Methylene Blue Metabolic Therapy Restrains In Vivo Ovarian Tumor Growth
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Cell Lines and Culture Medium
2.3. Xenograft Mouse Model
2.4. In Vitro Cell Proliferation Assay under the Methylene Blue, METABLOC, and Carboplatin Treatments
2.5. Flow Cytometry Analysis
2.6. Oxygen Consumption Assay
2.7. Quantitative PCR for Respiratory mRNA Complex Estimation
3. Results
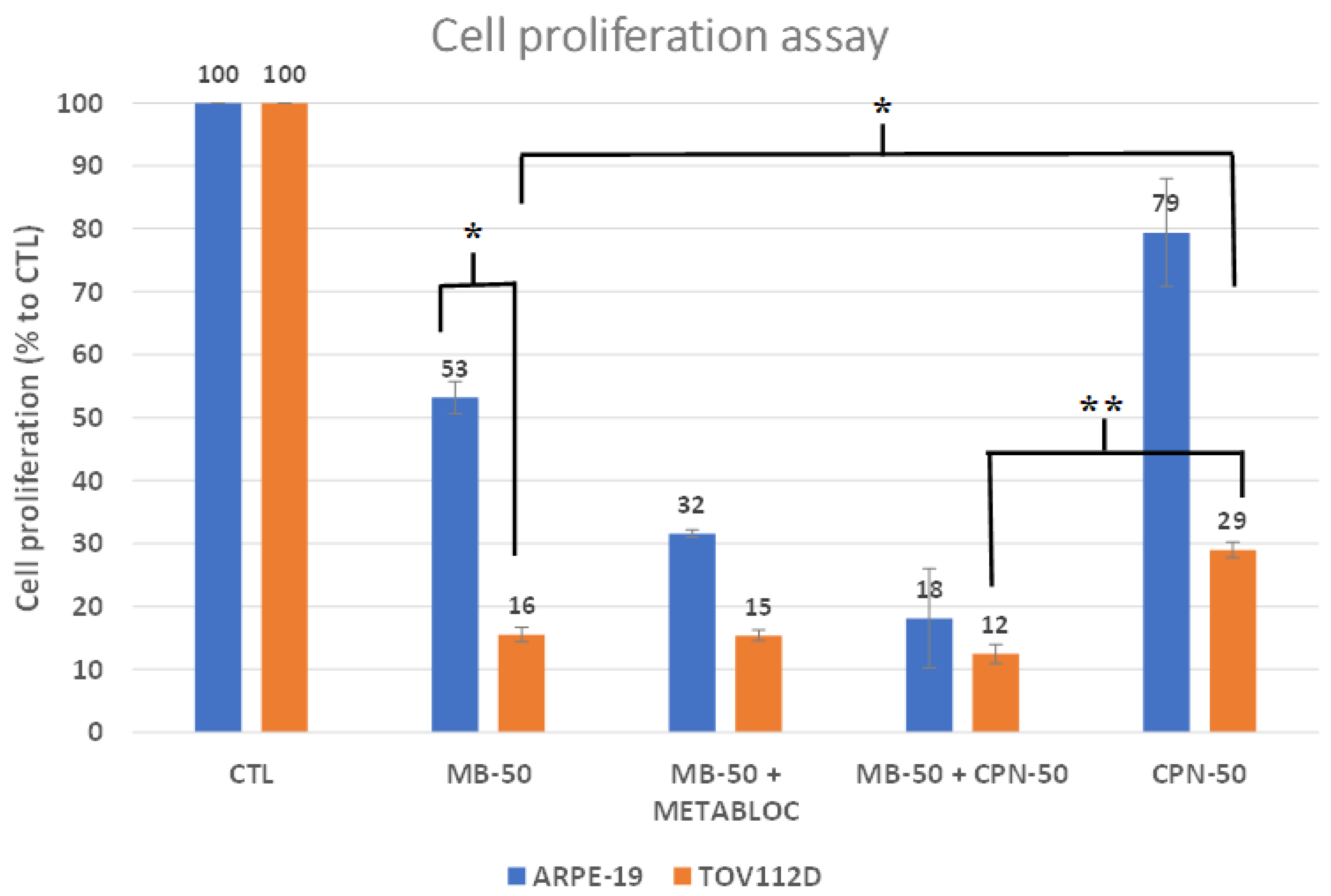
3.1. Metabolic Therapy Effects on Cell Proliferation
3.2. Impact of Metabolic Therapy on Oxygen Consumption Rate in Normal and Tumor Cell Lines
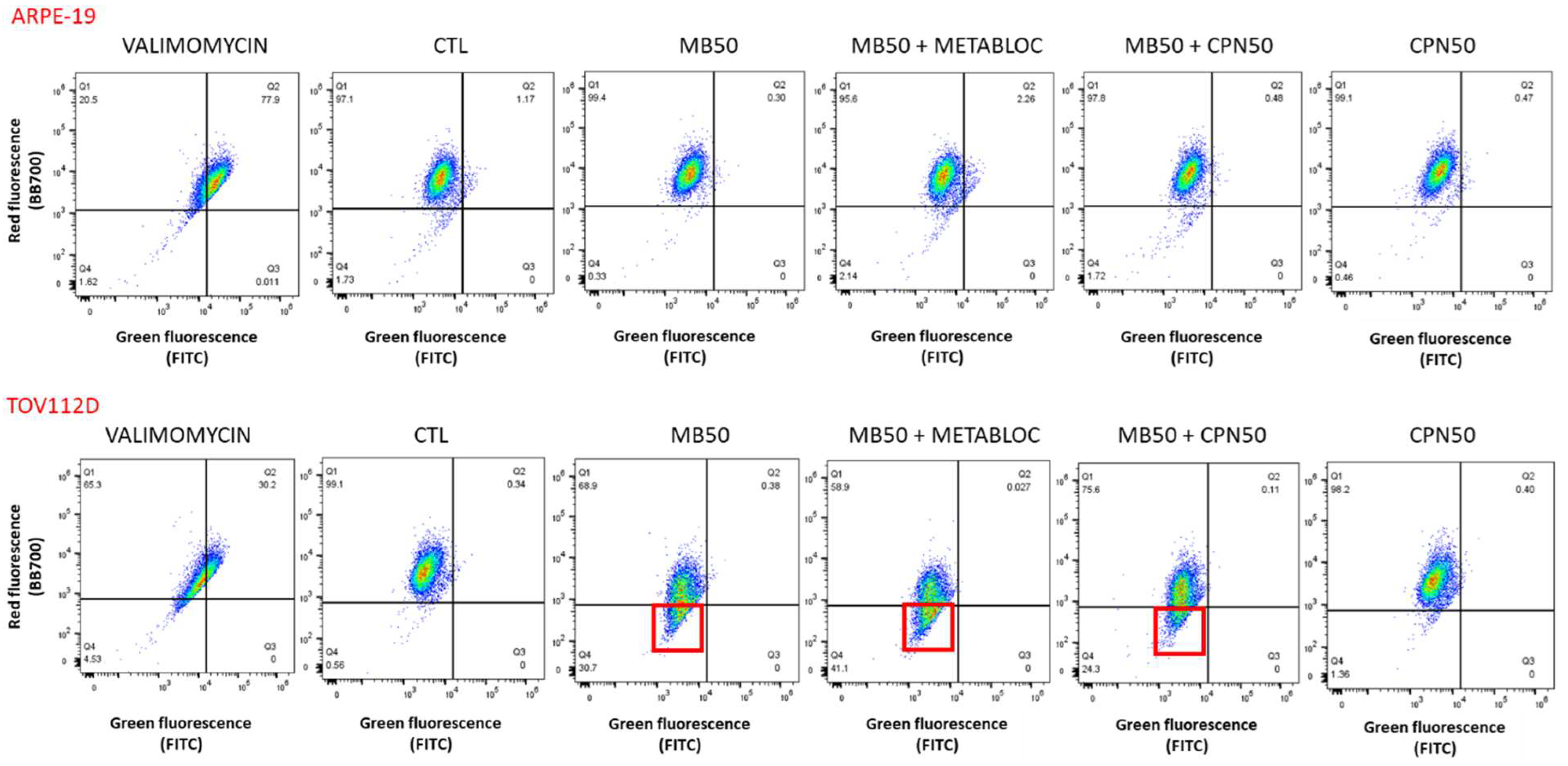
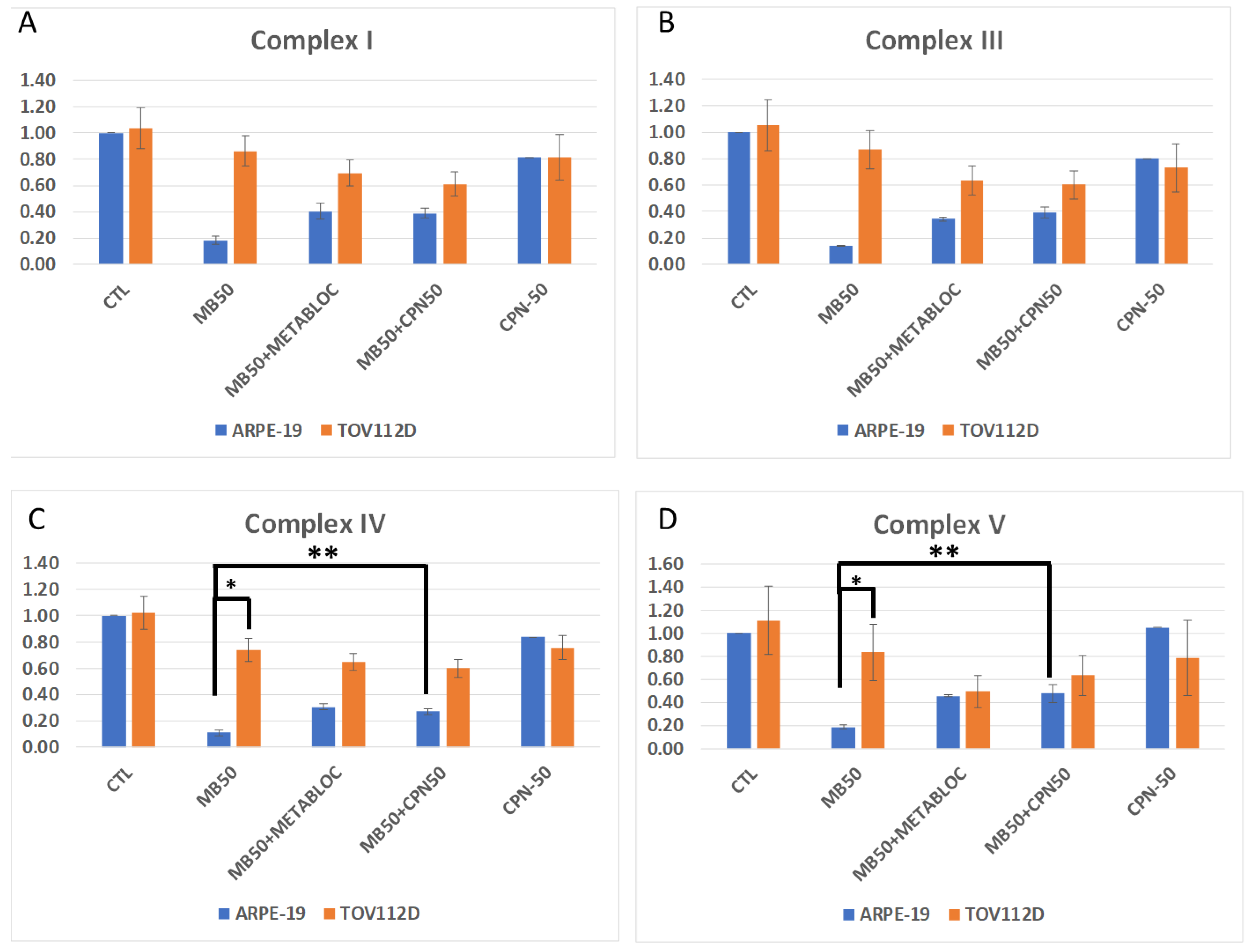
3.3. Evaluation of Mitochondrial Membrane Potential and Respiratory Complex Gene Expression under Metabolic Therapy
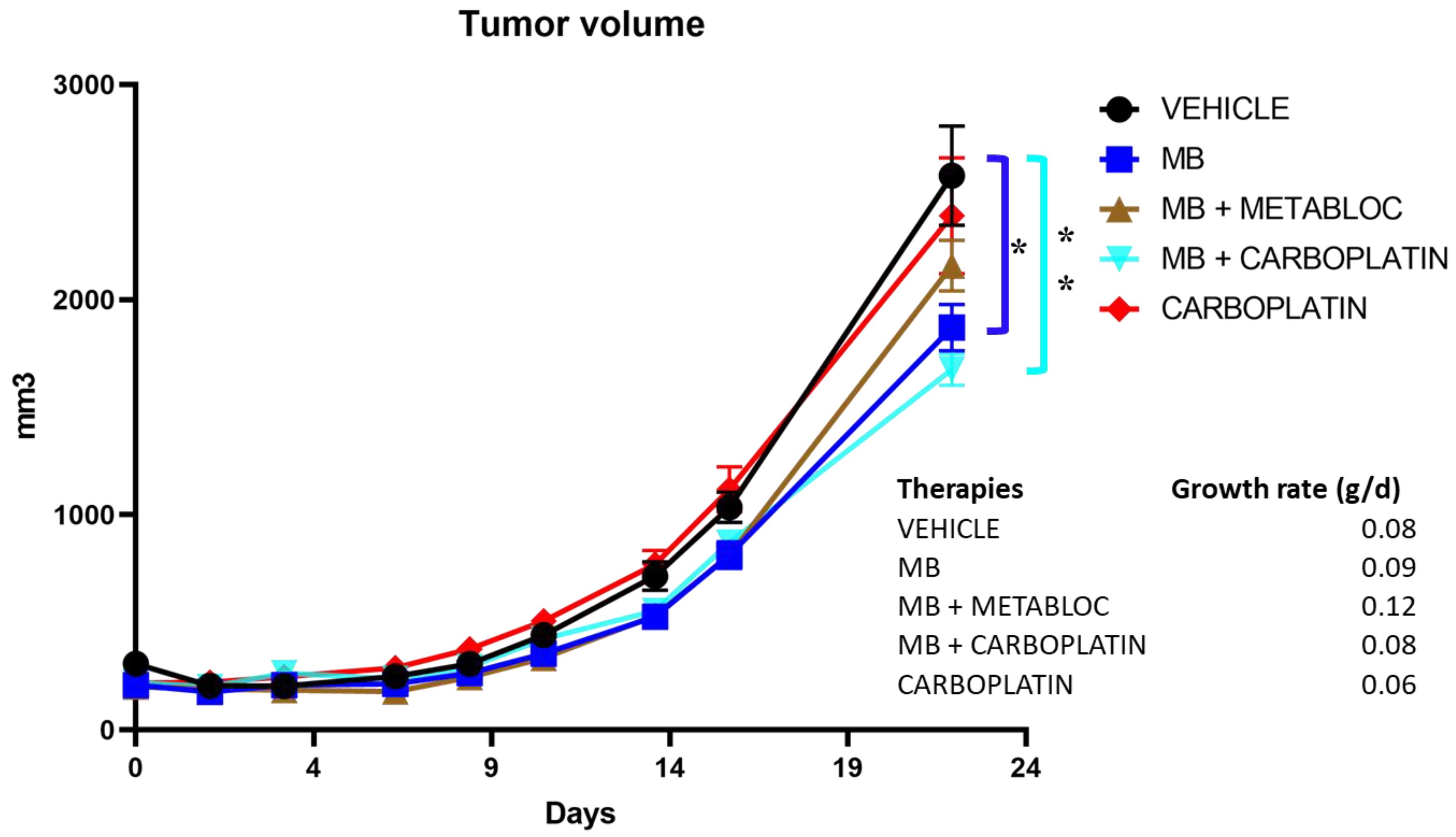
3.4. The Effect of Metabolic Therapy on Tumor Growth in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, H.-J.; Li, Y.-Y.; Wang, X.; Liu, X.-X.; Mai, J. Molecular Mechanisms of Platinum-Based Chemotherapy Resistance in Ovarian Cancer. Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial Ovarian Cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Taylor, M.H.; Kelly, K.; Beck, J.T.; Gordon, M.; Moore, K.M.; Patel, M.R.; Chaves, J.; Park, H.; Mita, A.C.; et al. Efficacy and Safety of Avelumab for Patients with Recurrent or Refractory Ovarian Cancer. JAMA Oncol. 