A Comprehensive Review of Immunotherapy Clinical Trials for Metastatic Urothelial Carcinoma: Immune Checkpoint Inhibitors Alone or in Combination, Novel Antibodies, Cellular Therapies, and Vaccines
Abstract
Simple Summary
Abstract
1. Introduction
2. The Current Role of Immunotherapy in the Standard of Care for Metastatic Urothelial Carcinoma
2.1. Pembrolizumab plus Enfortumab Vedotin
2.2. Nivolumab plus Gemcitabine plus Cisplatin in the First-Line Metastatic Setting
2.3. Maintenance Avelumab after First-Line Platinum Chemotherapy
2.4. Pembrolizumab in the First-Line Metastatic Setting for Platinum Chemotherapy Ineligible
2.5. Single-Agent ICIs in the Second-Line Metastatic Setting
2.5.1. Pembrolizumab
2.5.2. Nivolumab
2.5.3. Avelumab
3. Investigational Approaches Involving Immunotherapy for Metastatic Urothelial Carcinoma: Recent and Ongoing Clinical Trials
3.1. Novel Anti-PD-1, Anti-PD-L1, and Anti-CTLA-4 ICIs
3.2. Maintenance ICI-Containing Combination Regimens
3.3. Combination ICI Regimens for Metastatic Urothelial Carcinoma
3.3.1. Cytotoxic Chemotherapy in Combination with ICIs
3.3.2. Antibody–Drug Conjugates in Combination with ICIs
| Regimen | Drug Classes | Inclusion Criteria | Trial | Phase | Status (March 2023) | Identifier | ORR | mPFS (Months) | mOS (Months) |
|---|---|---|---|---|---|---|---|---|---|
| EV + pembrolizumab | ADC + anti-PD1 | Cohort B: 2L+ mUC | EV-103 | 1/2 | Active, not recruiting | NCT03288545 | NA | NA | NA |
| EV + pembrolizumab vs. chemo | ADC + anti-PD1 vs. chemo | 1L mUC | EV-302 | 3 | Recruiting | NCT04223856 | 67.7% vs. 44.4% | 12.5 vs. 6.3 | 31.5 vs. 16.1 |
| EV + platinum + pembrolizumab | ADC + chemo + anti-PD1 | Cohort G: 1L mUC | EV-103 | 1/2 | Active, not recruiting | NCT03288545 | NA | NA | NA |
| EV + platinum + pembrolizumab | ADC + chemo + anti-PD1 | Arm C: 1L mUC | EV-302 | 3 | Recruiting | NCT04223856 | NA | NA | NA |
| EV + sitravatinib + pembrolizumab | ADC + TKI + anti-PD1 | Cohort 9: mUC after platinum and ICI | 2 | Terminated | NCT03606174 | NA | NA | NA | |
| EV + pembrolizumab | ADC + anti-PD1 | Non-urothelial and variant histology mUC | 2 | Not yet recruiting | NCT05756569 | NA | NA | NA | |
| SG + pembrolizumab | ADC + anti-PD1 | Cohort 3: mUC after platinum | TROPHY U-01 | 2 | Recruiting | NCT03547973 | 41% | 5.3 | 12.7 |
| SG +/− zimberelimab +/− domvanalimab vs. gemcitabine + carboplatin | ADC +/− anti-PD1 +/− anti-TIGIT vs. chemo | Cohort 6: 1L mUC cisplatin-ineligible | TROPHY U-01 | 2 | Recruiting | NCT03547973 | NA | NA | NA |
| SG + ipilimumab + nivolumab | ADC + anti-CTLA-4 + anti-PD1 | 1L mUC cisplatin-ineligible | 1/2 | Active, not recruiting | NCT04863885 | NA | NA | NA | |
| T-DXd + nivolumab | ADC + anti-PD1 | HER2 expressing mUC after platinum; breast cancer | DS8201-A-U105 | 1b | Unknown | NCT03523572 | 36.7% | 6.9 | 11.0 |
| DV +/− pembrolizumab | ADC + anti-PD1 | HER2 expressing mUC | KEYNOTE-D78 | 2 | Recruiting | NCT04879329 | NA | NA | NA |
| DV + toripalimab | ADC + anti-PD1 | mUC | 1b/2 | Unknown | NCT04264936 | 73.2% | 9.2 | 63.2% at 2 years | |
| DV + toripalimab vs. platinum chemo | ADC + anti-PD1 vs. chemo | 1L HER2 expressing mUC | 3 | Recruiting | NCT05302284 | NA | NA | NA |
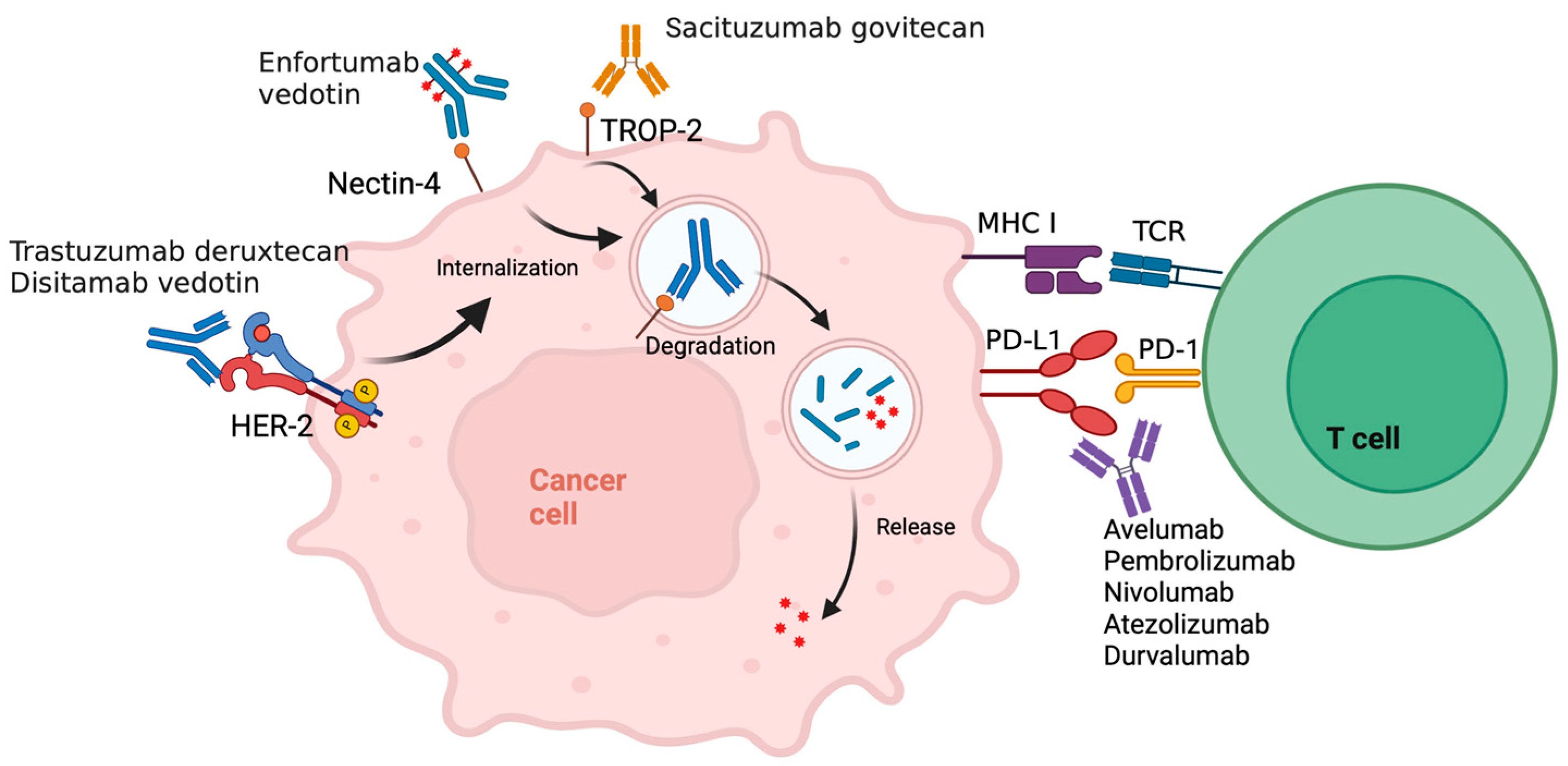
Enfortumab Vedotin plus ICI
Sacituzumab Govitecan plus ICI
HER2 Antibody–Drug Conjugate plus ICI
3.3.3. Tyrosine Kinase Inhibitors plus ICIs
Fibroblast Growth Factor Receptor (FGFR) Inhibitors plus ICIs
VEGFR and Multitargeted TKIs plus ICIs
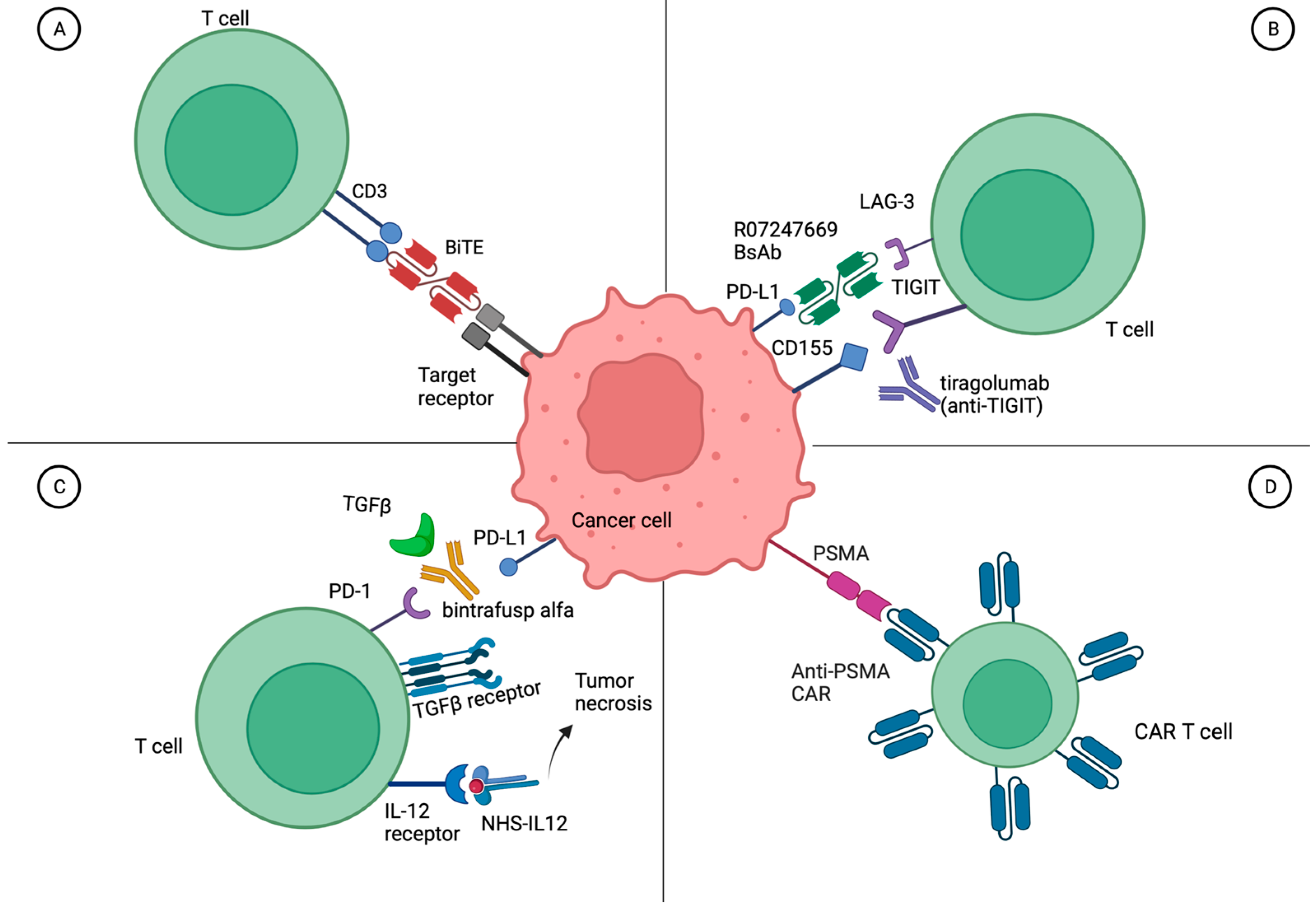
3.3.4. Novel Immunotherapeutic Antibodies plus ICIs
3.4. Bispecific Antibodies for Metastatic Urothelial Carcinoma
3.5. Cellular Therapy for Metastatic Urothelial Carcinoma
3.6. Neoantigen Vaccines for Metastatic Urothelial Carcinoma
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Common Cancer Sites—Cancer Stat Facts. SEER. (n.d.). Available online: https://seer.cancer.gov/statfacts/html/common.html#comparison (accessed on 2 April 2023).
