Chondrosarcoma of the Femur: Is Local Recurrence Influenced by the Presence of an Extraosseous Component?
Abstract
Simple Summary
Abstract
1. Introduction
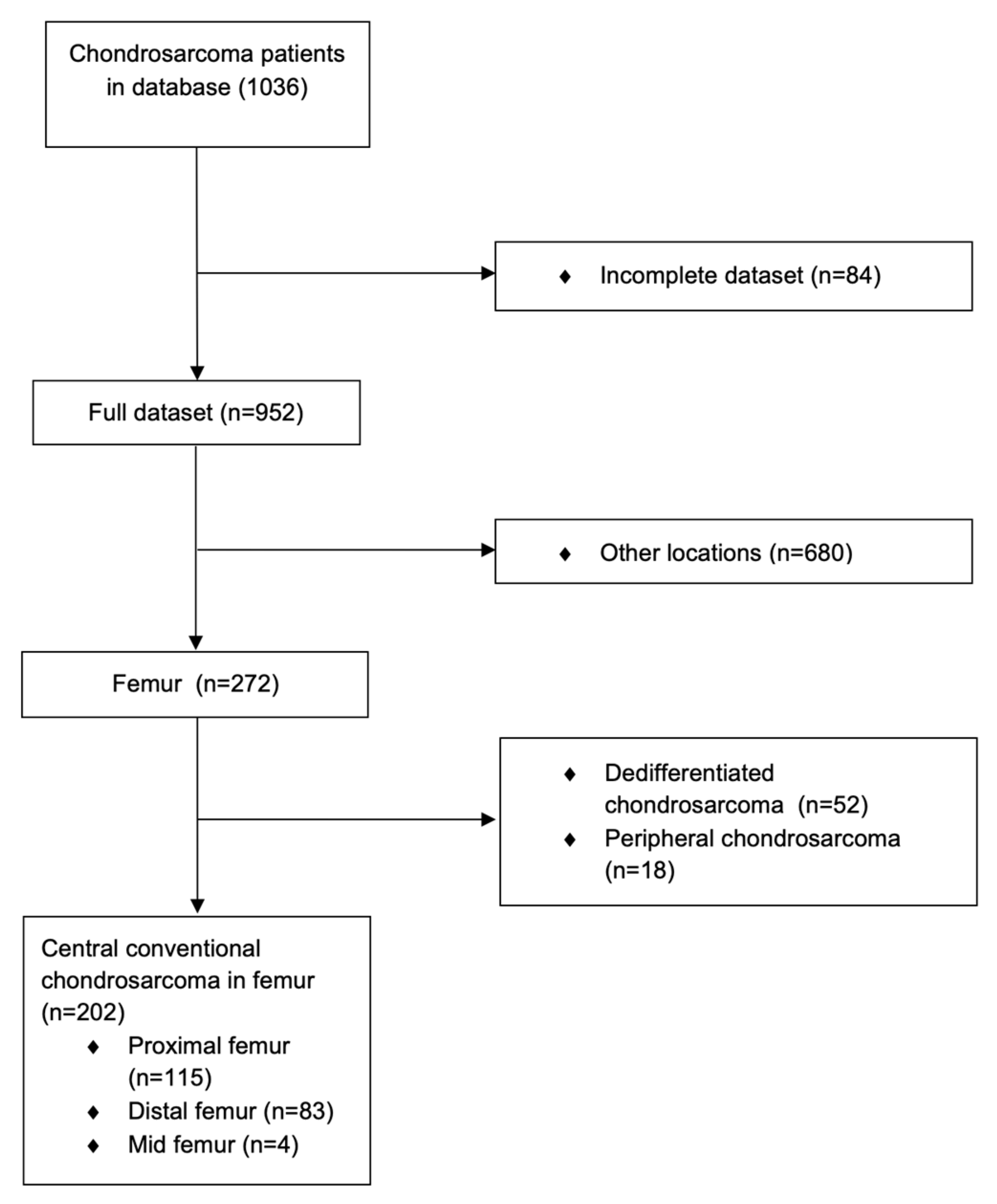
2. Materials and Methods
Statistical Analysis
3. Results
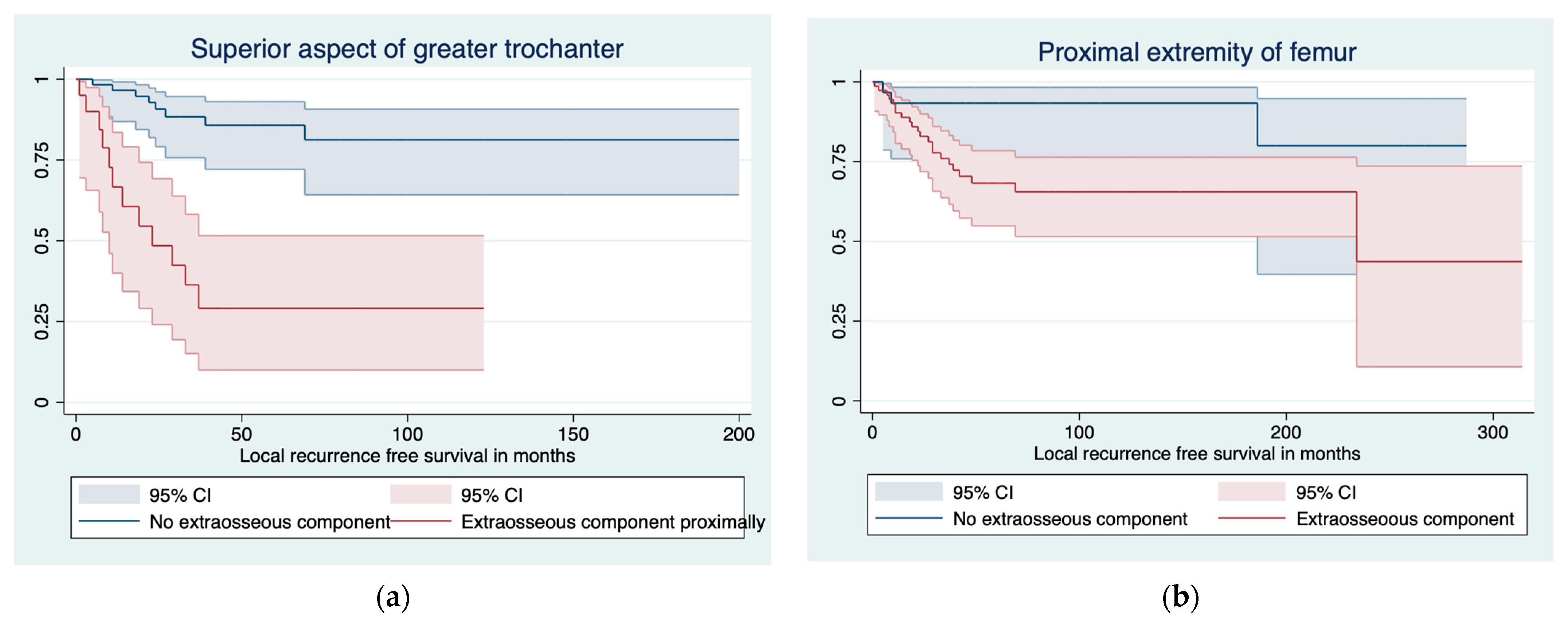
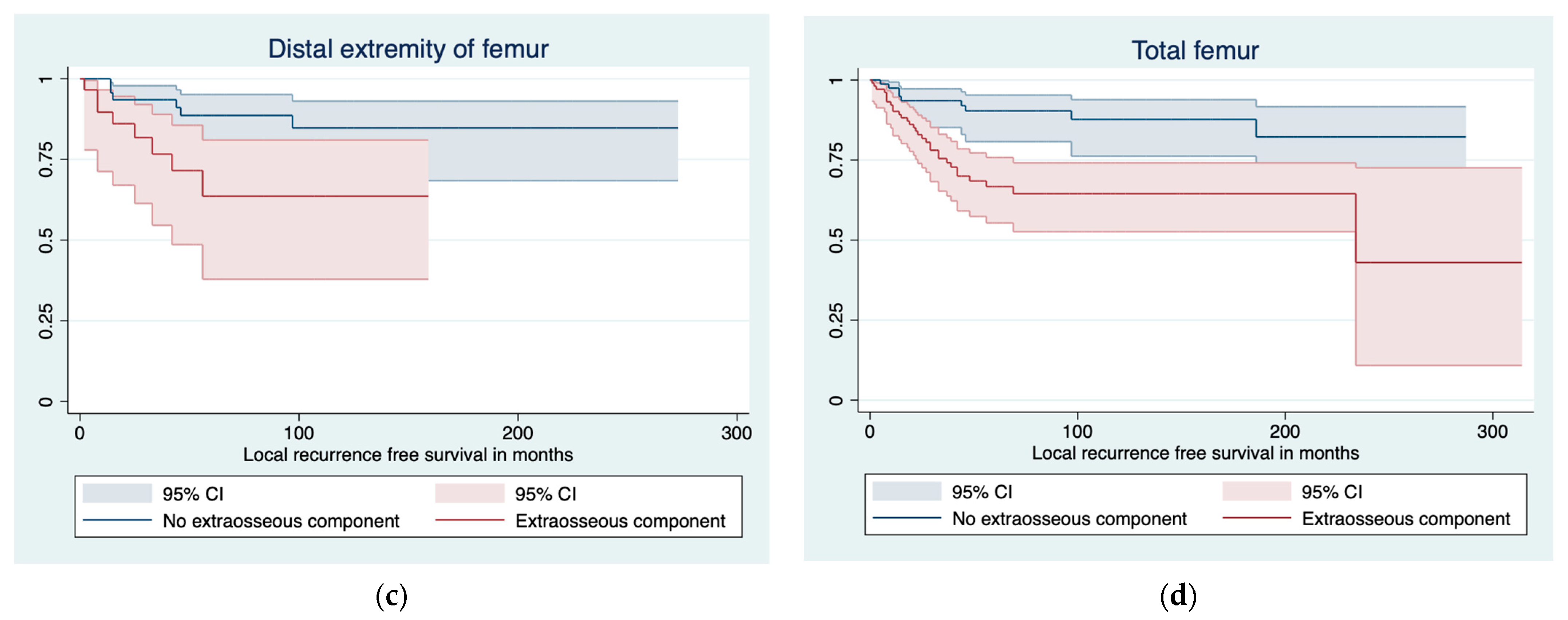
3.1. Predictors of LR and LRFS
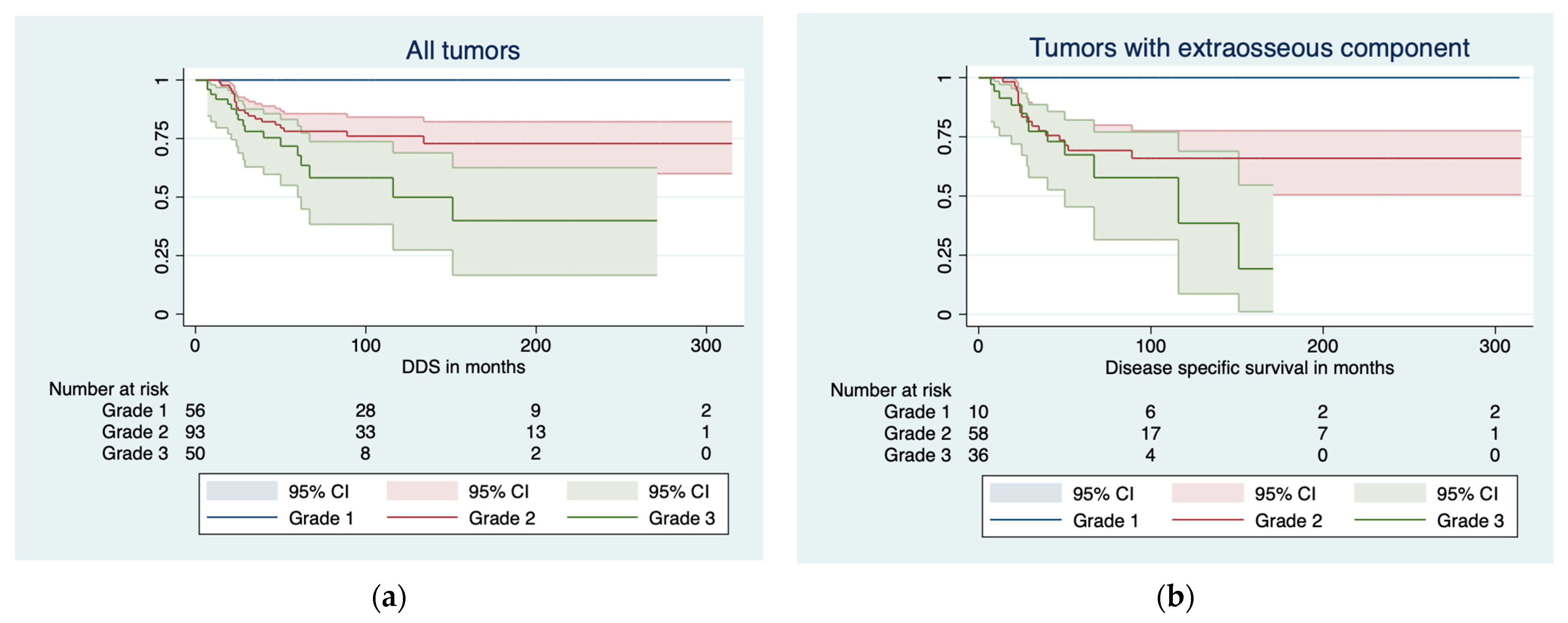
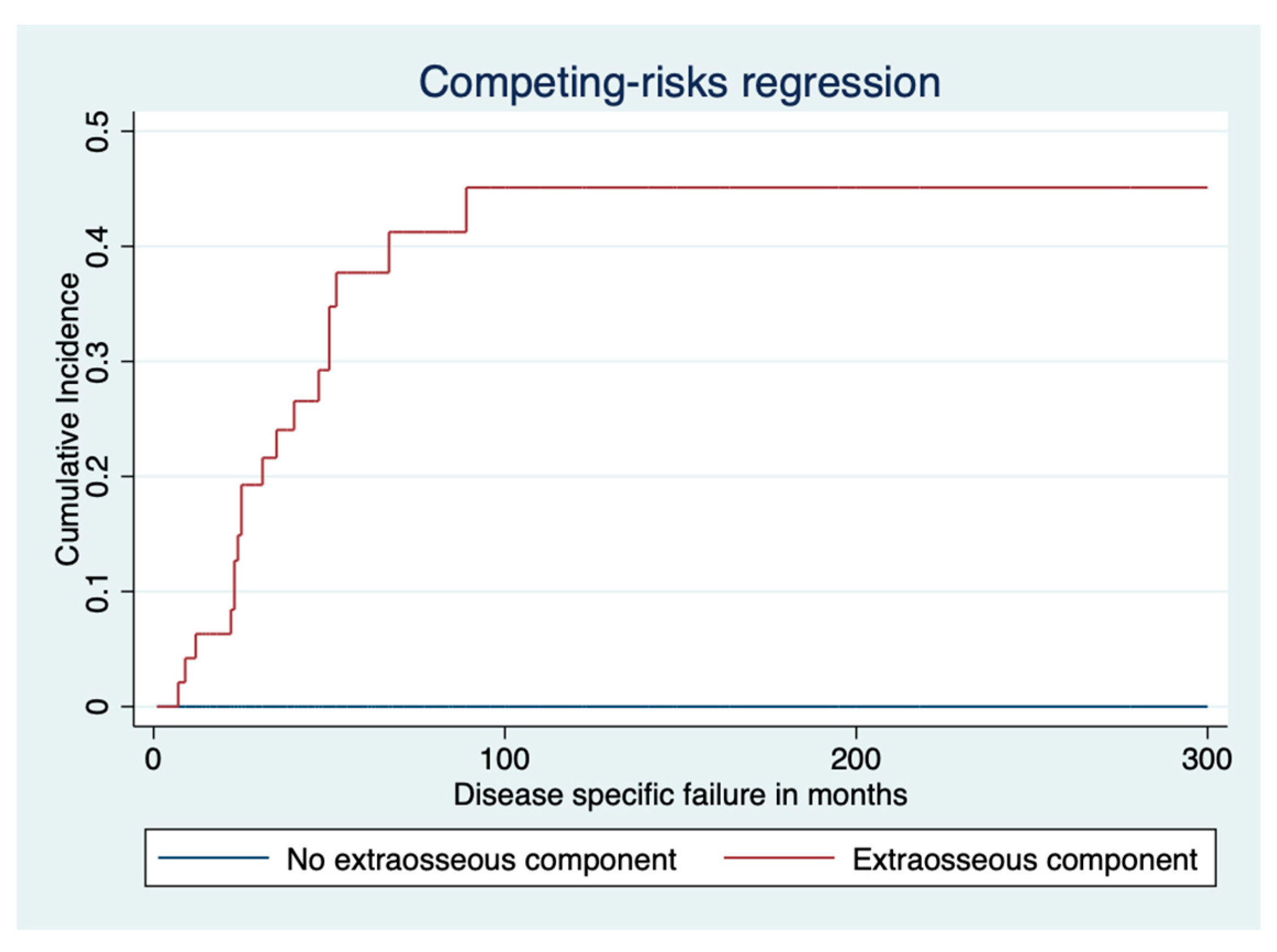
3.2. Disease-Specific Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.-H.; Lee, S.K. Classification of Chondrosarcoma: From Characteristic to Challenging Imaging Findings. Cancers 2023, 15, 1703. [Google Scholar] [CrossRef]
- Venneker, S.; Bovée, J.V.M.G. IDH Mutations in Chondrosarcoma: Case Closed or Not. Cancers 2023, 15, 3603. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.; Tudor, M.; Montanari, J.; Commenchail, K.; Savu, D.I.; Lesueur, P.; Chevalier, F. Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions. Cancers 2023, 15, 1962. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Hogendoorn, P.C.; Dijkstra, S.D.; van Rijswijk, C.S.; Krol, A.D.; Taminiau, A.H.; Bovée, J.V. The Clinical Approach Towards Chondrosarcoma. Oncologist 2008, 13, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, M.K.; Evans, S.; Stevenson, J.; Sumathi, V.; Kask, G.; Jeys, L.M.; Parry, M.C. Clinical differences between central and peripheral chondrosarcomas. Bone Jt. J. 2021, 103, 984–990. [Google Scholar] [CrossRef]
- Arshi, A.; Sharin, J.; Park, D.Y.; Park, H.Y.; Bernthal, N.M.; Yazdanshenas, H.; Shamie, A.N. Chondrosarcoma of the ossoeus spiine. J. Bone Jt. Surg. Br. 2005, 87, 1527–1530. [Google Scholar]