2019, 5, 393–401. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Sayed, N.; Ditsworth, D.; Thompson, C.B. Brick by Brick: Metabolism and Tumor Cell Growth. Curr. Opin. Genet. Dev. 2008, 18, 54–61. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer Metabolic Reprogramming: Importance, Main Features, and Potentials for Precise Targeted Anti-Cancer Therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic Reprogramming and Cancer Progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Schiffmann, L.M.; Werthenbach, J.P.; Heintges-Kleinhofer, F.; Seeger, J.M.; Fritsch, M.; Günther, S.D.; Willenborg, S.; Brodesser, S.; Lucas, C.; Jüngst, C.; et al. Mitochondrial Respiration Controls Neoangiogenesis during Wound Healing and Tumour Growth. Nat. Commun. 2020, 11, 3653. [Google Scholar] [CrossRef]
- Tan, Y.; Li, J.; Zhao, G.; Huang, K.-C.; Cardenas, H.; Wang, Y.; Matei, D.; Cheng, J.-X. Metabolic Reprogramming from Glycolysis to Fatty Acid Uptake and Beta-Oxidation in Platinum-Resistant Cancer Cells. Nat. Commun. 2022, 13, 4554. [Google Scholar] [CrossRef]
- Liu, Y.; Birsoy, K. Metabolic Sensing and Control in Mitochondria. Mol. Cell 2023, 83, 877–889. [Google Scholar] [CrossRef]
- Oliva, C.R.; Moellering, D.R.; Gillespie, G.Y.; Griguer, C.E. Acquisition of Chemoresistance in Gliomas Is Associated with Increased Mitochondrial Coupling and Decreased ROS Production. PLoS ONE 2011, 6, e24665. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Zimberlin, C.D.; Fessler, E.; Hogdal, L.; Prasetyanti, P.R.; Grandela, C.M.; Letai, A.; Medema, J.P. Decreased Mitochondrial Priming Determines Chemoresistance of Colon Cancer Stem Cells. Cell Death Differ. 2014, 21, 1170–1177. [Google Scholar] [CrossRef]
- Guerra, F.; Arbini, A.A.; Moro, L. Mitochondria and Cancer Chemoresistance. Biochim. Biophys. Acta Bioenergy 2017, 1858, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Bonetti, L.; Brenner, D. Metabolic Modulation of Immunity: A New Concept in Cancer Immunotherapy. Cell Rep. 2020, 32, 107848. [Google Scholar] [CrossRef] [PubMed]
- Martins Pinto, M.; Paumard, P.; Bouchez, C.; Ransac, S.; Duvezin-Caubet, S.; Mazat, J.P.; Rigoulet, M.; Devin, A. The Warburg Effect and Mitochondrial Oxidative Phosphorylation: Friends or Foes? Biochim. Biophys. Acta BBA—Bioenergy 2023, 1864, 148931. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Hudson, C.D.; Savadelis, A.; Nagaraj, A.B.; Joseph, P.; Avril, S.; DiFeo, A.; Avril, N. Altered Glutamine Metabolism in Platinum Resistant Ovarian Cancer. Oncotarget 2016, 7, 41637–41649. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine Reliance in Cell Metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Schwartz, L.; Seyfried, T.; Alfarouk, K.O.; Da Veiga Moreira, J.; Fais, S. Out of Warburg Effect: An Effective Cancer Treatment Targeting the Tumor Specific Metabolism and Dysregulated pH. Semin. Cancer Biol. 2017, 43, 134–138. [Google Scholar] [CrossRef] [PubMed]
