Mean Oral Cavity Organ-at-Risk Dose Predicts Opioid Use and Hospitalization during Radiotherapy for Patients with Head and Neck Tumors
Abstract
Simple Summary
Abstract
1. Introduction
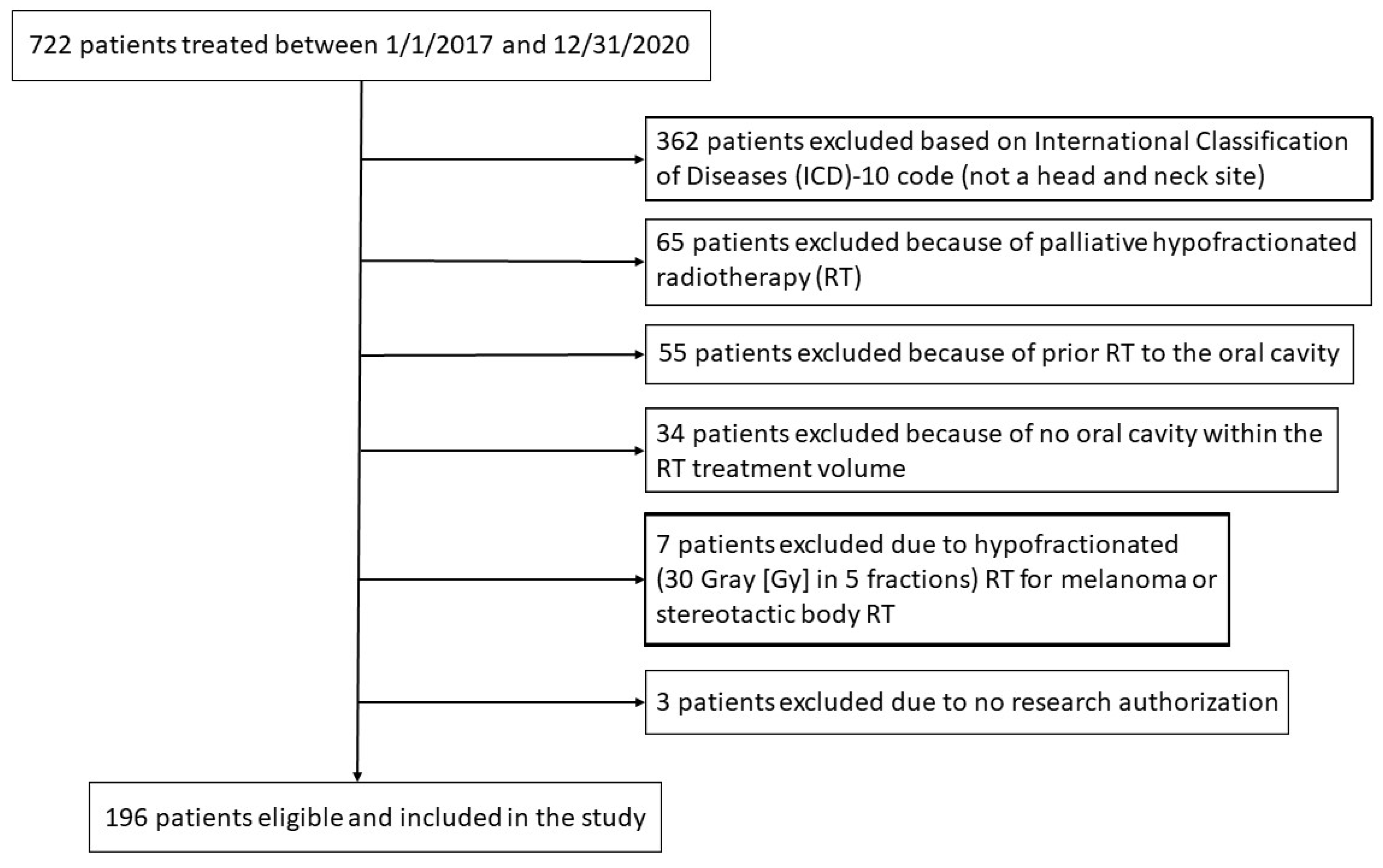
2. Materials and Methods
3. Results
3.1. Univariable Analysis
3.2. Multivariable Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ratko, T.A.; Douglas, G.W.; De Souza, J.A.; Belinson, S.E.; Aronson, N. Radiotherapy Treatments for Head and Neck Cancer Update [Internet]; (Comparative Effectiveness Review, No. 144.) Introduction; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK269010/ (accessed on 1 December 2023).
- Hand, A.R.; Pathmanathan, D.; Field, R.B. Morphological features of the minor salivary glands. Arch. Oral Biol. 1999, 44, S3–S10. [Google Scholar] [CrossRef]
- Schiffman, S.S. Taste and Smell in Disease. NEJM 1983, 308, 1275–1279. [Google Scholar] [PubMed]
- Deshpande, T.S.; Blanchard, P.; Wang, L.; Foote, R.L.; Zhang, X.; Frank, S.J. Radiation-Related Alterations of Taste Function in Patients with Head and Neck Cancer: A Systematic Review. Curr. Treat. Options Oncol. 2018, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, N.; Tahara, M.; Mizusawa, J.; Kodaira, T.; Fujii, H.; Yamazaki, T.; Mitani, H.; Iwae, S.; Fujimoto, Y.; Onozawa, Y.; et al. Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 1980–1990. [Google Scholar] [CrossRef]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+ Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311). J. Clin. Oncol. 2022, 40, 138–149. [Google Scholar] [CrossRef]
- Yom, S.S.; Torres-Saavedra, P.; Caudell, J.J.; Waldron, J.N.; Gillison, M.L.; Xia, P.; Truong, M.T.; Kong, C.; Jordan, R.; Subramaniam, R.M.; et al. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002). J. Clin. Oncol. 2021, 39, 956–965. [Google Scholar] [CrossRef]
- Chaukar, D.; Prabash, K.; Rane, P.; Patil, V.M.; Thiagarajan, S.; Ghosh-Laskar, S.; Sharma, S.; Pai, P.S.; Chaturvedi, P.; Pantvaidya, G.; et al. Prospective Phase II Open-Label Randomized Controlled Trial to Compare Mandibular Preservation in Upfront Surgery With Neoadjuvant Chemotherapy Followed by Surgery in Operable Oral Cavity Cancer. J. Clin. Oncol. 2021, 40, 272–281. [Google Scholar] [CrossRef]
- Chera, B.S.; Amdur, R.J.; Green, R.; Shen, C.; Gupta, G.; Tan, X.; Knowles, M.; Fried, D.; Hayes, N.; Weiss, J.; et al. Phase II Trial of De-Intensified Chemoradiotherapy for Human Papillomavirus–Associated Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2019, 37, 2661–2669. [Google Scholar] [CrossRef]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): An open-label, phase 2, randomised trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, G.-Q.; Zhang, N.; Zhu, X.-D.; Yang, K.-Y.; Jin, F.; Shi, M.; Chen, Y.P.; Hu, W.