- Key Statistics for Bladder Cancer. American Cancer Society. Available online: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html (accessed on 2 April 2023).
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Pérez-Valderrama, B.; Gurney, H.; Loriot, Y.; Sridhar, S.S.; Tsuchiya, N.; Sternberg, C.N.; et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): Long-term follow-up results from the JAVELIN Bladder 100 trial. J. Clin. Oncol. 2022, 40 (Suppl. S6), 487. [Google Scholar] [CrossRef]
- Sternberg, C.N.; de Mulder, P.H.; Schornagel, J.H.; Théodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, F.; Spina, M.; van Groeningen, C.J.; de Balincourt, C.; et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J. Clin. Oncol. 2001, 19, 2638–2646. [Google Scholar] [CrossRef]
- Sternberg, C.N.; de Mulder, P.; Schornagel, J.H.; Theodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, J.A.; Spina, M.; van Groeningen, C.J.; Duclos, B.; et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur. J. Cancer (Oxf. Engl. 1990) 2006, 42, 50–54. [Google Scholar] [CrossRef]
- von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Flaig, T.W.; Milowsky, M.I.; Friedlander, T.W.; Bilen, M.A.; Gupta, S.; Srinivas, S.; Merchan, J.R.; McKay, R.R.; Petrylak, D.P.; et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J. Clin. Oncol. 2023, 41, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Castellano, D.E.; Grivas, P.; Vaughn, D.J.; Powles, T.; Vuky, J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: Results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann. Oncol. 2023, 34, 289–299. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Vuky, J.; Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.; Hahn, N.M.; Savage, M.J.; et al. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients with Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2020, 38, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Laguerre, B.; Guadalupi, V.; Ku, J.H.; et al. Phase 3 THOR study: Results of erdafitinib (erda) versus chemotherapy (chemo) in patients (pts) with advanced or metastatic urothelial cancer (mUC) with select fibroblast growth factor receptor alterations (FGFRalt). J. Clin. Oncol. 2023, 41 (Suppl. S17), LBA4619. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Özgüroğlu, M.; Valderrama, B.P.; Laguerre, B.; Basso, U.; Triantos, S.; et al. Erdafitinib versus pembrolizumab in pretreated patients with advanced or metastatic urothelial cancer with select FGFR alterations: Cohort 2 of the randomized phase III THOR trial. Ann. Oncol. 2023. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing after Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Roth, B.J.; Dreicer, R.; Einhorn, L.H.; Neuberg, D.; Johnson, D.H.; Smith, J.L.; Hudes, G.R.; Schultz, S.M.; Loehrer, P.J. Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: A phase II trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 1994, 12, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, J.A.; Hilton, S.; Mazumdar, M.; Sadan, S.; Kelly, W.K.; Scher, H.I.; Bajorin, D.F. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J. Clin. Oncol. 1997, 15, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Roth, B.J.; Kabbinavar, F.F.; Vaughn, D.J.; Arning, M.; Curiel, R.E.; Obasaju, C.K.; Wang, Y.; Nicol, S.J.; Kaufman, D.S. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J. Clin. Oncol. 2006, 24, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.B.; Perez Valderrama, B.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. LBA6 EV-302/KEYNOTE-A39: Open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann. Oncol. 2023, 34, S1340. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Yu, E.Y.; Petrylak, D.P.; O’Donnell, P.H.; Lee, J.-L.; van der Heijden, M.S.; Loriot, Y.; Stein, M.N.; Necchi, A.; Kojima, T.; Harrison, M.R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 872–882. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; McKay, R.R.; Flaig, T.W.; Petrylak, D.P.; Hoimes, C.J.; Friedlander, T.W.; Bilen, M.A.; Srinivas, S.; Burgess, E.F.; Merchan, J.R.; et al. Study EV-103 dose escalation/cohort A: Long-term outcome of enfortumab vedotin + pembrolizumab in first-line (1L) cisplatin-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC) with nearly 4 years of follow-up. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4505. [Google Scholar] [CrossRef]
- Rosenberg, J.; Milowsky, M.; Ramamurthy, C.; Mar, N.; McKay, R.; Friedlander, T.; Ferrario, C.; Bracarda, S.; George, S.; Moon, H. LBA73 Study EV-103 Cohort K: Antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC). Ann. Oncol. 2022, 33, S1441. [Google Scholar] [CrossRef]
- Milowsky, M.I.; O’Donnell, P.H.; Petrylak, D.P.; Hoimes, C.J.; Flaig, T.W.; Mar, N.; Moon, H.H.; McKay, R.R.; Bilen, M.A.; Borchiellini, D.; et al. Enfortumab vedotin (EV) with or without pembrolizumab (P) in patients (pts) who are cisplatin-ineligible with previously untreated locally advanced or metastatic urothelial cancer (la/mUC): Additional 3-month follow-up on cohort K data. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4568. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. FDA Grants Accelerated Approval to Enfortumab Vedotin-ejfv. U.S. Food and Drug Administration. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic (accessed on 5 April 2023).
- van der Heijden, M.S.; Sonpavde, G.P.; Powles, T.B.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. LBA7 Nivolumab plus gemcitabine-cisplatin versus gemcitabine-cisplatin alone for previously untreated unresectable or metastatic urothelial carcinoma: Results from the phase III CheckMate 901 trial. Ann. Oncol. 2023, 34, S1341. [Google Scholar] [CrossRef]
- Galsky, M.D.; Mortazavi, A.; Milowsky, M.I.; George, S.; Gupta, S.; Fleming, M.T.; Dang, L.H.; Geynisman, D.M.; Walling, R.; Alter, R.S.; et al. Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo after First-Line Chemotherapy in Patients with Metastatic Urothelial Cancer. J. Clin. Oncol. 2020, 38, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Updated Indication for Merck’s KEYTRUDA® (pembrolizumab) for Treatment of Certain Patients with Urothelial Carcinoma (Bladder Cancer). 2021. Available online: https://www.merck.com/news/fda-approves-updated-indication-for-mercks-keytruda-pembrolizumab-for-treatment-of-certain-patients-with-urothelial-carcinoma-bladder-cancer/ (accessed on 10 October 2023).
- Seymour, C. ODAC Votes in Favor of Pembrolizumab Approval in Frontline Metastatic Urothelial Cancer. 2021. Available online: https://www.onclive.com/view/odac-votes-in-favor-of-pembrolizumab-approval-in-frontline-metastatic-urothelial-cancer (accessed on 10 October 2023).