- da Veiga Moreira, J.; Hamraz, M.; Abolhassani, M.; Bigan, E.; Pérès, S.; Paulevé, L.; Levy Nogueira, M.; Steyaert, J.-M.; Schwartz, L. The Redox Status of Cancer Cells Supports Mechanisms behind the Warburg Effect. Metabolites 2016, 6, 33. [Google Scholar] [CrossRef]
- Gureev, A.P.; Syromyatnikov, M.Y.; Ignatyeva, D.A.; Valuyskikh, V.V.; Solodskikh, S.A.; Panevina, A.V.; Gryaznova, M.V.; Kokina, A.V.; Popov, V.N. Effect of Long-Term Methylene Blue Treatment on the Composition of Mouse Gut Microbiome and Its Relationship with the Cognitive Abilities of Mice. PLoS ONE 2020, 15, e0241784. [Google Scholar] [CrossRef] [PubMed]
- Montégut, L.; Martínez-Basilio, P.C.; da Veiga Moreira, J.; Schwartz, L.; Jolicoeur, M. Combining Lipoic Acid to Methylene Blue Reduces the Warburg Effect in CHO Cells: From TCA Cycle Activation to Enhancing Monoclonal Antibody Production. PLoS ONE 2020, 15, e0231770. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hsu, C.-H.; Huang, C.-C.; Chang, P.-Y. Development of Therapeutic Au–Methylene Blue Nanoparticles for Targeted Photodynamic Therapy of Cervical Cancer Cells. ACS Appl. Mater. Interfaces 2015, 7, 432–441. [Google Scholar] [CrossRef]
- Jesus, V.P.S.; Raniero, L.; Lemes, G.M.; Bhattacharjee, T.T.; Caetano Júnior, P.C.; Castilho, M.L. Nanoparticles of Methylene Blue Enhance Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2018, 23, 212–217. [Google Scholar] [CrossRef]
