapist 1 Nurse specialist (oncology) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kočo, L.; Siebers, C.C.N.; Schlooz, M.; Meeuwis, C.; Oldenburg, H.S.A.; Prokop, M.; Mann, R.M. The Facilitators and Barriers of the Implementation of a Clinical Decision Support System for Breast Cancer Multidisciplinary Team Meetings—An Interview Study. Cancers 2024, 16, 401. https://doi.org/10.3390/cancers16020401
Kočo L, Siebers CCN, Schlooz M, Meeuwis C, Oldenburg HSA, Prokop M, Mann RM. The Facilitators and Barriers of the Implementation of a Clinical Decision Support System for Breast Cancer Multidisciplinary Team Meetings—An Interview Study. Cancers. 2024; 16(2):401. https://doi.org/10.3390/cancers16020401
Chicago/Turabian StyleKočo, Lejla, Carmen C. N. Siebers, Margrethe Schlooz, Carla Meeuwis, Hester S. A. Oldenburg, Mathias Prokop, and Ritse M. Mann. 2024. "The Facilitators and Barriers of the Implementation of a Clinical Decision Support System for Breast Cancer Multidisciplinary Team Meetings—An Interview Study" Cancers 16, no. 2: 401. https://doi.org/10.3390/cancers16020401
APA StyleKočo, L., Siebers, C. C. N., Schlooz, M., Meeuwis, C., Oldenburg, H. S. A., Prokop, M., & Mann, R. M. (2024). The Facilitators and Barriers of the Implementation of a Clinical Decision Support System for Breast Cancer Multidisciplinary Team Meetings—An Interview Study. Cancers, 16(2), 401. https://doi.org/10.3390/cancers16020401