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients with Advanced Urothelial Bladder Cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results from a Phase 1/2 Open-label Study. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Loriot, Y.; Durán, I.; Ravaud, A.; Retz, M.; Vogelzang, N.J.; Nelson, B.; Wang, J.; Shen, X.; Powles, T. Atezolizumab Versus Chemotherapy in Patients with Platinum-treated Locally Advanced or Metastatic Urothelial Carcinoma: A Long-term Overall Survival and Safety Update from the Phase 3 IMvigor211 Clinical Trial. Eur. Urol. 2021, 80, 7–11. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Loriot, Y.; James, N.; Choy, E.; Castellano, D.; Lopez-Rios, F.; Banna, G.L.; De Giorgi, U.; Masini, C.; Bamias, A.; et al. Primary Results from SAUL, a Multinational Single-arm Safety Study of Atezolizumab Therapy for Locally Advanced or Metastatic Urothelial or Nonurothelial Carcinoma of the Urinary Tract. Eur. Urol. 2019, 76, 73–81. [Google Scholar] [CrossRef]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Ellerton, J.A.; Infante, J.R.; Agrawal, M.; Gordon, M.S.; Aljumaily, R.; Gourdin, T.; Dirix, L.; Lee, K.W.; Taylor, M.H.; et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J. Immunother. Cancer 2020, 8, e001246. [Google Scholar] [CrossRef]
- Maio, M.; Schenker, M.; Medioni, J.; Mandziuk, S.; Majem, M.; Gravis, G.; Cornfeld, M.J.; Ranganathan, S.; Yao, Y.; Yeh, H.S.-H.; et al. Phase 2 study of retifanlimab (INCMGA00012) in patients (pts) with selected solid tumors (POD1UM-203). J. Clin. Oncol. 2021, 39 (Suppl. S15), 2571. [Google Scholar] [CrossRef]
- Johnson, M.L.; Braiteh, F.; Grilley-Olson, J.E.; Chou, J.; Davda, J.; Forgie, A.; Li, R.; Jacobs, I.; Kazazi, F.; Hu-Lieskovan, S. Assessment of Subcutaneous vs Intravenous Administration of Anti-PD-1 Antibody PF-06801591 in Patients with Advanced Solid Tumors: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2019, 5, 999–1007. [Google Scholar] [CrossRef]
- Cho, B.C.; Penkov, K.; Bondarenko, I.; Kurochkin, A.; Pikiel, J.; Ahn, H.K.; Korożan, M.E.; Osipov, M.; Odintsova, S.; Braiteh, F.; et al. A phase Ib/II dose expansion study of subcutaneous sasanlimab in patients with locally advanced or metastatic non-small-cell lung cancer and urothelial carcinoma. ESMO Open 2023, 8, 101589. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Liu, J.; Zhou, A.; Zou, Q.; Li, H.; Fu, C.; Hu, H.; Huang, J.; Zhu, S.; Jin, J.; et al. Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Sci. 2021, 112, 305–313. [Google Scholar] [CrossRef]
- Niu, Y.; Hu, H.; Wang, H.; Tian, D.; Zhao, G.; Shen, C.; Wu, Z.; Da, L.; Zhan, X.; Huang, S. Phase II clinical study of tislelizumab combined with nab-paclitaxel (TRUCE-01) for muscle-invasive urothelial bladder carcinoma: Bladder preservation subgroup analysis. J. Clin. Oncol. 2022, 40 (Suppl. S16), 4589. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, B.; Geng, Z.; Geng, Q. Toripalimab: The First Domestic Anti-Tumor PD-1 Antibody in China. Front. Immunol. 2021, 12, 730666. [Google Scholar] [CrossRef]
- Sheng, X.; Chen, H.; Hu, B.; Yao, X.; Liu, Z.; Guo, H.; Hu, Y.; Ji, Z.; Luo, H.; Shi, B.; et al. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Patients with Previously Treated Advanced Urothelial Carcinoma: Results from a Multicenter Phase II Trial POLARIS-03. Clin. Cancer Res. 2022, 28, 489–497. [Google Scholar] [CrossRef]
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and with Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J. Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef]
- Bristol Myers Squibb Provides Update on Checkmate -901 Trial Evaluating Opdivo (Nivolumab) Plus Yervoy (Ipilimumab) as First-Line Treatment for Patients with Unresectable or Metastatic Urothelial Carcinoma. News. 2022. Available online: https://news.bms.com/news/details/2022/Bristol-Myers-Squibb-Provides-Update-on-CheckMate--901-Trial-Evaluating-Opdivo-nivolumab-Plus-Yervoy-ipilimumab-as-First-Line-Treatment-for-Patients-with-Unresectable-or-Metastatic-Urothelial-Carcinoma/default.aspx (accessed on 2 April 2023).
- Galsky, M.D.; Powles, T.; Li, S.; Hennicken, D.; Sonpavde, G. A phase 3, open-label, randomized study of nivolumab plus ipilimumab or standard of care (SOC) versus SOC alone in patients (pts) with previously untreated unresectable or metastatic urothelial carcinoma (mUC; CheckMate 901). J. Clin. Oncol. 2018, 36 (Suppl. S6), TPS539. [Google Scholar] [CrossRef]
- Coquan, E.; Clarisse, B.; Lequesne, J.; Brachet, P.E.; Nevière, Z.; Meriaux, E.; Bonnet, I.; Castera, M.; Goardon, N.; Boutrois, J.; et al. TALASUR trial: A single arm phase II trial assessing efficacy and safety of TALazoparib and Avelumab as maintenance therapy in platinum-Sensitive metastatic or locally advanced URothelial carcinoma. BMC Cancer 2022, 22, 1213. [Google Scholar] [CrossRef]
- Sonpavde, G.P.; Alemany, C.A.; Mchayleh, W.; Pepe, J.W.; Coakley, S.; Young, A.; Jain, R.K. Phase II trial of lurbinectedin combined with avelumab as maintenance therapy for metastatic urothelial carcinoma with stable or responding disease following platinum-based chemotherapy. J. Clin. Oncol. 2023, 41 (Suppl. S6), TPS590. [Google Scholar] [CrossRef]
- Sonpavde, G.P.; Grivas, P.; Milowsky, M.I.; Galsky, M.D.; Malik, R.K.; Beelen, A.P. Trial in progress: A phase 2, randomized, open-label study of trilaciclib with first-line, platinum-based chemotherapy and avelumab maintenance in untreated patients with locally advanced or metastatic urothelial carcinoma (PRESERVE 3). J. Clin. Oncol. 2022, 40 (Suppl. S6), TPS585. [Google Scholar] [CrossRef]
- Shah, A.Y.; Gao, J.; Rezazadeh, A.; Cole, S.; Jain, R.K.; Stevenson, A.; Fyvie, G.; Gaw, N.J.; Pant, S. Trial in progress: A phase II switch maintenance study of live biotherapeutic MRx0518 and avelumab in patients with unresectable locally advanced or metastatic urothelial carcinoma (UC) who did not progress on first-line platinum-containing chemotherapy. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS4610. [Google Scholar] [CrossRef]
- G1 Therapeutics Provides Initial Update on Phase 2 Bladder Cancer Trial; Progression Free Survival (PFS) Data Expected in Mid-2023. G1 Therapeutics, Inc. 2023. Available online: http://investor.g1therapeutics.com/news-releases/news-release-details/g1-therapeutics-provides-initial-update-phase-2-bladder-cancer (accessed on 2 April 2023).
- Powles, T.; Necchi, A.; Duran, I.; Loriot, Y.; Ramamurthy, C.; Recio-Boiles, A.; Sweis, R.F.; Bedke, J.; Tonelli, J.; Sierecki, M.; et al. TROPHU-U-01 cohort 5: Evaluation of maintenance sacituzumab govitecan (SG) plus zimberelimab (ZIM), ZIM, or avelumab in cisplatin-eligible patients (pts) with unresectable or metastatic urothelial cancer (mUC). J. Clin. Oncol. 2023, 41 (Suppl. S6), TPS598. [Google Scholar] [CrossRef]
- Gupta, S.; Ballman, K.V.; Galsky, M.D.; Morris, M.J.; Chen, R.C.; Chan, T.A.; Dercle, L.; Wen, Y.; Sridhar, S.S.; Yen, A.E.; et al. MAIN-CAV: Phase III randomized trial of maintenance cabozantinib and avelumab versus avelumab after first-line platinum-based chemotherapy in patients with metastatic urothelial cancer (mUC) (Alliance A032001). J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS4607. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Bamias, A.; Davis, I.D.; Galsky, M.D.; Arija, J.A.A.; Kikuchi, E.; Grande, E.; Muro, X.G.d.; Park, S.H.; Giorgi, U.D.; Alekseev, B.; et al. Final overall survival (OS) analysis of atezolizumab (atezo) monotherapy vs chemotherapy (chemo) in untreated locally advanced or metastatic urothelial carcinoma (mUC) from the Phase 3 IMvigor130 study. J. Clin. Oncol. 2023, 41 (Suppl. S6), LBA441. [Google Scholar] [CrossRef]
- Bamias, A.; Galsky, M.D.; Kikuchi, E.; Davis, I.D.; Arranz, J.A.; Rezazadeh, A.; Muro, X.G.d.; Park, S.H.; Giorgi, U.D.; Alekseev, B.; et al. Overall survival (OS) by response to first-line (1L) induction treatment with atezolizumab (atezo) + platinum/gemcitabine (plt/gem) vs placebo + plt/gem in patients (pts) with metastatic urothelial carcinoma (mUC): Updated data from the IMvigor130 OS final analysis. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4503. [Google Scholar] [CrossRef]
- Velasco, G.D.; García-Carbonero, I.; Esteban-Gonzalez, E.; Pinto, A.; Lorente, D.; Lista, A.G.D.L.; Ortega, E.M.; Colomo, L.J.; Puente, J.; Gonzalez, I.; et al. Early efficacy results from atezolizumab (ATZ) with split doses of cisplatin plus gemcitabine in patients with locally advanced or metastatic urothelial carcinoma (SOGUG-AUREA). J. Clin. Oncol. 2023, 41 (Suppl. S6), 502. [Google Scholar] [CrossRef]