- Songsantiphap, C.; Vanichanan, J.; Chatsuwan, T.; Asawanonda, P.; Boontaveeyuwat, E. Methylene Blue–Mediated Antimicrobial Photodynamic Therapy against Clinical Isolates of Extensively Drug Resistant Gram-Negative Bacteria Causing Nosocomial Infections in Thailand, An In Vitro Study. Front. Cell. Infect. Microbiol. 2022, 12, 929242. [Google Scholar] [CrossRef]
- Guais, A.; Baronzio, G.; Sanders, E.; Campion, F.; Mainini, C.; Fiorentini, G.; Montagnani, F.; Behzadi, M.; Schwartz, L.; Abolhassani, M. Adding a Combination of Hydroxycitrate and Lipoic Acid (METABLOCTM) to Chemotherapy Improves Effectiveness against Tumor Development: Experimental Results and Case Report. Investig. New Drugs 2012, 30, 200–211. [Google Scholar] [CrossRef]
- Provencher, D.M.; Lounis, H.; Champoux, L.; Tétrault, M.; Manderson, E.N.; Wang, J.C.; Eydoux, P.; Savoie, R.; Tonin, P.N.; Mes-Masson, A.M. Characterization of Four Novel Epithelial Ovarian Cancer Cell Lines. In Vitro Cell. Dev. Biol. Anim. 2000, 36, 357–361. [Google Scholar] [CrossRef]
- Brodeur, M.N.; Simeone, K.; Leclerc-Deslauniers, K.; Fleury, H.; Carmona, E.; Provencher, D.M.; Mes-Masson, A.-M. Carboplatin Response in Preclinical Models for Ovarian Cancer: Comparison of 2D Monolayers, Spheroids, Ex Vivo Tumors and in Vivo Models. Sci. Rep. 2021, 11, 18183. [Google Scholar] [CrossRef]