-H.; et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N. Engl. J. Med. 2019, 381, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Hosni, A.; Chiu, K.; Huang, S.H.; Xu, W.; Huang, J.; Bayley, A.; Bratman, S.V.; Cho, J.; Giuliani, M.; Kim, J.; et al. Non-operative management for oral cavity carcinoma: Definitive radiation therapy as a potential alternative treatment approach. Radiother. Oncol. 2021, 154, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Cassidy, R.J.; Switchenko, J.M.; Kayode, O.A.; Saba, N.F.; Steuer, C.E.; Shin, D.M.; Wadsworth, J.T.; El-Deiry, M.; Patel, M.; et al. Development of Late Toxicities in Patients with Oral Tongue Cancer Treated with Surgical Resection and Adjuvant Radiation Therapy. Front. Oncol. 2017, 6, 272. [Google Scholar] [CrossRef]
- Sciubba, J.J.; Goldenberg, D. Oral complications of radiotherapy. Lancet Oncol. 2006, 7, 175–183. [Google Scholar] [CrossRef]
- Massa, S.T.; Osazuwa-Peters, N.; Boakye, E.A.; Walker, R.J.; Ward, G.M. Comparison of the Financial Burden of Survivors of Head and Neck Cancer With Other Cancer Survivors. JAMA Otolaryngol. Neck Surg. 2019, 145, 239–249. [Google Scholar] [CrossRef]
- Lang, K.; Sussman, M.; Friedman, M.; Su, J.; Kan, H.J.; Mauro, D.; Tafesse, E.; Menzin, J. Incidence and Costs of Treatment-Related Complications Among Patients With Advanced Squamous Cell Carcinoma of the Head and Neck. JAMA Otolaryngol. Neck Surg. 2009, 135, 582–588. [Google Scholar] [CrossRef]
- Elting, L.S.; Chang, Y.-C. Costs of Oral Complications of Cancer Therapies: Estimates and a Blueprint for Future Study. JNCI Monogr. 2019, 2019, lgz010. [Google Scholar] [CrossRef]
- Jacobson, J.J.; Epstein, J.B.; Eichmiller, F.C.; Gibson, T.B.; Carls, G.S.; Vogtmann, E.; Wang, S.; Murphy, B. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: Commercial insurance, medicare, and medicaid. Head Neck Oncol. 2012, 4, 15. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, Outcomes, and Costs of Radiation-Induced Oral Mucositis among Patients with Head-and-Neck Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120. [Google Scholar] [CrossRef]
- Rosenthal, D.I. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J. Support. Oncol. 2007, 5, 23–31. [Google Scholar]
- Sapir, E.; Tao, Y.; Feng, F.; Samuels, S.; El Naqa, I.; Murdoch-Kinch, C.A.; Feng, M.; Schipper, M.; Eisbruch, A. Predictors of Dysgeusia in Patients with Oropharyngeal Cancer Treated with Chemotherapy and Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. 2016, 96, 354–361. [Google Scholar] [CrossRef]
- Hoebers, F.; Yu, E.; Thorstad, W.; O’Sullivan, B.; Dawson, L.A.; Hope, A. A Pragmatic Contouring Guideline for Salivary Gland Structures in Head and Neck Radiation Oncology. The MOIST Target. Am. J. Clin. Oncol. 2013, 36, 70–76. [Google Scholar] [CrossRef]
- Brouwer, C.L.; Steenbakkers, R.J.; Bourhis, J.; Budach, W.; Grau, C.; Grégoire, V.; Van Herk, M.; Lee, A.; Maingon, P.; Nutting, C.; et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother. Oncol. 2015, 117, 83–90. [Google Scholar] [CrossRef]