- Galsky, M.D.; Wang, H.; Hahn, N.M.; Twardowski, P.; Pal, S.K.; Albany, C.; Fleming, M.T.; Starodub, A.; Hauke, R.J.; Yu, M.; et al. Phase 2 Trial of Gemcitabine, Cisplatin, plus Ipilimumab in Patients with Metastatic Urothelial Cancer and Impact of DNA Damage Response Gene Mutations on Outcomes. Eur. Urol. 2018, 73, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Necchi, A.; Sridhar, S.S.; Ogawa, O.; Angra, N.; Hois, S.; Xiao, F.; Goluboff, E.; Bellmunt, J. A phase III, randomized, open-label, multicenter, global study of first-line durvalumab plus standard of care (SoC) chemotherapy and durvalumab plus tremelimumab, and SoC chemotherapy versus SoC chemotherapy alone in unresectable locally advanced or metastatic urothelial cancer (NILE). J. Clin. Oncol. 2021, 39 (Suppl. S6), TPS504. [Google Scholar] [CrossRef]
- Duan, R.; Liu, Y.; Hu, X.; Cui, C.; Si, L.; Sheng, X.; Liu, Z.; Xiang, P.; Yan, X.; Li, S.; et al. Phase Ib study of anti-PD-L1 monoclonal antibody socazolimab in combination with nab-paclitaxel as first-line therapy for advanced urothelial carcinoma. J. Clin. Oncol. 2022, 40 (Suppl. S16), 4563. [Google Scholar] [CrossRef]
- Valderrama, B.P.; Gauna, D.C.; Pinto Marin, A.; Mellado Gonzalez, B.; Puente, J.; Climent Duran, M.A.; Domenech, M.; Vazquez Mazon, F.J.; Perez Gracia, J.L.; Morales Barrera, R.; et al. LBA27—Phase II multicenter, randomized study to evaluate efficacy and safety of avelumab with gemcitabine/carboplatin (CG) vs CG alone in patients with unresectable or metastatic urothelial carcinoma (mUC) who are ineligible to receive cisplatin-based therapy. Ann. Oncol. 2020, 31 (Suppl. S4), S1142–S1215. [Google Scholar]
- Parikh, M.; Pan, C.-X.; Beckett, L.; Li, Y.; Robles, D.; DeVisser, C.; Lara, P. Combination checkpoint immunotherapy and cytotoxic chemotherapy: Further results from a phase Ib/II trial of pembrolizumab and docetaxel or gemcitabine in patients with advanced or metastatic urothelial cancer. J. Clin. Oncol. 2018, 36 (Suppl. S6), 525. [Google Scholar] [CrossRef]
- Garje, R.; Packiam, V.T.; Koski, A.; Milhem, M.M.; O’Donnell, M.A.; Zakharia, Y. Phase Ib study of avelumab and taxane based chemotherapy in platinum-refractory or ineligible metastatic urothelial cancer (AVETAX study). J. Clin. Oncol. 2022, 40 (Suppl. S6), 503. [Google Scholar] [CrossRef]
- Giannatempo, P.; Raggi, D.; Marandino, L.; Bandini, M.; Farè, E.; Calareso, G.; Colecchia, M.; Gallina, A.; Ross, J.S.; Alessi, A.; et al. Pembrolizumab and nab-paclitaxel as salvage therapy for platinum-treated, locally advanced or metastatic urothelial carcinoma: Interim results of the open-label, single-arm, phase II PEANUT study. Ann. Oncol. 2020, 31, 1764–1772. [Google Scholar] [CrossRef]
- Bitting, R.L.; Vile, D.C.; Tooze, J.A.; Thomas, C.Y.; Neve, M.; Kooshki, M.; Brown, J.; Dubey, P.; Triozzi, P.; Goodman, M.M. Single-arm phase II study of low-dose paclitaxel and pembrolizumab in platinum-refractory metastatic urothelial carcinoma (UC). J. Clin. Oncol. 2021, 39 (Suppl. S6), 433. [Google Scholar] [CrossRef]
- Shah, A.Y.; Campbell, M.T.; Tidwell, R.; Siefker-Radtke, A.O.; Goswami, S.; Alhalabi, O.; Adriazola, A.C.; Shaw, L.; Ye, Y.; Chen, J.; et al. A phase II trial evaluating combination pemetrexed and avelumab in patients with MTAP-deficient advanced urothelial cancer. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS4612. [Google Scholar] [CrossRef]
- Alhalabi, O.; Chen, J.; Zhang, Y.; Lu, Y.; Wang, Q.; Ramachandran, S.; Tidwell, R.S.; Han, G.; Yan, X.; Meng, J.; et al. MTAP deficiency creates an exploitable target for antifolate therapy in 9p21-loss cancers. Nat. Commun. 2022, 13, 1797. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Pouessel, D.; Park, C.H.; Barthélémy, P.; Bupathi, M.; Petrylak, D.P.; Agarwal, N.; Flechon, A.; Ramamurthy, C.; Davis, N.B.; et al. TROPHY-U-01 Cohort 3: Sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) who progressed after platinum (PLT)-based regimens. J. Clin. Oncol. 2022, 40 (Suppl. S6), 434. [Google Scholar] [CrossRef]
- Grivas, P.; Pouessel, D.; Park, C.H.; Barthelemy, P.; Bupathi, M.; Petrylak, D.P.; Agarwal, N.; Gupta, S.; Flechon, A.; Ramamurthy, C.; et al. Primary analysis of TROPHY-U-01 cohort 3, a phase 2 study of sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) that progressed after platinum (PT)-based therapy. J. Clin. Oncol. 2023, 41 (Suppl. S6), 518. [Google Scholar] [CrossRef]
- Duran, I.; Necchi, A.; Powles, T.; Loriot, Y.; Ramamurthy, C.; Recio-Boiles, A.; Sweis, R.F.; Bedke, J.; Tonelli, J.; Sierecki, M.; et al. TROPHY-U-01 cohort 6: Sacituzumab govitecan (SG), SG plus zimberelimab (ZIM), SG plus ZIM plus domvanalimab (DOM), or carboplatin (CARBO) + gemcitabine (GEM) in cisplatin-ineligible patients (pts) with treatment-naive metastatic urothelial cancer (mUC). J. Clin. Oncol. 2023, 41 (Suppl. S6), TPS592. [Google Scholar] [CrossRef]
- Jain, R.K.; Chahoud, J.; Chatwal, M.; Kim, Y.; Dhillon, J.; Vosoughi, A.; Araujo, C.; Li, R.; Zhang, J.; Sonpavde, G.P. 720TiP Phase I/II study of ipilimumab plus nivolumab (IPI-NIVO) combined with sacituzumab govitecan in patients with metastatic cisplatin-ineligible urothelial carcinoma. Ann. Oncol. 2021, 32, S724. [Google Scholar] [CrossRef]
- Hussain, M.H.; MacVicar, G.R.; Petrylak, D.P.; Dunn, R.L.; Vaishampayan, U.; Lara, P.N., Jr.; Chatta, G.S.; Nanus, D.M.; Glode, L.M.; Trump, D.L.; et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: Results of a multicenter phase II National Cancer Institute trial. J. Clin. Oncol. 2007, 25, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, E.; Kang, A.; Bloudek, L.M.; Koshkin, V.S. HER2 expression in urothelial carcinoma, a systematic literature review. Front. Oncol. 2022, 12, 1011885. [Google Scholar] [CrossRef] [PubMed]
- Oudard, S.; Culine, S.; Vano, Y.; Goldwasser, F.; Théodore, C.; Nguyen, T.; Voog, E.; Banu, E.; Vieillefond, A.; Priou, F.; et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur. J. Cancer (Oxf. Engl. 1990) 2015, 51, 45–54. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Campanile, A.; Antic, T.; Yap, K.L.; Fitzpatrick, C.A.; Wade, J.L., 3rd; Karrison, T.; Stadler, W.M.; Nakamura, Y.; O’Donnell, P.H. Afatinib Activity in Platinum-Refractory Metastatic Urothelial Carcinoma in Patients With ERBB Alterations. J. Clin. Oncol. 2016, 34, 2165–2171. [Google Scholar] [CrossRef]
- Hamilton, E.; Shapiro, C.L.; Petrylak, D.; Boni, V.; Martin, M.; Conte, G.D.; Cortes, J.; Agarwal, L.; Arkenau, H.-T.; Tan, A.R.; et al. Abstract PD3-07: Trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: A 2-part, phase 1b, multicenter, open-label study. Cancer Res. 2021, 81 (Suppl. S4), PD3-07. [Google Scholar] [CrossRef]
- Galsky, M.D.; Conte, G.D.; Foti, S.; Yu, E.Y.; Machiels, J.-P.H.; Doger, B.; Necchi, A.; Braud, F.G.D.; Hamilton, E.P.; Hennequin, A.; et al. Primary analysis from DS8201-A-U105: A phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC). J. Clin. Oncol. 2022, 40 (Suppl. S6), 438. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, X.; Wang, L.; Shi, Y.; Yao, X.; Luo, H.; Shi, B.; Liu, J.; He, Z.; Yu, G.; et al. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, H.; Li, S.; Yan, X.; Li, J.; Wu, X.; Chi, Z.; Si, L.; Cui, C.; Kong, Y.; et al. Study RC48-C014: Preliminary results of RC48-ADC combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 2022, 40 (Suppl. S6), 515. [Google Scholar] [CrossRef]
- Sheng, X.; Zhou, L.; He, Z.; Guo, H.; Yan, X.; Li, S.; Xu, H.; Li, J.; Chi, Z.; Si, L.; et al. Preliminary results of a phase Ib/II combination study of RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate (ADC) with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 2022, 40 (Suppl. S16), 4518. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, K.; Zhang, S.; Xu, H.; Yan, X.; Li, S.; Li, J.; Cui, C.; Chi, Z.; Si, L.; et al. Disitamab vedotin, a novel humanized anti-HER2 antibody-drug conjugate (ADC), combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma: An open-label phase 1b/2 study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4566. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Loriot, Y.; Siena, S.; Beato, C.; Duran, M.A.C.; Varlamov, S.; Duran, I.; Tagawa, S.T.; Geoffrois, L.; Mellado, B.; et al. 752P Updated data from the NORSE trial of erdafitinib (ERDA) plus cetrelimab (CET) in patients (pts) with metastatic or locally advanced urothelial carcinoma (mUC) and specific fibroblast growth factor receptor (FGFR) alterations. Ann. Oncol. 2020, 31, S584. [Google Scholar] [CrossRef]