- Schwartz, L.; Summa, M.; Steyaert, J.M.; Guais-Vergne, A.; Baronzio, G.F.; Schwartz, L.; Summa, M.; Steyaert, J.M.; Guais-Vergne, A.; Baronzio, G.F. New Cancer Paradigm and New Treatment: The Example of METABLOC, New Cancer Paradigm and New Treatment: The Example of METABLOC. Conf. Pap. Sci. Conf. Pap. Sci. 2013, 2013, e827686. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.M.; Oliveira, T.C.; Gomes, V.d.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene Blue Photodynamic Therapy Induces Selective and Massive Cell Death in Human Breast Cancer Cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Pursell, R.T. Treatment of Cancer in Dogs by Intravenous Methylene Blue. Nature 1957, 180, 1300. [Google Scholar] [CrossRef] [PubMed]
- da Veiga Moreira, J.; De Staercke, L.; César Martínez-Basilio, P.; Gauthier-Thibodeau, S.; Montégut, L.; Schwartz, L.; Jolicoeur, M. Hyperosmolarity Triggers the Warburg Effect in Chinese Hamster Ovary Cells and Reveals a Reduced Mitochondria Horsepower. Metabolites 2021, 11, 344. [Google Scholar] [CrossRef]
- Shin, M.-K.; Cheong, J.-H. Mitochondria-Centric Bioenergetic Characteristics in Cancer Stem-like Cells. Arch. Pharm. Res. 2019, 42, 113–127. [Google Scholar] [CrossRef]
- Fiorillo, M.; Sotgia, F.; Lisanti, M.P. “Energetic” Cancer Stem Cells (e-CSCs): A New Hyper-Metabolic and Proliferative Tumor Cell Phenotype, Driven by Mitochondrial Energy. Front. Oncol. 2019, 8, 677. [Google Scholar] [CrossRef]