- Donati, D.; El Ghoneimy, A.; Bertoni, F.; Di Bella, C.; Mercuri, M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J. Bone Jt. Surg. 2013, 108, 19–27. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Angelini, A.; Drago, G.; Merlino, B.; Ruggieri, P. Survival analysis of patients with chondrosarcomas of the pelvis. Spine 2017, 42, 644–652. [Google Scholar] [CrossRef]
- Fletcher, C.D.M.; Bridge, J.A.; Hogendoorn, P.C.W. Chondrogenic Tumors. In Soft Tissue and Bone Tumors, 4th ed.; IARC Pub-lisher: Lyon, France, 2013. [Google Scholar]
- Riedel, R.F.; Larrier, N.; Dodd, L.; Kirsch, D.; Martinez, S.; Brigman, B.E. The Clinical Management of Chondrosarcoma. Curr. Treat. Options Oncol. 2009, 10, 94–106. [Google Scholar] [CrossRef]
- Healey, J.H.; Lane, J.M. Chondrosarcoma. Clin. Orthop. Relat. Res. 1986, 204, 119–129. [Google Scholar] [CrossRef]
- Laitinen, M.K.; Stevenson, J.D.; Parry, M.C.; Sumathi, V.; Grimer, R.J.; Jeys, L.M. The role of grade in local recurrence and the disease-specific survival in chondrosarcomas. Bone Jt. J. 2018, 100, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, F.; Abudu, A.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Ayoub, K.; Mangham, D.C.; Davies, A.M. Risk factors for survival and local control in chondrosarcoma of bone. J. Bone Jt. Surg. 2002, 84, 93–99. [Google Scholar] [CrossRef]