- Powles, T.; Chistyakov, V.; Beliakouski, V.; Semenov, A.; Everaert, E.; Baranau, Y.; Moreno, V.; Valderrama, B.P.; Vano, Y.; Del Conte, G. LBA27 Erdafitinib (ERDA) or ERDA plus cetrelimab (CET) for patients with metastatic or locally advanced urothelial carcinoma (mUC) and Fibroblast Growth Factor Receptor alterations (FGFRa): First phase (Ph) II results from the NORSE study. Ann. Oncol. 2021, 32, S1303. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Powles, T.; Moreno, V.; Kang, T.W.; Cicin, I.; Girvin, A.; Akapame, S.; Triantos, S.; O’Hagan, A.; Zhu, W.; et al. Erdafitinib (ERDA) vs ERDA plus cetrelimab (ERDA+CET) for patients (pts) with metastatic urothelial carcinoma (mUC) and fibroblast growth factor receptor alterations (FGFRa): Final results from the phase 2 Norse study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4504. [Google Scholar] [CrossRef]
- Koshkin, V.S.; Sonpavde, G.P.; Hwang, C.; Mellado, B.; Tomlinson, G.; Shimura, M.; Chisamore, M.J.; Gil, M.; Loriot, Y. Futibatinib plus pembrolizumab in patients (pts) with advanced or metastatic urothelial carcinoma (mUC): Preliminary safety results from a phase 2 study. J. Clin. Oncol. 2022, 40 (Suppl. S6), 501. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Gajate, P.; Morales-Barrera, R.; Lee, J.-L.; Necchi, A.; Penel, N.; Zagonel, V.; Sierecki, M.R.; Piciu, A.-M.; Ellinghaus, P.; et al. Safety and preliminary efficacy of rogaratinib in combination with atezolizumab in a phase Ib/II study (FORT-2) of first-line treatment in cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (UC) and FGFR mRNA overexpression. J. Clin. Oncol. 2020, 38 (Suppl. S15), 5014. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Gajate, P.; Morales-Barrera, R.; Lee, J.-L.; Necchi, A.; Penel, N.; Zagonel, V.; Sierecki, M.R.; Bao, W.; Zhou, Y.; et al. Safety and efficacy of rogaratinib in combination with atezolizumab in cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (UC) and FGFR mRNA overexpression in the phase Ib/II FORT-2 study. J. Clin. Oncol. 2021, 39 (Suppl. S15), 4521. [Google Scholar] [CrossRef]
- Chaudhry, A.; Sternberg, C.N.; Santis, M.D.; Bellmunt, J.; Necchi, A.; Powles, T.; Cantero, F.; Marszewska, M.; Grzyb, M.; McSheehy, P.; et al. FIDES-02, a phase Ib/II study of derazantinib (DZB) as monotherapy and combination therapy with atezolizumab (A) in patients with surgically unresectable or metastaticurothelial cancer (UC) and FGFR genetic aberrations. J. Clin. Oncol. 2020, 38 (Suppl. S6), TPS590. [Google Scholar] [CrossRef]
- Abdul-Karim, R.M.; Chaudhry, A.; Patrikidou, A.; Gonzalez, A.F.; Racca, F.; Loriot, Y.; Pouessel, D.; Deville, J.-L.; Lee, H.J.; Cantero, F.; et al. Derazantinib (DZB) in combination with atezolizumab (AZB) in patients with solid tumors: Results from the dose-finding phase Ib substudy of FIDES-02. J. Clin. Oncol. 2021, 39 (Suppl. S6), 437. [Google Scholar] [CrossRef]
- Necchi, A.; Todenhöfer, T.; Deville, J.-L.; Häckl, M.; Marszewska, M.; McKernan, P.; Saulay, M.; Engelhardt, M.; Santis, M.D. Efficacy and safety of derazantinib (DZB) in patients with metastatic urothelial carcinoma (mUC) with activating FGFR1/2/3 genetic aberrations (GA): Results from the phase 1b/2 FIDES-02 study. J. Clin. Oncol. 2023, 41 (Suppl. S6), 501. [Google Scholar] [CrossRef]
- Iyer, G.; Siefker-Radtke, A.; Milowsky, M.; Shore, N.; Gao, X.; Reimers, M.A.; Hahn, N.; Cosman, R.; Matsubara, N.; Necchi, A.; et al. Abstract CT119: A first-in-human phase 1 study of LOXO-435, a potent, highly isoform-selective FGFR3 inhibitor in advanced solid tumors with FGFR3 alterations (trial in progress). Cancer Res. 2023, 83 (Suppl. S8), CT119. [Google Scholar] [CrossRef]
- Apolo, A.B.; Nadal, R.; Girardi, D.M.; Niglio, S.A.; Ley, L.; Cordes, L.M.; Steinberg, S.M.; Sierra Ortiz, O.; Cadena, J.; Diaz, C.; et al. Phase I Study of Cabozantinib and Nivolumab Alone or with Ipilimumab for Advanced or Metastatic Urothelial Carcinoma and Other Genitourinary Tumors. J. Clin. Oncol. 2020, 38, 3672–3684. [Google Scholar] [CrossRef]
- Girardi, D.M.; Niglio, S.A.; Mortazavi, A.; Nadal, R.; Lara, P.; Pal, S.K.; Saraiya, B.; Cordes, L.; Ley, L.; Ortiz, O.S.; et al. Cabozantinib plus Nivolumab Phase I Expansion Study in Patients with Metastatic Urothelial Carcinoma Refractory to Immune Checkpoint Inhibitor Therapy. Clin. Cancer Res. 2022, 28, 1353–1362. [Google Scholar] [CrossRef]
- Guadalupi, V.; Marandino, L.; Raggi, D.; Stellato, M.; Rametta, A.; Baciarello, G.; Bottiglieri, A.; Sepe, P.; Claps, M.; Buti, S.; et al. Activity of cabozantinib (CABO) plus durvalumab (DURVA) in patients (pts) with advanced urothelial carcinoma (UC) or non-UC variant histologies (VH) after platinum chemotherapy: Interim results from the phase 2 ARCADIA trial. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4578. [Google Scholar] [CrossRef]
- Loriot, Y.; Grivas, P.; Wit, R.D.; Balar, A.V.; Siefker-Radtke, A.O.; Zolnierek, J.; Csoszi, T.; Shin, S.J.; Park, S.H.; Atduev, V.; et al. First-line pembrolizumab (pembro) with or without lenvatinib (lenva) in patients with advanced urothelial carcinoma (LEAP-011): A phase 3, randomized, double-blind study. J. Clin. Oncol. 2022, 40 (Suppl. S6), 432. [Google Scholar] [CrossRef]
- Doshi, G.K.; Vogelzang, N.J.; Richards, D.A.; Chong, D.; Shaffer, D.R.; Nordquist, L.T.; Picus, J.; Alvarez, D.; Der-Torossian, H.; Christensen, J.; et al. Phase II study of sitravatinib in combination with nivolumab in patients with advanced or metastatic urothelial carcinoma (UC) after checkpoint inhibitor therapy (CIT). J. Clin. Oncol. 2019, 37 (Suppl. S7), TPS498. [Google Scholar] [CrossRef]
- Msaouel, P.; Siefker-Radtke, A.; Sweis, R.; Mao, S.; Rosenberg, J.; Vaishampayan, U.; Kalebasty, A.R.; Pili, R.; Bupathi, M.; Nordquist, L. 705MO Sitravatinib (sitra) in combination with nivolumab (nivo) demonstrates clinical activity in checkpoint inhibitor (CPI) naïve, platinum-experienced patients (pts) with advanced or metastatic urothelial carcinoma (UC). Ann. Oncol. 2020, 31, S556. [Google Scholar] [CrossRef]
- Msaouel, P.; Siefker-Radtke, A.; Sweis, R.; Mortazavi, A.; Vogelzang, N.; Vaishampayan, U.; Bradley, T.; Bupathi, M.; Nordquist, L.; Shaffer, D.; et al. O23 Sitravatinib in combination with nivolumab demonstrates clinical activity in platinum-experienced patients with urothelial carcinoma (UC) who progressed on prior immune checkpoint inhibitor (CPI). JITC 2019, 7 (Suppl. S1). [Google Scholar] [CrossRef]
- Qu, Y.Y.; Sun, Z.; Han, W.; Zou, Q.; Xing, N.; Luo, H.; Zhang, X.; He, C.; Bian, X.J.; Cai, J.; et al. Camrelizumab plus famitinib for advanced or metastatic urothelial carcinoma after platinum-based therapy: Data from a multicohort phase 2 study. J. Immunother. Cancer 2022, 10, e004427. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.-Y.; Zou, Q.; Guo, H.; Xing, N.; Sun, Z.; Han, W.; Luo, H.; Xia, S.; Zhang, X.; He, C.; et al. Camrelizumab plus famitinib for advanced renal cell carcinoma or unresectable urothelial carcinoma: Updated results from a phase II trial. J. Clin. Oncol. 2021, 39 (Suppl. S15), 4550. [Google Scholar] [CrossRef]
- Choueiri, T.K.; McGregor, B.A.; Shah, N.J.; Bajaj, A.; Chahoud, J.; O’Neil, B.; Michalski, J.; Garmezy, B.; Jin, L.; Oliver, J.W.; et al. A phase 1b study (STELLAR-002) of XL092 administered in combination with nivolumab (NIVO) with or without ipilimumab (IPI) or bempegaldesleukin (BEMPEG) in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS4600. [Google Scholar] [CrossRef]
- Powderly, J.; Shimizu, T.; LoRusso, P.; Razak, A.; Miller, K.; Balar, A.; Bruix, J.; Michel, L.; Blaney, M.; Guan, X.; et al. Abstract CT207: Phase 1 first-in-human study of ABBV-151 as monotherapy or in combination with budigalimab in patients with locally advanced or metastatic solid tumors. Cancer Res. 2021, 81 (Suppl. S13), CT207. [Google Scholar] [CrossRef]
- Tolcher, A.; Roda-Perez, D.; He, K.; Moreno, V.; Gomez-Roca, C.; Machiels, J.-P.; Razak, A.; Sahtout, M.; Guan, X.; Jaryno-Daly, S.; et al. 770 Safety, efficacy, and pharmacokinetic results from a phase I first-in-human study of ABBV-151 with or without anti-PD1 mAb (budigalimab) in patients with locally advanced or metastatic solid tumors. J. ImmunoTherapy Cancer 2022, 10 (Suppl. S2), A801. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Diab, M.; Van Tine, B.A.; Shields, A.F.; Razak, A.A.; Hilton, J.F.; Santana-Davila, R.; Sanai, E.; Zeron-Medina, J.; Bragulat, V.; et al. Abstract CT116: First-in-human study of AZD8853, an anti-growth and differentiation factor 15 (GDF15) antibody, in patients (pts) with advanced/metastatic solid tumors. Cancer Res. 2023, 83 (Suppl. S8), CT116. [Google Scholar] [CrossRef]