- Li, K.; Yang, L.; Hu, Q.-Y.; Chen, X.-Z.; Chen, M.; Chen, Y. Oral Mucosa Dose Parameters Predicting Grade ≥ 3 Acute Toxicity in Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Concurrent Intensity-Modulated Radiation Therapy and Chemotherapy: An Independent Validation Study Comparing Oral Cavity versus Mucosal Surface Contouring Techniques. Transl. Oncol. 2017, 10, 752–759. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.-L.; Luo, W.; Lee, A.W.; Wee, J.T.S.; Lee, N.; Zhou, G.-Q.; Tang, L.-L.; Tao, C.-J.; Guo, R.; et al. Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Radiother. Oncol. 2014, 110, 390–397. [Google Scholar] [CrossRef]
- Dean, J.A.; Welsh, L.C.; Gulliford, S.L.; Harrington, K.J.; Nutting, C.M. A novel method for delineation of oral mucosa for radiotherapy dose–response studies. Radiother. Oncol. 2015, 115, 63–66. [Google Scholar] [CrossRef]
- Kaae, J.K.; Johnsen, L.; Hansen, C.R.; Kristensen, M.H.; Brink, C.; Eriksen, J.G. Relationship between patient and physician-rated xerostomia and dose distribution to the oral cavity and salivary glands for head and neck cancer patients after radiotherapy. Acta Oncol. 2019, 58, 1366–1372. [Google Scholar] [CrossRef]
- Fried, D.V.; Das, S.K.; Shen, C.; Marks, L.B.; Chera, B.S. Impact of Oral Cavity Dosimetry on Patient Reported Xerostomia and Dysgeusia in the Setting of Deintensified Chemoradiotherapy. Adv. Radiat. Oncol. 2022, 7, 100952. [Google Scholar] [CrossRef]
- Chen, W.-C.; Hsu, C.-M.; Tsai, Y.-T.; Lin, M.-H.; Tsai, M.-S.; Chang, G.-H.; Lai, C.-H.; Fang, F.; Chen, M.-F. Prospective Evaluation of Taste Function in Patients With Head and Neck Cancer Receiving Intensity-Modulated Radiotherapy. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 604–611. [Google Scholar] [CrossRef]
- Wang, X.; Eisbruch, A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J. Radiat. Res. 2016, 57, i69–i75. [Google Scholar] [CrossRef]
- Gunn, L.; Gilbert, J.; Nenclares, P.; Soliman, H.; Newbold, K.; Bhide, S.; Wong, K.H.; Harrington, K.; Nutting, C. Taste dysfunction following radiotherapy to the head and neck: A systematic review. Radiother. Oncol. 2021, 157, 130–140. [Google Scholar] [CrossRef]
- Jellema, A.P.; Doornaert, P.; Slotman, B.J.; Rene Leemans, C.; Langendijk, J.A. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother. Oncol. 2005, 77, 164–171. [Google Scholar] [CrossRef]
- Eisbruch, A.; Kim, H.M.; Terrell, J.E.; Marsh, L.H.; Dawson, L.A.; Ship, J.A. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 695–704. [Google Scholar] [CrossRef]
- Blanco, A.I.; Chao, K.C.; El Naqa, I.; Franklin, G.E.; Zakarian, K.; Vicic, M.; Deasy, J.O. Dose–volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int. J. Radiat. Oncol. 2005, 62, 1055–1069. [Google Scholar] [CrossRef]
- Riva, G.; Raimondo, L.; Ravera, M.; Moretto, F.; Boita, M.; Potenza, I.; Rampino, M.; Ricardi, U.; Garzaro, M. Late Sensorial Alterations in Different Radiotherapy Techniques for Nasopharyngeal Cancer. Chem. Senses 2015, 40, 285–292. [Google Scholar] [CrossRef]