- Turchiello, R.F.; Oliveira, C.S.; Fernandes, A.U.; Gómez, S.L.; Baptista, M.S. Methylene Blue-Mediated Photodynamic Therapy in Human Retinoblastoma Cell Lines. J. Photochem. Photobiol. B 2021, 222, 112260. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Veiga Moreira, J.; Nleme, N.; Schwartz, L.; Leclerc-Desaulniers, K.; Carmona, E.; Mes-Masson, A.-M.; Jolicoeur, M. Methylene Blue Metabolic Therapy Restrains In Vivo Ovarian Tumor Growth. Cancers 2024, 16, 355. https://doi.org/10.3390/cancers16020355
da Veiga Moreira J, Nleme N, Schwartz L, Leclerc-Desaulniers K, Carmona E, Mes-Masson A-M, Jolicoeur M. Methylene Blue Metabolic Therapy Restrains In Vivo Ovarian Tumor Growth. Cancers. 2024; 16(2):355. https://doi.org/10.3390/cancers16020355
Chicago/Turabian Styleda Veiga Moreira, Jorgelindo, Nancy Nleme, Laurent Schwartz, Kim Leclerc-Desaulniers, Euridice Carmona, Anne-Marie Mes-Masson, and Mario Jolicoeur. 2024. "Methylene Blue Metabolic Therapy Restrains In Vivo Ovarian Tumor Growth" Cancers 16, no. 2: 355. https://doi.org/10.3390/cancers16020355
APA Styleda Veiga Moreira, J., Nleme, N., Schwartz, L., Leclerc-Desaulniers, K., Carmona, E., Mes-Masson, A.-M., & Jolicoeur, M. (2024). Methylene Blue Metabolic Therapy Restrains In Vivo Ovarian Tumor Growth. Cancers, 16(2), 355. https://doi.org/10.3390/cancers16020355