- Rohrberg, K.S.; Garralda, E.; Calvo, E.; Moreno Garcia, V.; Guidi, M.; Kraus, D.G.; McIntyre, C.; Kao, H.; Codarri Deak, L.; Michielin, F.; et al. 745P Clinical activity, safety, and PK/PD from the first in human study (NP41300) of RO7247669, a PD1-LAG3 bispecific antibody. Ann. Oncol. 2022, 33, S884–S885. [Google Scholar] [CrossRef]
- Niglio, S.A.; Girardi, D.d.M.; Cordes, L.M.; Ley, L.; Mallek, M.; Ortiz, O.S.; Cadena, J.; Diaz, C.; Chalfin, H.; Kydd, A.; et al. A phase I study of bintrafusp alfa (M7824) and NHS-IL12 (M9241) alone and in combination with stereotactic body radiation therapy (SBRT) in adults with metastatic non-prostate genitourinary malignancies. J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS4599. [Google Scholar] [CrossRef]
- Strauss, J.; Heery, C.R.; Kim, J.W.; Jochems, C.; Donahue, R.N.; Montgomery, A.S.; McMahon, S.; Lamping, E.; Marté, J.L.; Madan, R.A.; et al. First-in-Human Phase I Trial of a Tumor-Targeted Cytokine (NHS-IL12) in Subjects with Metastatic Solid Tumors. Clin. Cancer Res. 2019, 25, 99–109. [Google Scholar] [CrossRef]
- Grivas, P.; Morales-Barrera, R.; Sridhar, S.S.; Loriot, Y.; Heijden, M.S.V.D.; Galsky, M.D.; Baxter, D.; Khaled, A.H.; Hug, B.A. A phase Ib single-arm study of bintrafusp alfa for the treatment of pretreated, locally advanced/unresectable or metastatic urothelial cancer. J. Clin. Oncol. 2021, 39 (Suppl. S6), TPS501. [Google Scholar] [CrossRef]
- Simon, N.I.; Niglio, S.A.; Chandran, E.; Iannantuono, G.M.; Boudjadi, S.; Banday, R.; Girardi, D.d.M.; Cordes, L.M.; Ley, L.; Gurram, S.; et al. A phase I study of bintrafusp alfa and NHS-IL12 (M9241) alone and in combination with stereotactic body radiation therapy (SBRT) in patients with metastatic genitourinary (GU) malignancies. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4571. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Railkar, R.; Sanford, T.; Crooks, D.R.; Eckhaus, M.A.; Haines, D.; Choyke, P.L.; Kobayashi, H.; Agarwal, P.K. Targeting Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (HER2) Expressing Bladder Cancer Using Combination Photoimmunotherapy (PIT). Sci. Rep. 2019, 9, 2084. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Shin, S.; Lee, J.L.; Shim, B.; Lee, H.; Yun, N.; Ham, M.; Jeong, Y.; Lee, W.; Kang, H.; et al. 718 Preliminary results of GII-101-P101 (Keynote-B59): GI-101 (CD80-IgG4 Fc-IL2v) as a single agent and in combination with pembrolizumab in patients with advanced and/or metastatic solid tumors. J. ImmunoTher. Cancer 2022, 10 (Suppl. S2), A751. [Google Scholar] [CrossRef]
- Ogasawara, M.; Miyashita, M.; Ota, S. Vaccination of Urological Cancer Patients with WT1 Peptide-Pulsed Dendritic Cells in Combination with Molecular Targeted Therapy or Conventional Chemotherapy Induces Immunological and Clinical Responses. Ther. Apher. Dial. 2018, 22, 266–277. [Google Scholar] [CrossRef]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: Long-term follow-up and correlates with response. Clin. Cancer Res. 2015, 21, 1019–1027. [Google Scholar] [CrossRef]
- Ou, Y.; Liu, M.; Zhan, K.; Wu, W.; Huang, J.; Sun, H.; Zheng, H.; Yu, X.; Gong, H.; Han, Z.; et al. Development of an affinity-enhanced clinical candidate TCR targeting NY-ESO-1 with optimal potency and high specificity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Parriott, G.; Deal, K.; Crean, S.; Richardson, E.; Nylen, E.; Barber, A. T-cells expressing a chimeric-PD1-Dap10-CD3zeta receptor reduce tumour burden in multiple murine syngeneic models of solid cancer. Immunology 2020, 160, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, C.M.; Haist, C.; König, C.; Petzsch, P.; Bister, A.; Nößner, E.; Wiek, C.; Scheckenbach, K.; Köhrer, K.; Niegisch, G.; et al. Epigenetic Priming of Bladder Cancer Cells with Decitabine Increases Cytotoxicity of Human EGFR and CD44v6 CAR Engineered T-Cells. Front. Immunol. 2021, 12, 782448. [Google Scholar] [CrossRef] [PubMed]
- Greco, B.; Malacarne, V.; De Girardi, F.; Scotti, G.M.; Manfredi, F.; Angelino, E.; Sirini, C.; Camisa, B.; Falcone, L.; Moresco, M.A.; et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies. Sci. Transl. Med. 2022, 14, eabg3072. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.; Chakrabarti, S.; Padul, V.; Jones, L.D.; Ashili, S. Designing neoantigen cancer vaccines, trials, and outcomes. Front. Immunol. 2023, 14, 1105420. [Google Scholar] [CrossRef] [PubMed]
- Obara, W.; Eto, M.; Mimata, H.; Kohri, K.; Mitsuhata, N.; Miura, I.; Shuin, T.; Miki, T.; Koie, T.; Fujimoto, H.; et al. A phase I/II study of cancer peptide vaccine S-288310 in patients with advanced urothelial carcinoma of the bladder. Ann. Oncol. 2017, 28, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kitamura, H.l.; Inoue, R.; Nishida, S.; Takahashi-Takaya, A.; Kawami, S.; Torigoe, T.; Hirohashi, Y.; Tsukamoto, T.; Sato, N.; et al. Potential Survival Benefit of Anti-Apoptosis Protein: Survivin-Derived Peptide Vaccine with and without Interferon Alpha Therapy for Patients with Advanced or Recurrent Urothelial Cancer—Results from Phase I Clinical Trials. J. Immunol. Res. 2013, 2013, 262967. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Matsumoto, K.; Uemura, H.; Arai, G.; Eto, M.; Naito, S.; Ohyama, C.; Nasu, Y.; Tanaka, M.; Moriya, F.; et al. An Open-Label, Randomized Phase II Trial of Personalized Peptide Vaccination in Patients with Bladder Cancer that Progressed after Platinum-Based Chemotherapy. Clin. Cancer Res. 2016, 22, 54–60. [Google Scholar] [CrossRef]
- Matsumoto, K.; Noguchi, M.; Satoh, T.; Tabata, K.-I.; Fujita, T.; Iwamura, M.; Yamada, A.; Komatsu, N.; Baba, S.; Itoh, K. A phase I study of personalized peptide vaccination for advanced urothelial carcinoma patients who failed treatment with methotrexate, vinblastine, adriamycin and cisplatin. BJU Int. 2011, 108, 831–838. [Google Scholar] [CrossRef]
- Suekane, S.; Ueda, K.; Nishihara, K.; Sasada, T.; Yamashita, T.; Koga, N.; Yutani, S.; Shichijo, S.; Itoh, K.; Igawa, T.; et al. Personalized peptide vaccination as second-line treatment for metastatic upper tract urothelial carcinoma. Cancer Sci. 2017, 108, 2430–2437. [Google Scholar] [CrossRef]
- Sonpavde, G.P.; Maughan, B.L.; McGregor, B.A.; Wei, X.X.; Kilbridge, K.L.; Lee, R.J.; Yu, E.Y.; Schweizer, M.T.; Montgomery, R.B.; Cheng, H.H.; et al. Phase II trial of CV301 vaccine combined with atezolizumab in advanced urothelial carcinoma. Cancer Immunol. Immunother. 2023, 72, 775–782. [Google Scholar] [CrossRef]
- Kodysh, J.; Rubinsteyn, A. OpenVax: An Open-Source Computational Pipeline for Cancer Neoantigen Prediction. Methods Mol. Biol. 2020, 2120, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, J.; Hahn, N.; Bellmunt, J.; Rosencranz, S.; Diehl, M.; Morrow, M.; Kraynyak, K.; McMullan, T. Abstract CT177: INO-5401 + INO-9012 in combination with atezolizumab for locally advanced unresectable or metastatic/recurrent urothelial carcinoma. Cancer Res. 2019, 79 (Suppl. S13), CT177. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Deng, J.; Yang, Z.; Chen, J.; Tan, Q.; Guo, M.; Jin, Y. Phospholipid-Membrane-Based Nanovesicles Acting as Vaccines for Tumor Immunotherapy: Classification, Mechanisms and Applications. Pharmaceutics 2022, 14, 2446. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183, 347–362.e324. [Google Scholar] [CrossRef] [PubMed]
- Mørk, S.K.; Kadivar, M.; Bol, K.F.; Draghi, A.; Westergaard, M.C.W.; Skadborg, S.K.; Overgaard, N.; Sørensen, A.B.; Rasmussen, I.S.; Andreasen, L.V.; et al. Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF®09b, in patients with metastatic melanoma. Oncoimmunology 2022, 11, 2023255. [Google Scholar] [CrossRef]
- Riess, J.W.; Shaw, P.; Srinivasan, D.; Garrido, P.; Vuky, J.; Chaney, M.F.; O’Neill, S.; Alavi, A.; McDowell, D.O.; Ehrnrooth, E.; et al. Phase 2 study of the IDO/PD-L1-targeted immune-modulatory vaccine, IO102-IO103, plus pembrolizumab as first-line treatment for metastatic non–small cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), or urothelial bladder cancer (UBC). J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS2699. [Google Scholar] [CrossRef]
- IO Biotech. IO Biotech Provides Business Update. IO Biotech. Available online: https://www.iobiotech.com/2023/01/09/io-biotech-provides-business-update/ (accessed on 12 April 2023).