- Maes, A.; Huygh, I.; Weltens, C.; Vandevelde, G.; Delaere, P.; Evers, G.; Bogaert, W.V.D. De Gustibus: Time scale of loss and recovery of tastes caused by radiotherapy. Radiother. Oncol. 2002, 63, 195–201. [Google Scholar] [CrossRef]
- Mirza, N.; Machtay, M.; Devine, P.A.; Troxel, A.; Abboud, S.K.; Doty, R.L. Gustatory Impairment in Patients Undergoing Head and Neck Irradiation. Laryngoscope 2008, 118, 24–31. [Google Scholar] [CrossRef]
- Chen, W.-C.; Tsai, M.-S.; Tsai, Y.-T.; Lai, C.-H.; Lee, C.-P.; Chen, M.-F. Long-Term Taste Impairment after Intensity-Modulated Radiotherapy to Treat Head-and-Neck Cancer: Correlations with Glossectomy and the Mean Radiation Dose to the Oral Cavity. Chem. Senses 2019, 44, 319–326. [Google Scholar] [CrossRef]
- Yamashita, H.; Nakagawa, K.; Nakamura, N.; Abe, K.; Asakage, T.; Ohmoto, M.; Okada, S.; Matsumoto, I.; Hosoi, Y.; Sasano, N.; et al. Relation between acute and late irradiation impairment of four basic tastes and irradiated tongue volume in patients with head-and-neck cancer. Int. J. Radiat. Oncol. 2006, 66, 1422–1429. [Google Scholar] [CrossRef]
- Yamashita, H.; Nakagawa, K.; Tago, M.; Nakamura, N.; Shiraishi, K.; Eda, M.; Nakata, H.; Nagamatsu, N.; Yokoyama, R.; Onimura, M.; et al. Taste dysfunction in patients receiving radiotherapy. Head Neck 2006, 28, 508–516. [Google Scholar] [CrossRef]
- Baharvand, M.; ShoalehSaadi, N.; Barakian, R.; Jalali Moghaddam, E. Taste alteration and impact on quality of life after head and neck radiotherapy. J. Oral Pathol. Med. 2013, 42, 106–112. [Google Scholar] [CrossRef]
- Negi, P.; Kingsley, P.-A.; Thomas, M.; Sachdeva, J.; Srivastava, H.; Kalra, B. Pattern of Gustatory Impairment and its Recovery after Head and Neck Irradiation. Iran. J. Otorhinolaryngol. 2017, 29, 319–327. [Google Scholar]
- Fernando, I.; Patel, T.; Billingham, L.; Hammond, C.; Hallmark, S.; Glaholm, J.; Henk, J. The effect of head and neck irradiation on taste dysfunction: A prospective study. Clin. Oncol. 1995, 7, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kamprad, F.; Ranft, D.; Weber, A.; Hildebrandt, G. Functional Changes of the Gustatory Organ Caused by Local Radiation Exposure during Radiotherapy of the Head-and-Neck Region. Strahlenther. Onkol. 2008, 184, 157–162. [Google Scholar] [CrossRef]
- Sandow, P.; Hejrat-Yazdi, M.; Heft, M. Taste Loss and Recovery Following Radiation Therapy. J. Dent. Res. 2006, 85, 608–611. [Google Scholar] [CrossRef]
- da Silva, J.L.B.; Doty, R.L.; Miyazaki, J.V.M.K.; Borges, R.; Pinna, F.d.R.; Voegels, R.L.; Fornazieri, M.A. Gustatory disturbances occur in patients with head and neck cancer who undergo radiotherapy not directed to the oral cavity. Oral Oncol. 2019, 95, 115–119. [Google Scholar] [CrossRef]