- Angelini, A.; Guerra, G.; Mavrogenis, A.F.; Pala, E.; Picci, P.; Ruggieri, P. Clinical outcome of central conventional chondrosarcoma. J. Surg. Oncol. 2012, 106, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.D.; Laitinen, M.K.; Parry, M.C.; Sumathi, V.; Grimer, R.J.; Jeys, L.M. The role of surgical margins in chondrosarcoma. Eur. J. Surg. Oncol. 2018, 44, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Andreou, D.; Fehlberg, S.; Pink, D.; Werner, M.; Tunn, P.-U. Survival amnd prognostic factors in chondrosarcoma. Results in 115 patients with long-term follow-up. Acta Orthop. 2011, 82, 749–755. [Google Scholar] [CrossRef]
- Kim, H.; Bindiganavile, S.S.; Han, I. Oncologic outcome after local recurrence of chondrosarcoma: Analysis of prognostic factors. J. Surg. Oncol. 2015, 111, 957–961. [Google Scholar] [CrossRef]
- Kask, G.; Laitinen, M.K.; Stevenson, J.; Evans, S.; Jeys, L.M.; Parry, M.C. Chondrosarcoma of the hands and feet. Bone Jt. J. 2021, 103, 562–568. [Google Scholar] [CrossRef]
- Wellings, E.P.; Mallett, K.E.; Parkes, C.W.; Labott, J.R.; Rose, P.S.; Houdek, M.T. Impact of tumour stage on the surgical outcomes of scapular chondrosarcoma. Int. Orthop. 2022, 46, 1175–1180. [Google Scholar] [CrossRef]
- Banskota, N.; Fang, X.; Yuan, D.; Lei, S.; Zhang, W.; Duan, H. Comparative Study of Proximal Femur Bone Tumor Patients Undergoing Hemiarthroplasty versus Total Hip Arthroplasty: A Meta-Analysis. J. Clin. Med. 2023, 12, 1209. [Google Scholar] [CrossRef]
- ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014, 25, 113–123. [Google Scholar] [CrossRef]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980, 415, 106–120. [Google Scholar] [CrossRef]
- Houdek, M.T.; Watts, C.D.; Wyles, C.C.; Rose, P.S.; Taunton, M.J.; Sim, F.H. Functional and oncologic outcome of cemented endoprosthesis for malignant proximal femoral tumors. J. Surg. Oncol. 2016, 114, 501–506. [Google Scholar] [CrossRef]