- Merck. Merck’s KEYTRUDA® (pembrolizumab) Met Primary Endpoint of Disease-Free Survival (DFS) in Certain Patients with Muscle-Invasive Urothelial Carcinoma (MIUC) after Surgery. 2023. Available online: https://www.merck.com/news/mercks-keytruda-pembrolizumab-met-primary-endpoint-of-disease-free-survival-dfs-in-certain-patients-with-muscle-invasive-urothelial-carcinoma-miuc-after-surgery/ (accessed on 28 November 2023).
- Kaur, J.; Choi, W.; Geynisman, D.M.; Plimack, E.R.; Ghatalia, P. Role of immunotherapy in localized muscle invasive urothelial cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211045858. [Google Scholar] [CrossRef]
- Day, D.; Ganju, V.; Chung, K.; Si, L.; Mao, L.; Aghmesheh, M.; Hoyer, R.; Brewin, K.; Zeng, S.; Lu, Q.; et al. 1028P Preliminary phase I results from a first-in-human study of EMB-02, a PD-1xLAG-3 bispecific antibody, in patients (pts) with advanced solid tumors. Ann. Oncol. 2023, 34, S625. [Google Scholar] [CrossRef]
- Coward, J.; Mislang, A.R.A.; Frentzas, S.; Lemech, C.R.; Nagrial, A.; Jin, X.; Li, B.; Wang, Z.M.; Kwek, K.Y.; Xia, Y. Safety and efficacy of AK112, an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors in a phase I dose escalation study. J. Clin. Oncol. 2021, 39, 2515. [Google Scholar] [CrossRef]
- Shum, E.; Reilley, M.; Najjar, Y.; Daud, A.; Thompson, J.; Baranda, J.; Harvey, R.D.; Shields, A.; Cohen, E.; Pant, S.; et al. 523 Preliminary clinical experience with XmAb20717, a PD-1 x CTLA-4 bispecific antibody, in patients with advanced solid tumors. J. ImmunoTherapy Cancer 2021, 9 (Suppl. S2), A553. [Google Scholar] [CrossRef]
- Tolcher, A.; Gabrail, N.; Barve, M.; Wu, X.; Zhang, J.; Shi, M.; Qi, C.; Chen, L.; Yu, S.; Yao, J.; et al. A phase 1, first in human, open-label, dose escalation and dose expansion study of TST005 in patients with locally advanced or metastatic solid. J. Clin. Oncol. 2023, 41, 2531. [Google Scholar] [CrossRef]
- Yap, T.A.; LoRusso, P.M.; Wong, D.J.; Hu-Lieskovan, S.; Papadopoulos, K.P.; Holz, J.B.; Grabowska, U.; Gradinaru, C.; Leung, K.M.; Marshall, S.; et al. A Phase 1 first-in-human study of FS118, a tetravalent bispecific antibody targeting LAG-3 and PD-L1 in patients with advanced cancer and PD-L1 resistance. Clin. Cancer Res. 2023, 29, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Liu, J.; Luo, S.X.; Li, Q.; Zou, W.; Wang, Z.; Peng, Y.; Xiao, S.; Wang, H.; et al. SI-B003 (PD-1/CTLA-4) in patients with advanced solid tumors: A phase I study. J. Clin. Oncol. 2023, 41, e14668. [Google Scholar] [CrossRef]
- Akce, M.; Hu-Lieskovan, S.; Reilley, M.; Strauss, J.F.; Specht, J.M.; Stein, M.N.; Wang, J.S.; Choe, J.H.; Leidner, R.; Davar, D.; et al. A phase 1 multiple-ascending dose study to evaluate the safety and tolerability of XmAb23104 (PD-1 x ICOS) in subjects with selected advanced solid tumors (DUET-3). J. Clin. Oncol. 2022, 40, 2604. [Google Scholar] [CrossRef]
- Sanborn, R.E.; Bordoni, R.E.; Fleming, G.F.; Khasraw, M.; Hawthorne, T.; Thomas, L.J.; Rawls, T.; Young, D.C.; Golden, P.; Keler, T.; et al. A phase 1 dose-escalation study of a PD-L1xCD27 bispecific antibody CDX-527 in patients with advanced malignancies. J. Clin. Oncol. 2021, 39, 2585. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Chu, Q.; Duan, J.; Wan, R.; Wang, Z.; Zhao, J.; Li, H.; Guo, Y.; Chen, Y.; et al. Abstract CT513: Phase I study of IBI322 (anti-CD47/PD-L1 bispecific antibody) monotherapy therapy in patients with advanced solid tumors in China. Cancer Res. 2022, 82, CT513. [Google Scholar] [CrossRef]
- Ku, G.; Bendell, J.C.; Tolcher, A.W.; Hurvitz, S.A.; Krishnamurthy, A.; El-Khoueiry, A.B.; Patnaik, A.; Shroff, R.T.; Noonan, A.; Hahn, N.M.; et al. 525O A phase I dose escalation study of PRS-343, a HER2/4-1BB bispecific molecule, in patients with HER2-positive malignancies. Ann. Oncol. 2020, 31, S462–S463. [Google Scholar] [CrossRef]
- Gong, J.; Dong, Z.; Liu, D.; Xu, J.; Yang, J.; Yang, Y.; Qi, Y.; Men, J.; Kong, P.; Xu, T.; et al. 339 Preliminary safety, tolerability and efficacy results of KN026 (a HER2-targeted Bispecific Antibody) in combination with KN046 (an anti-PD-L1/CTLA-4 Bispecific Antibody) in patients (pts) with HER2 aberrated solid tumors. J. ImmunoTherapy Cancer 2020, 8 (Suppl. S3), A207. [Google Scholar]
- Qing, Z.; Gabrail, N.; Uprety, D.; Rotow, J.; Han, B.; Jänne, P.A.; Nagasaka, M.; Zheng, M.; Zhang, Y.; Yang, G.; et al. 22P EMB-01: An EGFR-cMET bispecific antibody, in advanced/metastatic solid tumors phase I results. Ann. Oncol. 2022, 33, S39–S40. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hernando-Calvo, A.; Carvajal, R.D.; Morris, V.K.; Paik, P.K.; Zandberg, D.P.; Kaczmar, J.M.; Niculescu, L.; Bohr, D.; Reiners, R.; et al. A phase 1 trial of the bifunctional EGFR/TGFβ fusion protein BCA101 alone and in combination with pembrolizumab in patients with advanced solid tumors. J. Clin. Oncol. 2022, 40, 2513. [Google Scholar] [CrossRef]
- Hanna, G.J.; Kaczmar, J.M.; Zandberg, D.P.; Wong, D.J.; Yilmaz, E.; Sherman, E.J.; Hernando-Calvo, A.; Sacco, A.G.; Chung, C.H.; Bohr, D.; et al. Dose expansion results of the bifunctional EGFR/TGFβ inhibitor BCA101 with pembrolizumab in patients with recurrent, metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2023, 41, 6005. [Google Scholar] [CrossRef]
| Regimen | Drug Class | Inclusion Criteria | Phase | Status (March 2023) | Identifier | ORR | mPFS (Months) | mOS (Months) |
|---|---|---|---|---|---|---|---|---|
| Retifanlimab | Anti-PD1 | mUC, RCC, melanoma, NSCLC | 2 | Completed | NCT03679767 | 37.9% | 5.7 | 15.2 |
| Sasanlimab | Anti-PD1 | Advanced solid tumors | 1 | Completed | NCT02573259 | 21.1% | 2.9 | 10.9 |
| Tislelizumab | Anti-PD1 | 2L+ mUC after platinum | 2 | Completed | NCT04004221 | 24% | 2.1 | 9.8 |
| Rulonilimab | Anti-PD1 | 1L cis-ineligible or 2L+ mUC | 2 | Unknown | NCT04636515 | NA | NA | NA |
| Toripalimab | Anti-PD1 | 1L mUC | 2 | Unknown | NCT03113266 | 25.8% | 2.3 | 14.4 |
| Socazolimab | Anti-PDL1 | mUC | 1/2 | Unknown | NCT03676946 | NA | NA | NA |
| Tremelimumab | Anti-CTLA4 | mUC after PD1/PDL1 | 2 | Active, not recruiting | NCT03557918 | NA | NA | NA |
| Regimen | Drug Classes | Trial Name | Phase | Status (March 2023) | Identifier |
|---|---|---|---|---|---|
| Nivolumab + Ipilimumab | Anti-PD1 + Anti-CTLA4 | VEXILLUM | 2 | Recruiting | NCT05219435 |
| Avelumab + Talazoparib | Anti-PDL1 + PARP inhibitor | TALASUR | 2 | Recruiting | NCT04678362 |
| Avelumab + Lurbinectedin | Anti-PDL1 + chemotherapy | 2 | Recruiting | NCT05574504 | |
| Avelumab + Copanlisib | Anti-PDL1 + PI3K inhibitor | 1/2 | Not yet recruiting | NCT05687721 | |
| Avelumab + MRx0518 | Anti-PDL1 + Enterococcus gallinarum | AVENU | 2 | Withdrawn | NCT05107427 |
| Avelumab + Trilaciclib | Anti-PDL1 + CDK4/6 inhibitor | PRESERVE 3 | 2 | Active, not recruiting | NCT04887831 |
| Avelumab +/− SG or M6223 or NKTR-255 | Anti-PDL1 +/− ADC or anti-TIGIT or IL-15 receptor agonist | JAVELIN Bladder Medley | 2 | Recruiting | NCT05327530 |
| Cohort 4: Cisplatin + SG → maintenance SG + either Avelumab or Zimberelimab Cohort 5: Zimberelimab +/− SG vs. Avelumab | Chemo + ADC then ADC + PD1 or PDL1 Anti-PD1 +/− ADC | TROPHY U-01 | 2 | Recruiting | NCT03547973 |
| Avelumab +/− Cabozantinib | Anti-PDL1 + multi-TKI | MAIN-CAV | 3 | Recruiting | NCT05092958 |