- Martini, S.; Iorio, G.C.; Arcadipane, F.; Olivero, F.; Silvetti, P.; Rampino, M.; Demo, P.G.; Fasolis, M.; Pecorari, G.; Airoldi, M.; et al. Prospective assessment of taste impairment and nausea during radiotherapy for head and neck cancer. Med. Oncol. 2019, 36, 44. [Google Scholar] [CrossRef] [PubMed]
- Mossman, K.L. Quantitative Radiation Dose-Response Relationships for Normal Tissues in Man: I. Gustatory Tissue Response during Photon and Neutron Radiotherapy. Radiat. Res. 1982, 91, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.; Lehmann, J.; Coleman, M.A.; Vaughan, A.; Yang, C.C.; Enepekides, D.; Farwell, G.; Purdy, J.A.; Laredo, G.; Nolan, K.; et al. Prospective evaluation to establish a dose response for clinical oral muscositis in patients undergoing head-and-neck conformal radiotherpy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 73, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Zhang, S.-Z.; Zhang, Z.-Y.; Zhang, C.-P.; Hu, H.-S.; Tu, W.-Y.; Kirwan, J.; Mendenhall, W.M. Protecting the oral mucosa in patients with oral tongue squamous cell carcinoma treated postoperatively with intensity-modulated radiotherapy: A randomized study. Laryngoscope 2012, 122, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, R.; Ricchetti, F.; Fersino, S.; Fiorentino, A.; Levra, N.G.; Di Paola, G.; Ruggieri, R.; Alongi, F. Predictors of mucositis in oropharyngeal and oral cavity cancer in patients treated with volumetric modulated radiation treatment: A dose–volume analysis. Head Neck 2015, 38, E815–E819. [Google Scholar] [CrossRef]
- Bjordal, K.; Ahlner-Elmqvist, M.; Tollesson, E.; Jensen, A.B.; Razavi, D.; Maher, E.J.; Kaasa, S. Development of a European Organization for Research and Treatment of Cancer (EORTC) questionnaire module to be used in quality of life assessments in head and neck cancer patients. EORTC Quality of Life Study Group. Acta Oncol. 1994, 33, 879–885. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Atallah, E.; Schiffer, C.A.; Weinfurt, K.P.; Zhang, M.-J.; Radich, J.P.; Oehler, V.G.; Pinilla-Ibarz, J.; Deininger, M.W.N.; Lin, L.; Larson, R.A.; et al. Design and rationale for the life after stopping tyrosine kinase inhibitors (LAST) study, a prospective, single-group longitudinal study in patients with chronic myeloid leukemia. BMC Cancer 2018, 18, 359. [Google Scholar] [CrossRef]
- King, M.T. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual. Life Res. 1996, 5, 555–567. [Google Scholar] [CrossRef]
- Brodin, N.P.; Kabarriti, R.; Garg, M.K.; Guha, C.; Tomé, W.A. Systematic Review of Normal Tissue Complication Models Relevant to Standard Fractionation Radiation Therapy of the Head and Neck Region Published After the QUANTEC Reports. J. Radiat. Oncol. Biol. Phys. 2017, 100, 391–407. [Google Scholar] [CrossRef]
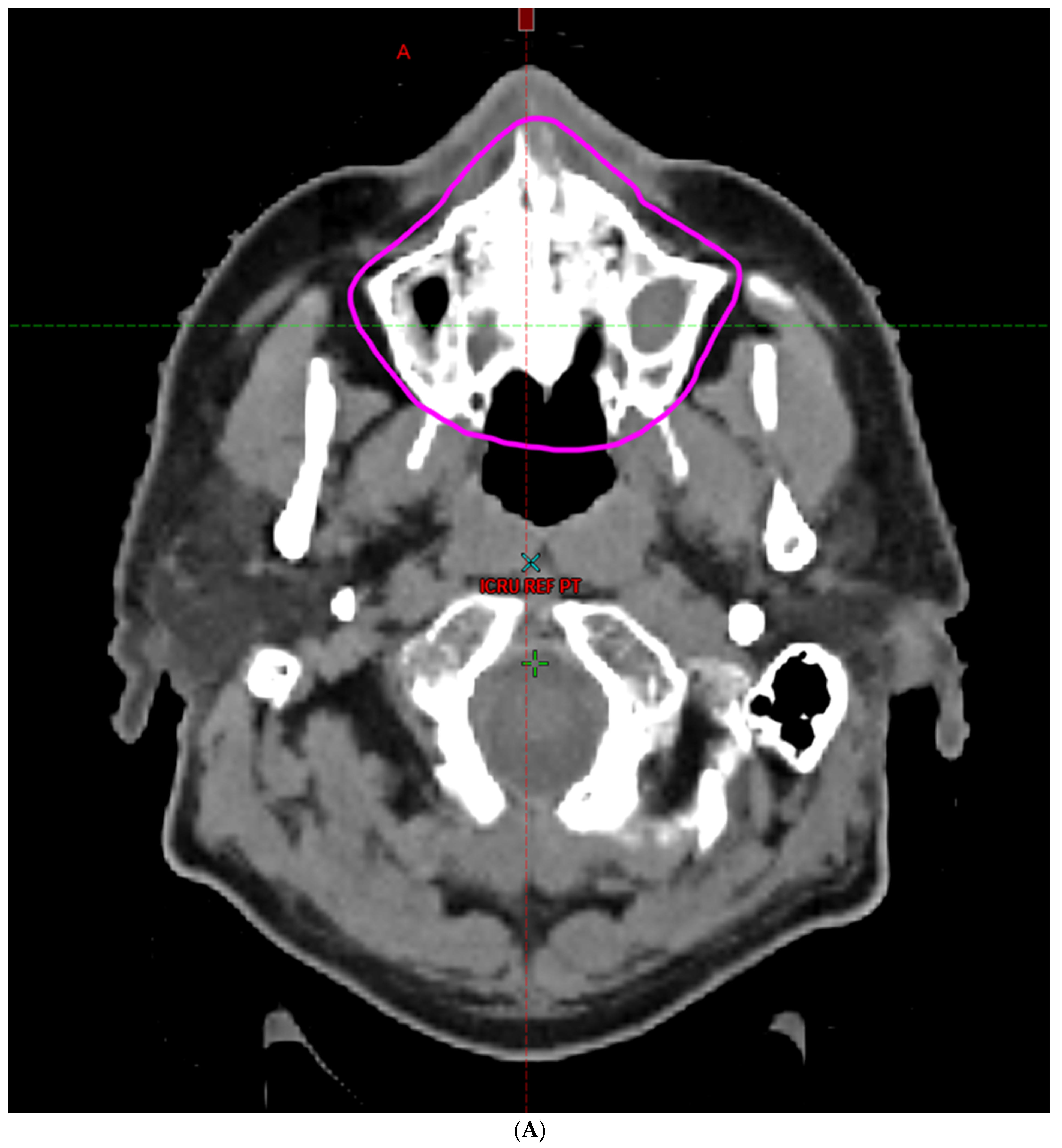


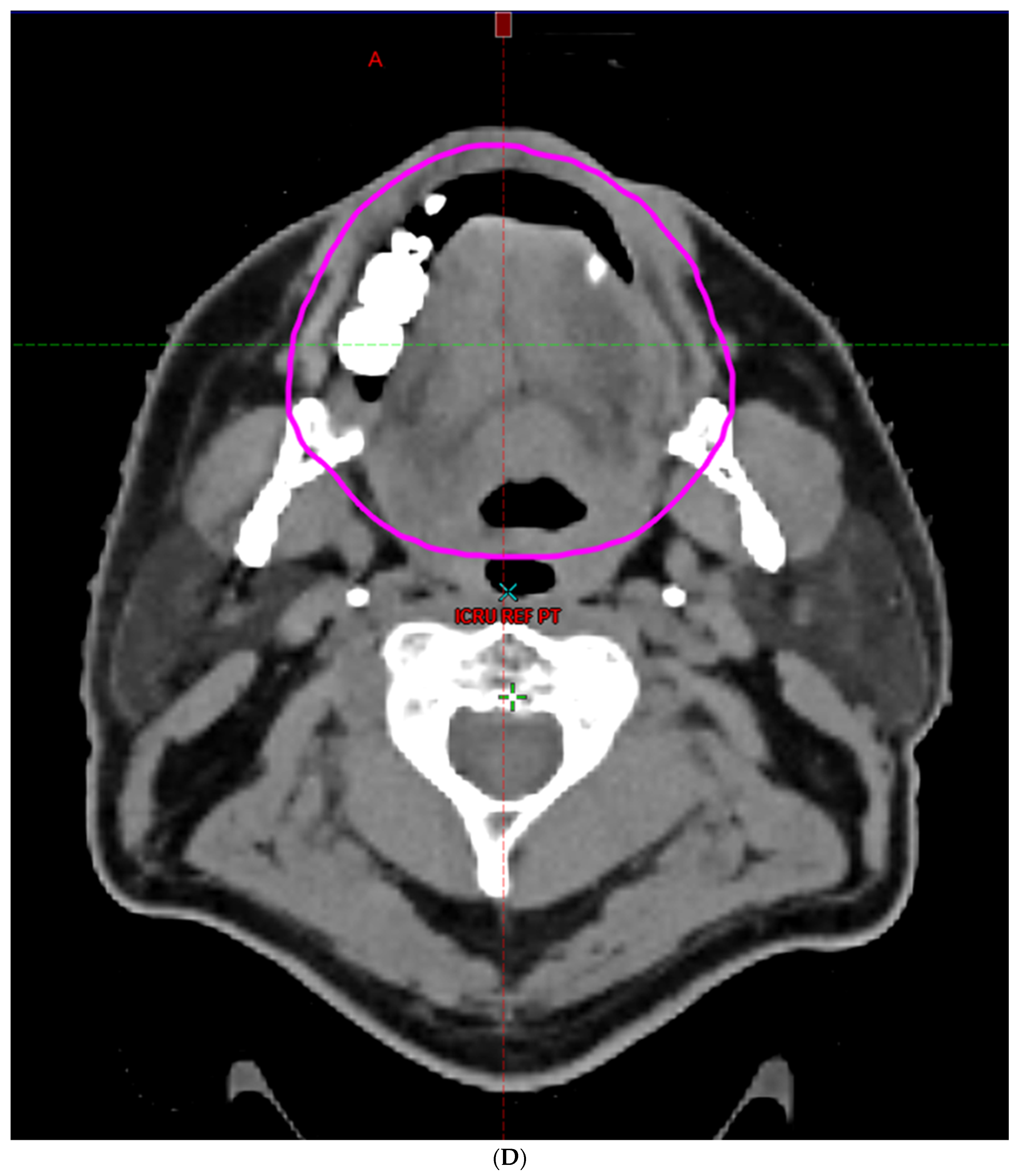
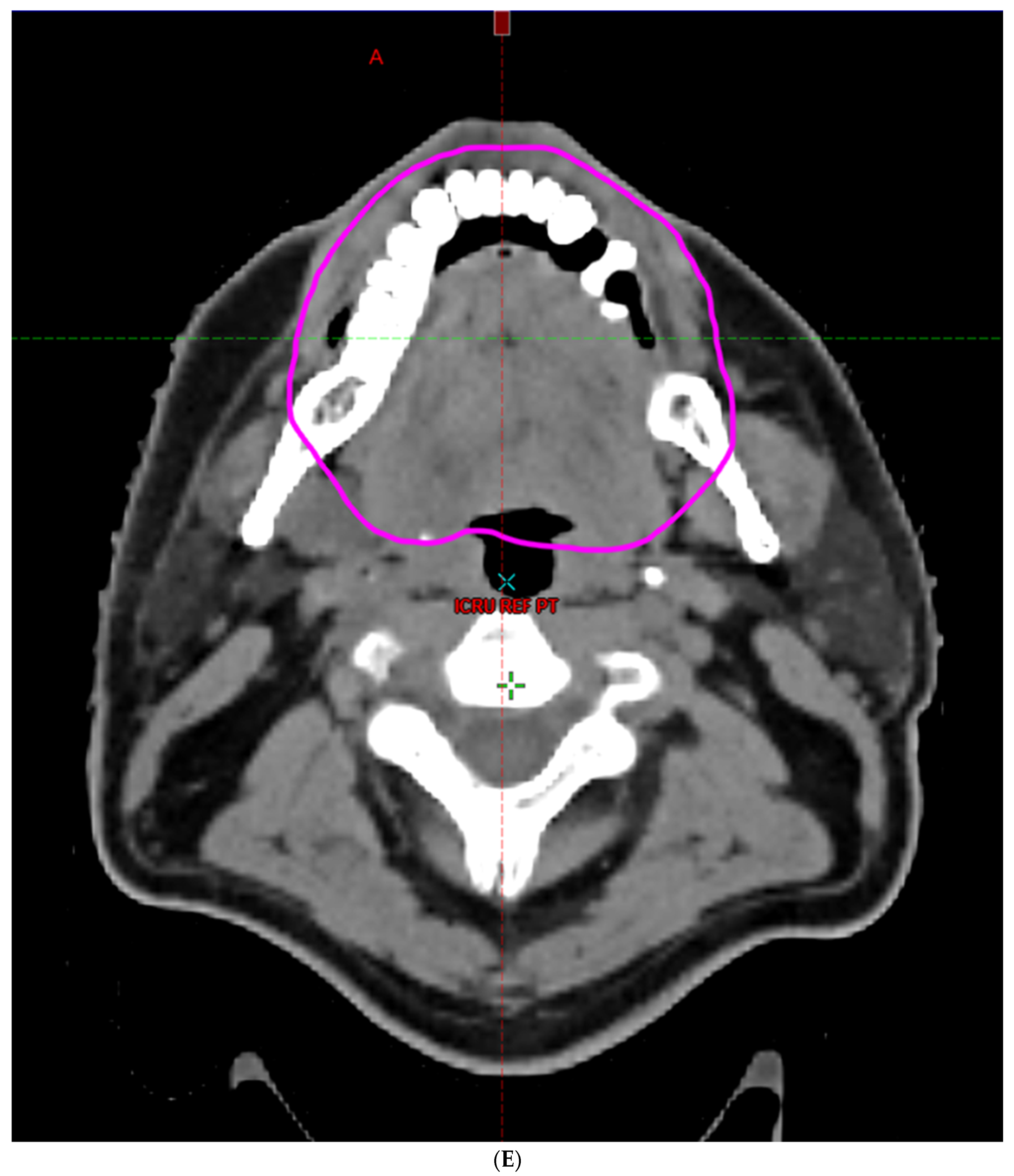
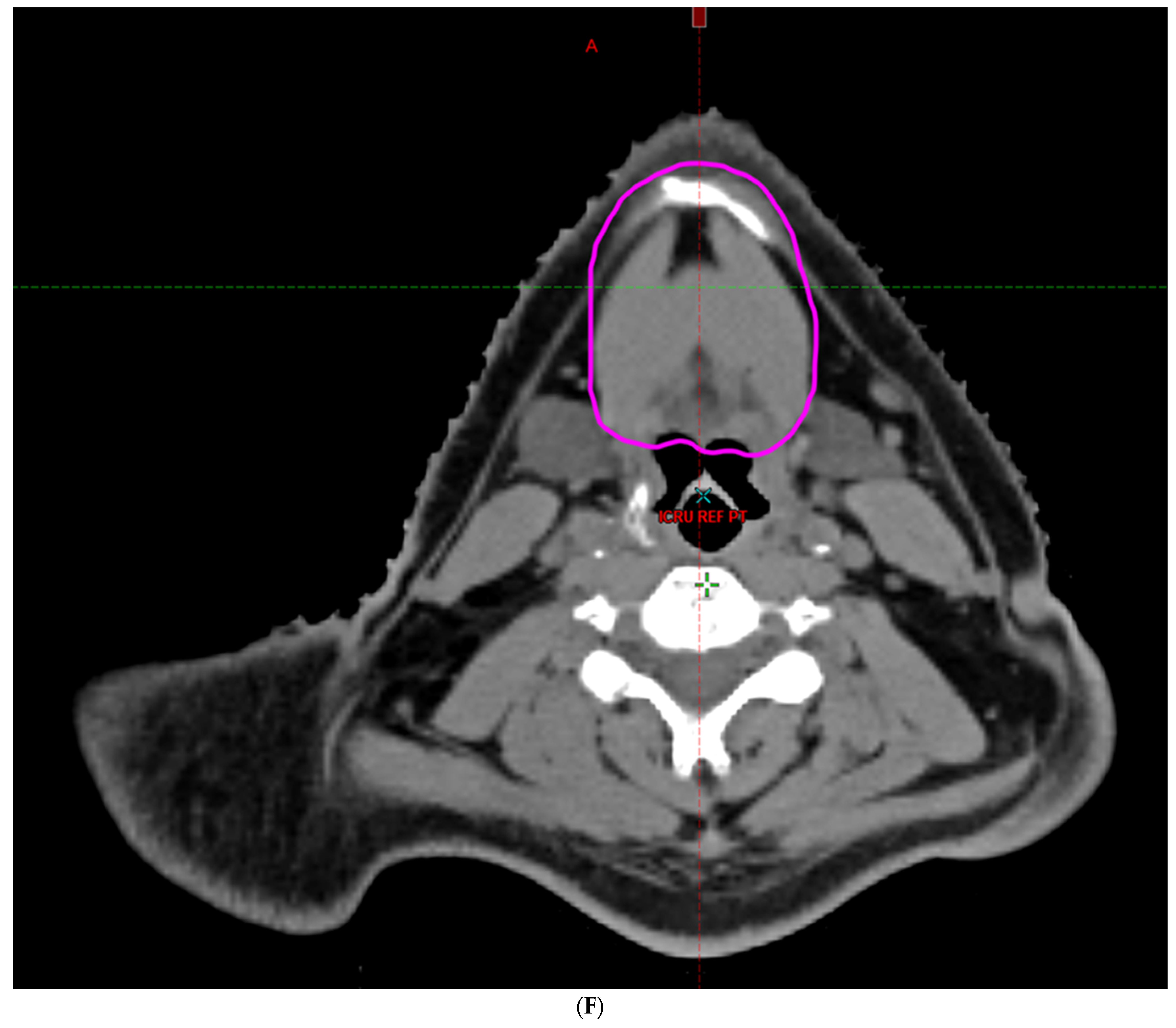
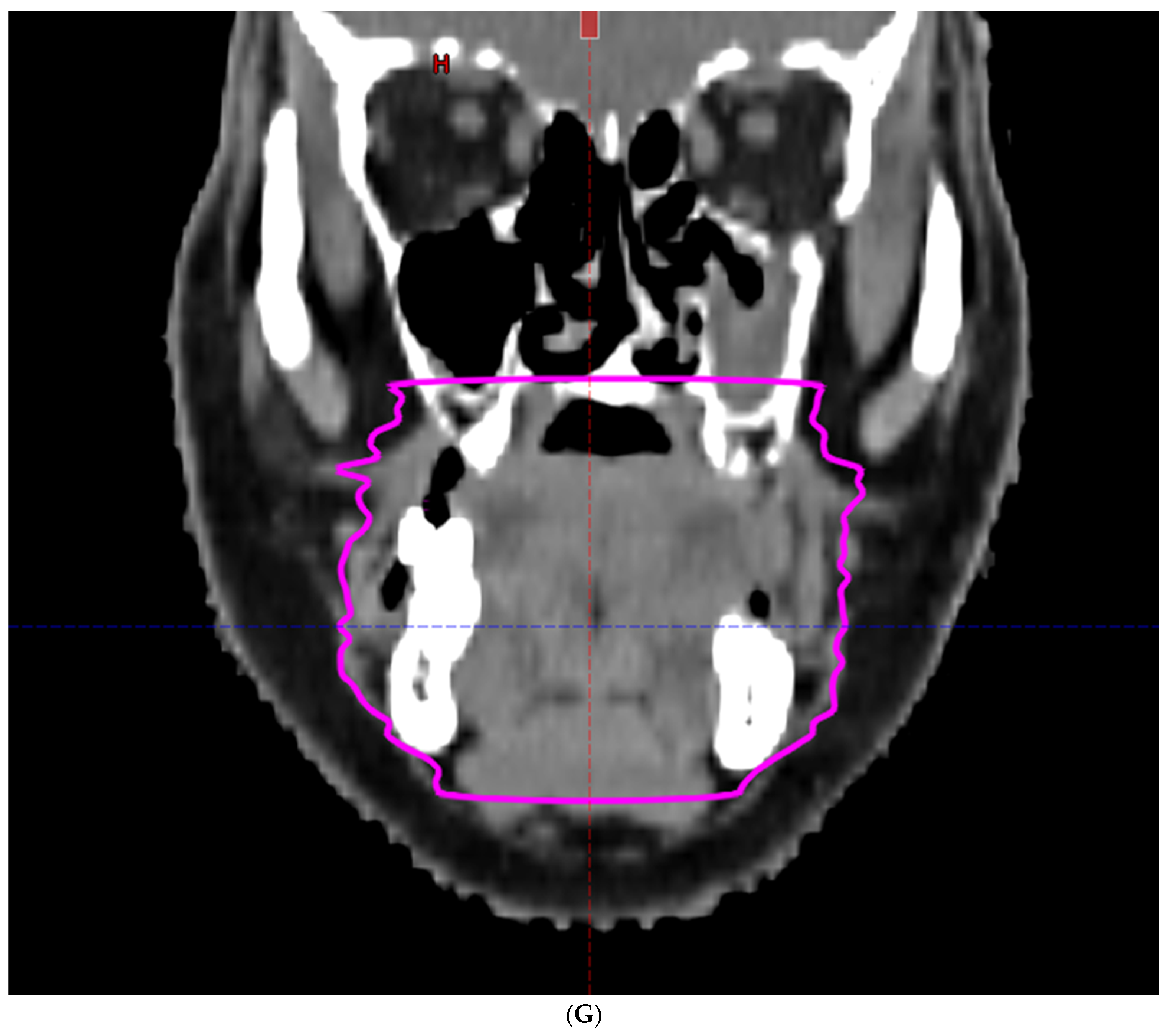