- Davies, A.; Patel, A.; Azzopardi, C.; James, S.; Botchu, R.; Jeys, L. The influence of site on the incidence and diagnosis of solitary central cartilage tumours of the femur. A 21st century perspective. J. Clin. Orthop. Trauma 2022, 32, 101953. [Google Scholar] [CrossRef]
- Kurisunkal, V.; Laitinen, M.K.; Kaneuchi, Y.; Kapanci, B.; Stevenson, J.; Parry, M.C.; Reito, A.; Fujiwara, T.; Jeys, L.M. Is 2 mm a wide margin in high-grade conventional chondrosarcomas of the pelvis? Bone Jt. J. 2021, 103, 1150–1154. [Google Scholar] [CrossRef]
- Thorkildsen, J.; Norum, O.J.; Myklebust, T.A.; Zaikova, O. Chondrosarcoma local recurrence in the Cancer Registry of Norway cohort (1990–2013): Patterns and impact. J. Surg. Oncol. 2021, 123, 510–520. [Google Scholar] [CrossRef]
- Fromm, J.; Klein, A.; Baur-Melnyk, A.; Knösel, T.; Lindner, L.; Birkenmaier, C.; Roeder, F.; Jansson, V.; Dürr, H.R. Survival and prognostic factors in conventional central chondrosarcoma. BMC Cancer 2018, 18, 849. [Google Scholar] [CrossRef]
- Frassica, F.J.; Unni, K.K.; Beabout, J.W.; Sim, F.H. Dedifferentiated chondrosarcoma. A report of the clinicopathological features and treatment of seventy-eight cases. J. Bone Jt. Surg. Am 1986, 68, 1197–1205. [Google Scholar] [CrossRef]
- Staals, E.L.; Bacchini, P.; Bertoni, F. Dedifferentiated central chondrosarcoma. Cancer 2006, 106, 2682–2691. [Google Scholar] [CrossRef]
- El Beaino, M.; Hoda, S.T.; Eldeib, A.J.; Masrouha, K. Dedifferentiated Chondrosarcoma: Diagnostic Controversies and Emerging Therapeutic Targets. Curr. Oncol. Rep. 2023, 25, 1117–1126. [Google Scholar] [CrossRef]