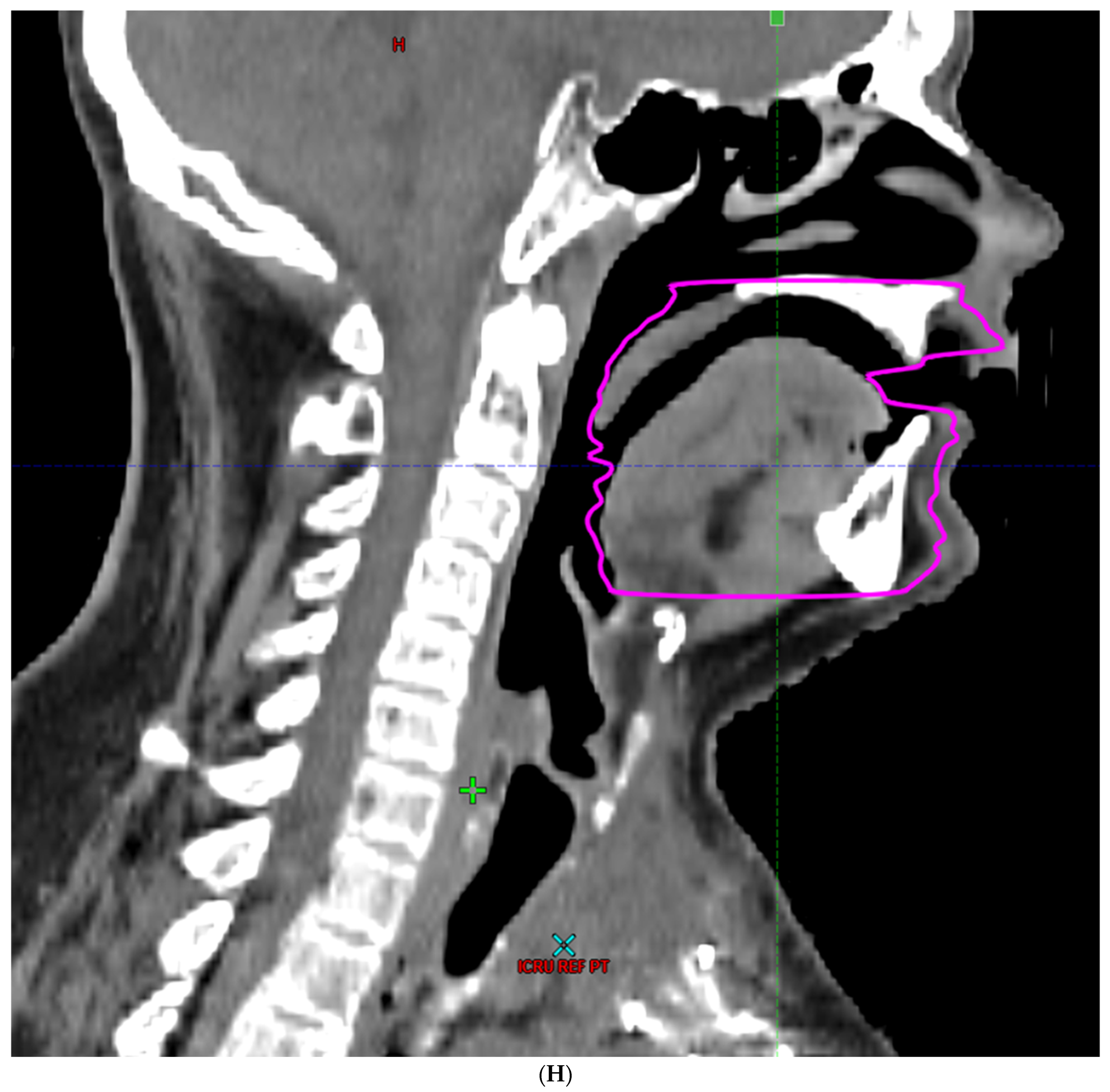
| Characteristic | |
| Male/female, n, (%) | 142 (72.4)/54 (27.6) |
| Median age in years (IQR) | 63.0 (55.1, 72.5) |
| Diabetes mellitus, n, (%) | 15 (7.7) |
| Hypertension, n, (%) | 83 (42.3) |
| Smoking status (none, former, current) | 99, 87, 9 |
| Ever smoker (no, yes) | 99, 96 |
| Leukoplakia | 2 |
| Site | |
| Oropharynx | 53 |
| Nasal cavity/paranasal sinus | 27 |
| Skin | 27 |
| Oral cavity | 25 |
| Major salivary gland | 19 |
| Larynx | 12 |
| Thyroid gland | 12 |
| Nasopharynx | 8 |
| Lacrimal gland/sac | 3 |
| Unknown primary (cervical lymph node metastasis) | 3 |
| Bone | 2 |
| Vascular (neck) | 2 |
| Conjunctiva | 1 |
| Hypopharynx | 1 |
| Trachea | 1 |
| Histology | |
| Squamous cell carcinoma | 124 |
| Adenoid cystic carcinoma | 11 |
| Adenocarcinoma | 11 |
| Carcinoma, NOS | 9 |
| Anaplastic carcinoma | 7 |
| Esthesioneuroblastoma | 6 |
| Melanoma | 4 |
| Merkel cell carcinoma | 4 |
| Unknown | 4 |
| Acinic cell carcinoma | 3 |
| Basal cell carcinoma | 3 |
| Epithelial–myoepithelial carcinoma | 2 |
| Paraganglioma | 2 |
| Undifferentiated carcinoma | 2 |
| Ameloblastoma | 1 |
| Angiosarcoma | 1 |
| Ewing sarcoma | 1 |
| Sinonasal undifferentiated carcinoma | 1 |
| American Joint Committee on Cancer (AJCC) stage (7th and 8th edition) * | |
| No AJCC staging system for site | 22 |
| Unknown | 7 |
| 0 | 2 |
| I | 34 |
| IA | 1 |
| IB | 1 |
| II | 30 |
| III | 26 |
| IIIA | 3 |
| IV | 1 |
| IVA | 42 |
| IVB | 24 |
| IVC | 3 |
| Treatment | |
| Glossectomy | 22 |
| Postoperative radiotherapy (RT) | 110 |
| Primary RT | 86 |
| Dose delivered, gray (Gy) †, median (IQR) | 60 (30, 80.4) |
| Dose per fraction, Gy, median (IQR) | 2.0 (1.2, 2.25) |
| Total number of fractions, median (IQR) | 30 (15, 35) |
| Protons | 134 |
| Photons | 62 |
| Cytotoxic chemotherapy, n, (%) | 89 (45.4) |
| Mean oral cavity organ-at-risk (OAR) dose, Gy, median (IQR) | 19.9 (9.65, 30.57) |
| Mean oral cavity OAR volume, cubic centimeters (cc), median (IQR) | 309 (264, 356) |
| Mean total parotid gland dose, Gy median (IQR) | 21.01 (5.89, 31.05) |
| Mean right parotid gland dose, Gy, median (IQR) | 19.45 (3.38, 29.45) |
| Mean left parotid gland dose, Gy, median (IQR) | 19.57 (4.65, 33.85) |
| Mean right submandibular gland dose, Gy, median (IQR) | 39.98 (0.48, 61.14) |
| Mean left submandibular gland dose, Gy, median (IQR) | 44.08 (0.28, 61.13) |
| Pearson Correlations * | V10Gy | V20Gy | V30Gy | V40Gy | V50Gy | V60Gy | V70Gy |
|---|---|---|---|---|---|---|---|
| Dmax | 0.33243 | 0.42786 | 0.46508 | 0.47391 | 0.43368 | 0.37524 | 0.30862 |
| Mean | 0.82351 | 0.90894 | 0.92801 | 0.89423 | 0.86955 | 0.82091 | 0.46076 |
| Toxicity | Time Point | Variable | OR (95% CI) | p-Value | C-Statistic |
|---|---|---|---|---|---|
| CTCAE v4.03 dehydration > grade 1 | End of treatment | Cytotoxic chemotherapy | 5 (3–12) | <0.001 | 0.77 |
| Mean total parotid dose | 1 (1–2) | 0.044 | 0.77 | ||