- Grimer, R.J.; Gosheger, G.; Taminiau, A.; Biau, D.; Matejovsky, Z.; Kollender, Y.; San-Julian, M.; Gherlinzoni, F.; Ferrari, C. Dedifferentiated chondrosarcoma: Prognostic factors and outcome from a European group. Eur. J. Cancer 2007, 43, 2060–2065. [Google Scholar]
- Bickels, J.; Meller, I.; Henshaw, R.M.; Malawer, M.M. Reconstruction of Hip Stability after Proximal and Total Femur Resections. Clin. Orthop. Relat. Res. 2000, 375, 218–230. [Google Scholar] [CrossRef]
- Elbuluk, A.M.; Coxe, F.R.; Schimizzi, G.V.; Ranawat, A.S.; Bostrom, M.P.; Sierra, R.J.; Sculco, P.K. Abductor Deficiency-Induced Recurrent Instability after Total Hip Arthroplasty. JBJS Rev. 2020, 8, e0164. [Google Scholar] [CrossRef]
- Henderson, E.R.; Keeney, B.J.; Pala, E.; Funovics, P.T.; Eward, W.C.; Groundland, J.S.; Ehrlichman, L.K.; Puchner, S.S.E.; Brigman, B.E.; Ready, J.E.; et al. The stability of the hip after the use of a proximal femoral endoprosthesis for oncological indications: Analysis of variables relating to the patient and the surgical technique. Bone Jt. J. 2017, 99, 531–537. [Google Scholar] [CrossRef]
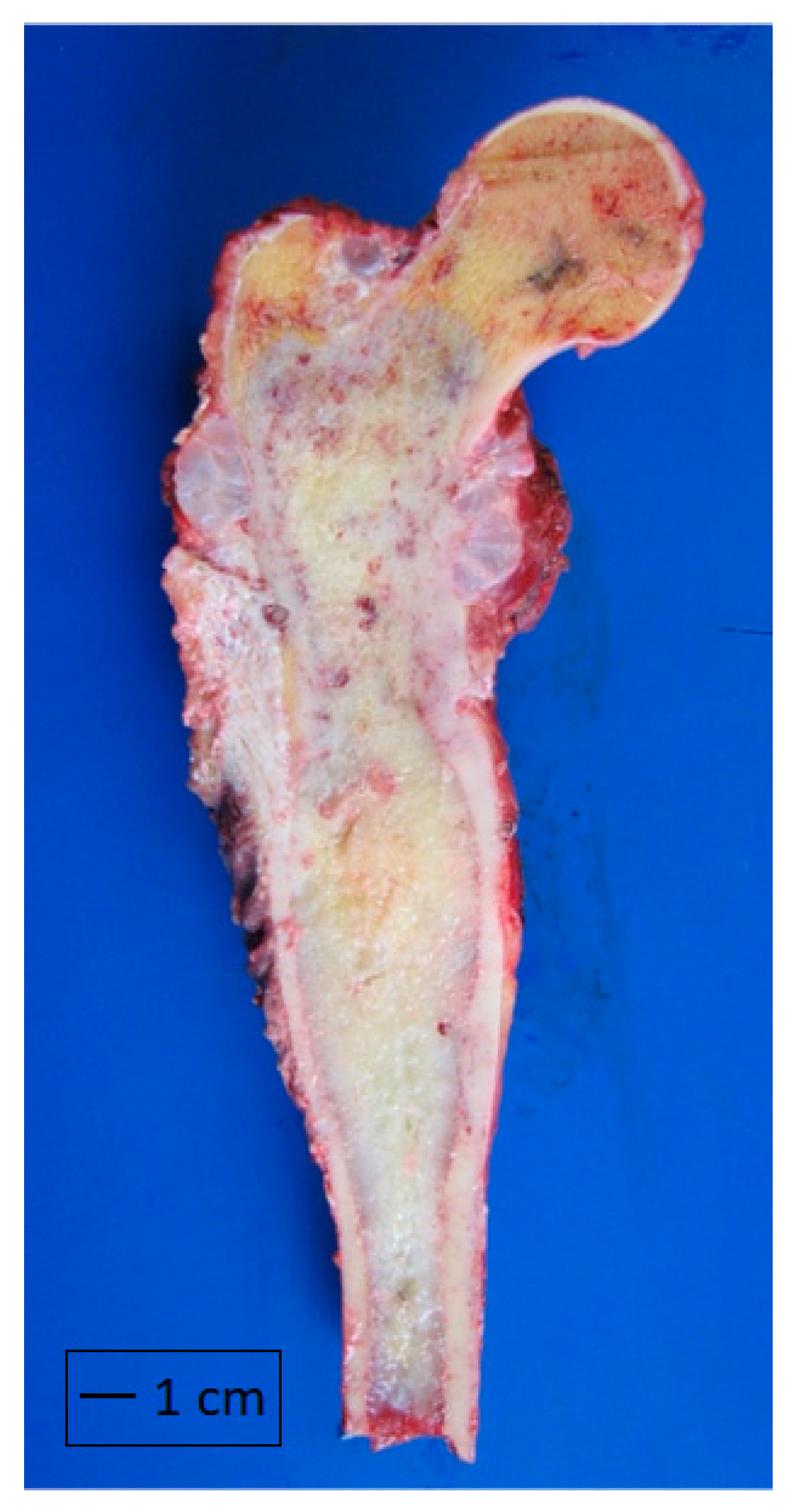
| Characteristics | Total | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Eligible cases | 202 | 57 (28%) | 94 (46%) | 51 (26%) |
| Site | ||||
| Proximal extremity | 115 (57%) | 20 (17%) | 60 (52%) | 35 (30%) |
| Distal extremity | 83 (41%) | 36 (43%) | 32 (38%) | 15 (19%) |
| Corpus | 4 (1.9%) | 1 (25%) | 2 (50%) | 1 (25%) |
| Male sex | 110 (55%) | 27 (25%) | 50 (46%) | 33 (30%) |