| CTCAE v4.03 dry mouth > grade 1 | End of treatment | Mean right SMG dose | 1 (1–1) | <0.001 | 0.68 |
| CTCAE v4.03 dysgeusia > grade 1 | End of treatment | Mean right SMG dose | 1 (1–2) | <0.001 | 0.71 |
| CTCAE v4.03 dysphagia > grade 1 | End of treatment | Mean left SMG dose | 1 (1–2) | 0.029 | 0.84 |
| Mean right SMG dose | 1 (1–2) | 0.006 | 0.84 | ||
| Protons | 0.3 (0.1–0.6) | 0.003 | 0.84 | ||
| 3 months | Mean left SMG dose | 1 (1–2) | 0.001 | 0.76 | |
| Protons | 0.4 (0.1–0.9) | 0.029 | 0.76 | ||
| CTCAE v4.03 salivary duct inflammation > grade 1 | End of treatment | Mean left SMG dose | 2 (1–2) | 0.002 | 0.87 |
| Mean right SMG dose | 2 (1–2) | 0.002 | 0.87 | ||
| Protons | 0.4 (0.2–1) | 0.047 | 0.87 | ||
| Weight loss ≥ 10% | 3 months | Mean total parotid dose | 2 (1–2) | <0.001 | 0.71 |
| 6 months | Mean total parotid dose | 2 (1–3) | 0.002 | 0.79 | |
| Mean right SMG dose | 1 (1–2) | 0.037 | 0.79 | ||
| 1 year | Mean total parotid dose | 2 (1–3) | 0.001 | 0.79 | |
| Mean right SMG dose | 1 (1–2) | 0.04 | 0.8 | ||
| 2 years | Mean left SMG dose | 1 (1–2) | 0.005 | 0.7 | |
| EORTC saliva scale decrease ≥ 10 pts | 1 year | Mean right SMG dose | 1 (1–1) | 0.028 | 0.67 |
| 2 years | Mean right SMG dose | 1 (1–2) | 0.032 | 0.71 | |
| Start of opioid pain medication | During or within 30 days of completing RT | Cytotoxic chemotherapy | 4 (2, 11) | 0.002 | 0.75 |
| Oral cavity OAR mean dose | 2 (1–2) | 0.008 | 0.75 | ||
| Hospitalization | During or within 30 days of completing RT | Cytotoxic chemotherapy | 2 (1–5) | 0.022 | 0.68 |
| Oral cavity OAR mean dose | 1 (1–2) | 0.036 | 0.68 |
| DVH Statistic | Photon (n = 62) | Proton (n = 134) | Total (n = 196) | p-Value 1 |
|---|---|---|---|---|
| Dmax (cGy) | 0.035 | |||
| Mean (SD) | 5853 (1595) | 6317 (1342) | 6170 (1439) | |
| Median | 6384 | 6399 | 6392 | |
| Q1, Q3 | 5864, 6782 | 6255, 7136 | 6208, 6991 | |
| Range | (178–7526) | (0.1–8543) | (0.1–8543) | |
| Mean (cGy) | <0.001 | |||
| Mean (SD) | 2878 (1565) | 1805 (1198) | 2145 (1412) | |
| Median | 2767 | 1801 | 1990 | |
| Q1, Q3 | 1793, 4108 | 830, 2515 | 965, 3057 | |
| Range | (31–5915) | (0.0–5271) | (0.0–5915) | |
| V1000cGy | <0.001 | |||
| Mean (SD) | 238 (106) | 136 (83) | 168 (102) | |
| Median | 251 | 124 | 160 | |
| Q1, Q3 | 175, 315 | 74, 207 | 87, 239 | |
| Range | (0–401) | (0–335) | (0–401) | |
| V2000cGy | <0.001 | |||
| Mean (SD) | 178 (110) | 104 (71) | 128 (92) | |
| Median | 176 | 93 | 117 | |
| Q1, Q3 | 90, 267 | 47, 159 | 59, 183 | |
| Range | (0–381) | (0–302) | (0–381) | |
| V3000cGy | <0.001 | |||
| Mean (SD) | 127 (103) | 83 (61) | 97 (80) | |
| Median | 101 | 71 | 81 | |
| Q1, Q3 | 34, 210 | 35, 127 | 35, 143 | |
| Range | (0–368) | (0–270) | (0–368) | |
| V4000cGy | 0.028 | |||
| Mean (SD) | 90 (98) | 66 (53) | 73 (71) | |
| Median | 55 | 55 | 55 | |
| Q1, Q3 | 5, 155 | 26, 103 | 15, 109 | |
| Range | (0–345) | (0–247) | (0–345) | |
| V5000cGy | 0.037 | |||
| Mean (SD) | 69 (87) | 49 (46) | 55 (62) | |
| Median | 17 | 40 | 38 | |
| Q1, Q3 | 1, 126 | 16, 77 | 5, 87 | |
| Range | (0–310) | (0–225) | (0–310) | |
| V6000cGy | 0.004 | |||
| Mean (SD) | 49 (70) | 27 (36) | 34 (50) | |
| Median | 4 | 12 | 10 | |
| Q1, Q3 | 0, 78 | 2, 37 | 0.4, 48 | |
| Range | (0–255) | (0–200) | (0–255) | |
| V7000cGy | 0.872 | |||
| Mean (SD) | 7 (26) | 7 (20) | 7 (22) | |
| Median | 0 | 0 | 0 | |
| Q1, Q3 | 0, 0 | 0, 0.1 | 0, 0 | |
| Range | (0–158) | (0–159) | (0–159) |
| Mean Oral Cavity OAR Dose Cut Point (cGy) | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Accuracy |
|---|---|---|---|---|---|
| <460, ≥460 | 91% (72/79) | 16% (7/43) | 67% (72/108) | 50% (7/14) | 65% (79/122) |
| <1050, ≥1050 | 81% (64/79) | 40% (17/43) | 71% (64/90) | 53% (17/32) | 66% (81/122) |
| <1610, ≥1610 | 71% (56/79) | 54% (27/43) | 78% (56/72) | 54% (27/50) | 68% (83/122) |
| Mean Oral Cavity OAR Dose Cut Point (cGy) | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Accuracy |
|---|---|---|---|---|---|
| <685, ≥685 | 90% (36/40) | 19% (29/156) | 22% (36/163) | 88% (29/33) | 33% (65/196) |
| <1450, ≥1450 | 80% (32/40) | 38% (60/156) | 25% (32/128) | 88% (60/68) | 47% (92/196) |
| <1960 ≥1960 | 70% (28/40) | 53% (83/156) | 28% (28/101) | 87% (83/95) | 57% (111/196) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foote, R.L.; Harmsen, W.S.; Amundson, A.C.; Carr, A.B.; Gamez, M.E.; Garces, Y.I.; Lester, S.C.; Ma, D.J.; McGee, L.A.; Moore, E.J.; et al. Mean Oral Cavity Organ-at-Risk Dose Predicts Opioid Use and Hospitalization during Radiotherapy for Patients with Head and Neck Tumors. Cancers 2024, 16, 349. https://doi.org/10.3390/cancers16020349
Foote RL, Harmsen WS, Amundson AC, Carr AB, Gamez ME, Garces YI, Lester SC, Ma DJ, McGee LA, Moore EJ, et al. Mean Oral Cavity Organ-at-Risk Dose Predicts Opioid Use and Hospitalization during Radiotherapy for Patients with Head and Neck Tumors. Cancers. 2024; 16(2):349. https://doi.org/10.3390/cancers16020349
Chicago/Turabian StyleFoote, Robert L., W. Scott Harmsen, Adam C. Amundson, Alan B. Carr, Mauricio E. Gamez, Yolanda I. Garces, Scott C. Lester, Daniel J. Ma, Lisa A. McGee, Eric J. Moore, and et al. 2024. "Mean Oral Cavity Organ-at-Risk Dose Predicts Opioid Use and Hospitalization during Radiotherapy for Patients with Head and Neck Tumors" Cancers 16, no. 2: 349. https://doi.org/10.3390/cancers16020349
APA StyleFoote, R. L., Harmsen, W. S., Amundson, A. C., Carr, A. B., Gamez, M. E., Garces, Y. I., Lester, S. C., Ma, D. J., McGee, L. A., Moore, E. J., Neben Wittich, M. A., Patel, S. H., Routman, D. M., Rwigema, J.-C. M., Van Abel, K. M., Yin, L. X., Muller, O. M., & Shiraishi, S. (2024). Mean Oral Cavity Organ-at-Risk Dose Predicts Opioid Use and Hospitalization during Radiotherapy for Patients with Head and Neck Tumors. Cancers, 16(2), 349. https://doi.org/10.3390/cancers16020349