| Proximal extremity | 65 (57%) | 10 (15%) | 33 (51%) | 22 (34%) |
| Distal extremity | 43 (52%) | 17 (40%) | 16 (37%) | 10 (23%) |
| Corpus | 2 (50%) | - | 1 (100%) | 1 (100%) |
| Age at surgery, median (range) | 55 (8–95) | 46 (10–85) | 59 (8–95) | 59 (23–88) |
| Proximal extremity | 59 (8–95) | 50 (10–85) | 60 (8–85) | 62 (25–87) |
| Distal extremity | 51 (16–90) | 44 (16–85) | 57 (28–90) | 54 (23–88) |
| Corpus | 53 (28–81) | 28 | 64 (47–81) | 55 |
| Mean tumor size, cm (range) | 12 (1.5–46) | 7.8 (1.5–46) | 12 (3.0–30) | 16 (4–40) |
| Proximal extremity | 13 (2.0–40) | 7.3 (2.0–17) | 13 (3.5–30) | 17 (6.0–40) |
| Distal extremity | 9.3 (1.5–60) | 8.0 (1.5–46) | 10 (3.0–25) | 11 (4.0–40) |
| Corpus | 13 (6.0–19) | 6.0 | 17 (16–19) | 13 |
| Extraosseous component | 105 (52%) | 10 (10%) | 58 (55%) | 37 (35%) |
| Proximal extremity | 77 (67%) | 6 (8%%) | 42 (55%) | 28 (37%) |
| Distal extremity | 28 (38%) | 4 (14%) | 16 (57%) | 8 (29%) |
| Corpus | 1 (25%) | - | - | 1 (100%) |
| Extraosseous component in cranial part of proximal extremity of femur | 28 (24%) | 1 (5%) | 17 (28%) | 10 (29%) |
| Median follow-up, months (range) | 90 (0–315) | 114 (0–314) | 92 (0–315) | 58 (0–271) |
| Proximal extremity | 84 (0–315) | 117 (13–314) | 93 (0–315) | 50 (0–182) |
| Distal extremity | 100 (0–273) | 114 (0–264) | 93 (8–273) | 78 (7–271) |
| Corpus | 55 (10–104) | 78 | 57 (10–104) | 29 |
| Pathologic fracture | 20 (10%) | 1 (1.8%) | 12 (14%) | 7 (14%) |
| Proximal extremity | 13 (11%) | - | 10 (17%) | 3 (8.6%) |
| Distal extremity | 6 (8.0%) | 1 (2.9%) | 2 (7.4%) | 3 (21%) |
| Corpus | 1 (25%) | - | - | 1 (100%) |
| Metastasis | 39 (19%) | - | 21 (22%) | 18 (35%) |
| Proximal extremity | 26 (23%) | - | 14 (23%) | 12 (34%) |
| Distal extremity | 12 (14%) | - | 7 (22%) | 5 (33%) |
| Corpus | 1 | - | - | 1 |
| Median time to metastasis in months (range) | 22 (0–189) | - | 26 (0–189) | 16 (4–41) |
| Proximal extremity | 19 (0–73) | - | 20 (0–73) | 18 (6–41) |
| Distal extremity | 27 (0–189) | - | 38 (0–189) | 11 (4–16) |
| Corpus | 21 | - | - | 21 |
| Local recurrence | 47 (23%) | 7 (12%) | 25 (27%) | 15 (29%) |
| Proximal extremity | 31 (27%) | 2 (10%) | 20 (33%) | 9 (26%) |
| Distal extremity | 15 (18%) | 5 (11%) | 5 (16%) | 5 (33%) |
| Corpus | 1 (25%) | - | - | 1 (100%) |
| Median time to LR in months (range) | 33 (0–234) | 54 (14–186) | 39 (0–234) | 12 (1–22) |
| Proximal extremity | 36 (0–234) | 113 (39–186) | 40 (0–234) | 13 (1–22) |
| Distal extremity | 28 (2–97) | 35 (14–97) | 38 (15–56) | 8 (2–18) |
| Corpus | 21 | - | - | 21 |
| Dead for disease | 37 (18%) | - | 20 (21%) | 17 (33%) |
| Proximal extremity | 25 (22%) | - | 14 (23%) | 11 (31%) |
| Distal extremity | 11 (13%) | - | 6 (19%) | 5 (33%) |
| Corpus | 1 (25%) | - | - | 1 (100%) |
| Total | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|
| Curettage | ||||
| Proximal extremity | 9 (8%) [5, 45%] | 9 (45%) [2, 22%] | - | - |
| Distal extremity | 18 (22%) [2, 11%] | 16 (44%) [4, 25%] | 2 (6%) [1, 50%] | - |
| Corpus | - | - | - | - |
| Resection | ||||
| Proximal extremity | 96 (85%) [29, 30%] | 11 (55%) [0] | 58 (97%) [20, 34%] | 27 (82%) [9, 33%] |
| Distal extremity | 58 (70%) [8, 32%] | 20 (56%) [1, 5%] | 27 (84%) [4, 15%] | 11 (73%) [3, 27%] |
| Corpus | 4 (100%) [1] | 1 (100%) [0] | 2 (100%) [0] | 1 (100%) [1, 100%] |
| Amputation | ||||
| Proximal extremity | 6 (5%) [0] | - | 2 (3%) [0] | 4 (12%) [0] |
| Distal extremity | 7 (8%) [2, 35%] | - | 3 (9%) [0] | 4 (27%) [2, 50%] |
| Corpus | - | - | - | - |
| Hindquarter | ||||
| Proximal extremity | 2 (2%) [0] | - | - | 2 (6%) [0] |
| Distal extremity | - | - | - | - |
| Corpus | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laitinen, M.K.; Parry, M.C.; Morris, G.V.; Grimer, R.J.; Sumathi, V.; Stevenson, J.D.; Jeys, L.M. Chondrosarcoma of the Femur: Is Local Recurrence Influenced by the Presence of an Extraosseous Component? Cancers 2024, 16, 363. https://doi.org/10.3390/cancers16020363
Laitinen MK, Parry MC, Morris GV, Grimer RJ, Sumathi V, Stevenson JD, Jeys LM. Chondrosarcoma of the Femur: Is Local Recurrence Influenced by the Presence of an Extraosseous Component? Cancers. 2024; 16(2):363. https://doi.org/10.3390/cancers16020363
Chicago/Turabian StyleLaitinen, Minna K., Michael C. Parry, Guy V. Morris, Robert J. Grimer, Vaiyapuri Sumathi, Jonathan D. Stevenson, and Lee M. Jeys. 2024. "Chondrosarcoma of the Femur: Is Local Recurrence Influenced by the Presence of an Extraosseous Component?" Cancers 16, no. 2: 363. https://doi.org/10.3390/cancers16020363
APA StyleLaitinen, M. K., Parry, M. C., Morris, G. V., Grimer, R. J., Sumathi, V., Stevenson, J. D., & Jeys, L. M. (2024). Chondrosarcoma of the Femur: Is Local Recurrence Influenced by the Presence of an Extraosseous Component? Cancers, 16(2), 363. https://doi.org/10.3390/cancers16020363