| Regimen | Drug Classes | Line | Trial Name | Phase | Status (March 2023) | Identifier |
|---|---|---|---|---|---|---|
| Pembrolizumab +/− chemo vs. chemo | Anti-PD1, chemo | 1L | KEYNOTE-361 | 3 | Completed | NCT02853305 |
| Atezolizumab +/− chemo vs. chemo | Anti-PDL1, chemo | 1L | IMvigor130 | 3 | Active, not recruiting | NCT02807636 |
| Atezolizumab + gemcitabine/cisplatin | Anti-PDL1 + chemo | 1L | 2 | Active, not recruiting | NCT03093922 | |
| Atezolizumab + gemcitabine/split-cisplatin | Anti-PDL1 + chemo | 1L | AUREA | 2 | Active, not recruiting | NCT04602078 |
| Nivolumab + chemo vs. ipilimumab/nivolumab vs. chemo | Anti-PD1, anti-CTLA4, chemo | 1L | CHECKMATE-901 | 3 | Active, not recruiting | NCT03036098 |
| Ipilimumab + gemcitabine/cisplatin | Anti-CTLA4 + chemo | 1L | 2 | Completed | NCT01524991 | |
| Durvalumab + chemo +/− tremelimumab vs. chemo | Anti-PDL1, anti-CTLA4, chemo | 1L | NILE | 3 | Recruiting | NCT03682068 |
| Gemcitabine/cisplatin +/− avelumab | Chemo +/− anti-PDL1 | 1L | GCISAVE | 2 | Terminated | NCT03324282 |
| Gemcitabine/platinum +/− tislelizumab | Chemo +/− anti-PD1 | 1L | 3 | Recruiting | NCT03967977 | |
| Gemcitabine/platinum +/− toripalimab | Chemo +/− anti-PD1 | 1L | 3 | Not yet recruiting | NCT04568304 | |
| Toripalimab + Nab-paclitaxel +/− cisplatin | Anti-PD1 + chemo | 1L | 2 | Unknown | NCT04211012 | |
| Socazolimab + Nab-paclitaxel | Anti-PDL1 + chemo | 1L | 1 | Active, not recruiting | NCT04603846 | |
| Gemcitabine/carboplatin +/− avelumab | Chemo +/− anti-PDL1 | 1L | INDUCOMAIN | 2 | Completed | NCT03390595 |
| Nivolumab + gemcitabine + either carboplatin or oxaliplatin | Anti-PD1 + chemo | 1L | 2 | Active, not recruiting | NCT03451331 | |
| Pembrolizumab + either docetaxel or gemcitabine | Anti-PD1 + chemo | 2L+ | 1b/2 | Completed | NCT02437370 | |
| Avelumab + docetaxel | Anti-PDL1 + chemo | 2L+ | AVETAX | 1b | Active, not recruiting | NCT03575013 |
| Pembrolizumab + nab-paclitaxel | Anti-PD1 + chemo | 2L+ | PEANUT | 2 | Completed | NCT03464734 |
| Pembrolizumab + paclitaxel | Anti-PD1 + chemo | 2L+ | 2 | Completed | NCT02581982 | |
| Atezolizumab +/− eribulin | Anti-PDL1 +/− chemo | 2 | Active, not recruiting | NCT03237780 | ||
| Anti-PD1 + liposomal doxorubicin | Anti-PD1 + chemo | 2L+ | 2 | Unknown | NCT04101812 | |
| Avelumab + pemetrexed in MTAP-deficient | Anti-PDL1 + chemo | 2L+ | 2 | Active, not recruiting | NCT03744793 | |
| Zimberelimab + pemetrexed + etrumadenant in MTAP-deficient | Anti-PD1 + chemo + adenosine receptor antagonist | 2L+ | 2 | Recruiting | NCT05335941 |
| Regimen | Drug Classes | Inclusion Criteria | Trial | Phase | Status (March 2023) | Identifier | ORR | mPFS (Months) | mOS (Months) |
|---|---|---|---|---|---|---|---|---|---|
| Erdafitinib +/− cetrelimab +/− platinum chemo | FGFRi +/− anti-PD1 +/− chemo | mUC with FGFR2/3 alterationPhase 2: 1L cisplatin-ineligible mUC | NORSE | 1b/2 | Active, not recruiting | NCT03473743 | 54.5% | 10.97 | NA |
| Futibatinib + pembrolizumab | FGFRi + anti-PD1 | Platinum-ineligible mUC | 2 | Recruiting | NCT04601857 | NA | NA | NA | |
| Rogaratinib + atezolizumab vs. atezolizumab | FGFRi + anti-PDL1 vs. anti-PDL1 | 1L mUC cisplatin-ineligible, FGFR1/3 (+) by RNAscope | FORT-2 | 1b/2 | Active, not recruiting | NCT03473756 | 54% | NA | NA |
| Derazantinib +/− atezolizumab | FGFR/CSF1R inhibitor +/− anti-PDL1 | 2L+ mUC, 1L cisplatin-ineligible mUC | FIDES-02 | 1b/2 | Completed | NCT04045613 | NA | NA | NA |
| LOXO-435 +/− pembrolizumab | FGFR3i +/− anti-PD1 | FGFR3-altered advanced solid tumors including mUC | 1 | Recruiting | NCT05614739 | NA | NA | NA | |
| Pemigatinib +/− pembrolizumab vs. gemcitabine + carboplatin | FGFRi +/− anti-PD1 vs. chemo | 1L cisplatin-ineligible mUC with an FGFR3 mutation or rearrangement | FIGHT-205 | 2 | Terminated | NCT04003610 | NA | NA | NA |
| Cabozantinib + nivolumab +/− ipilimumab | Multi-TKI + anti-PD1 +/− anti-CTLA4 | Metastatic GU cancers including mUC | 1 | Active, not recruiting | NCT02496208 | 38.5%, 16.0% | 12.8 | 25.4 | |
| Cabozantinib + nivolumab + ipilimumab | Multi-TKI + anti-PD1 + anti-CTLA4 | Rare GU tumors, metastatic bladder cancer histologic variants | ICONIC | 2 | Recruiting | NCT03866382 | NA | NA | NA |
| Cabozantinib + durvalumab | Multi-TKI + anti-PDL1 | mUC and non-UC histologies | ARCADIA | 2 | Unknown | NCT03824691 | 39.7% | 7.6 | 11.6 |
| Cabozantinib + pembrolizumab | Multi-TKI + anti-PD1 | 1L cisplatin-ineligible mUC | PemCab | 2 | Active, not recruiting | NCT03534804 | NA | NA | NA |
| Lenvatinib + pembrolizumab vs. placebo + pembrolizumab | Multi-TKI + anti-PD1 | 1L cisplatin-ineligible PD-L1 (-) or platinum- ineligible mUC | LEAP-011 | 3 | Active, not recruiting | NCT03898180 | NA | HR 0.91 (0.71–1.16) | HR 1.25 (0.94–1.67) |
| Sitravatinib + nivolumab | Multi-TKI + anti-PD1 | 8 cohorts of mUC | 2 | Terminated | NCT03606174 | 0–33% | 3.5–7.8 | NA | |
| Famitinib + camrelizumab | Multi-TKI + anti-PD1 | Advanced GU and gynecologic cancers | 2 | Recruiting | NCT03827837 | 30.6% | 4.1 | 12.9 | |
| XL092 +/− nivolumab +/− either ipilimumab or relatlimab | Multi-TKI +/− anti-PD1 +/− anti-CTLA5 or anti-LAG3 | Advanced solid tumors including mUC | STELLAR-002 | 1b | Recruiting | NCT05176483 | NA | NA | NA |
| Regimen | Drug Classes | Inclusion Criteria | Phase | Status (March 2023) | Identifier |
|---|---|---|---|---|---|
| INCAGN01949 +/− nivolumab +/− ipilimumab | Anti-OX40 Ab +/− anti-PD1 +/− anti-CTLA4 | Advanced solid tumors | 1/2 | Completed | NCT03241173 |
| Atezolizumab +/− vonlerolizumab | Anti-PDL1 +/− anti-OX40 Ab | 1L mUC cisplatin-ineligible | 2 | Terminated for slow accrual | NCT03029832 |
| BGB-A445 +/− tislelizumab | Anti-OX40 Ab +/− anti-PD1 | 2L+ metastatic UC, RCC, melanoma | 1b/2 | Recruiting | NCT05661955 |
| ICAGN01876 +/− nivolumab +/− ipilimumab | Anti-GITR Ab +/− anti-PD1 +/− anti-CTLA4 | Advanced solid tumors | 1/2 | Completed | NCT03126110 |
| LYT-200 +/− tislelizumab or gemcitabine + nab-paclitaxel | Anti-α-GAL-9 +/− anti-PD1 +/− chemo | Advanced solid tumors | 1/2 | Recruiting | NCT04666688 |
| ABBV-151 + budigalimab | Anti-GARP-TGFβ1 + anti-PD1 | Advanced solid tumors | 1 | Recruiting | NCT03821935 |
| NGM831 +/− pembrolizumab | Anti-ILT3 +/− anti-PD1 | Advanced solid tumors | 1/1b | Recruiting | NCT05215574 |
| NGM438 +/− pembrolizumab | Anti-LAIR1 +/− anti-PD1 | Advanced solid tumors | 1/1b | Recruiting | NCT05311618 |
| AZD8853 | Anti-MIC-1 | 2L+ mUC, NSCLC, colorectal | 1/2a | Terminated | NCT05397171 |
| Regimen | Drug Classes | Target | Inclusion Criteria | Phase | Status (March 2023) | Identifier |
|---|---|---|---|---|---|---|
| WT-1 dendritic cells | Autologous dendritic cells | WT-1 | mUC | 1 | Completed | Japan (UMIN 000027279) [125] |
| TILs | Autologous TILs | mUC after platinum, ICI | 2 | Unknown | NCT04383067 | |
| LN-145 + pembrolizumab | Autologous TILs + anti-PD1 | mUC after cisplatin | 2 | Withdrawn (no accrual) | NCT03935347 | |
| Autologous central memory T cells | Autologous central memory T cells | mUC after 1L gemcitabine + cisplatin | 2 | Terminated (shortage of funds) | NCT03389438 | |
| 4SCAR-T cells | CAR-T cells | PSMA + FRα | mUC without SOC option | 1/2 | Unknown | NCT03185468 |
| PD-1 knockout engineered T cells | Autologous engineered T cells | PD-1 knockout | mUC | 1 | Withdrawn (no funding) | NCT02863913 |
| Autologous cytokine-induced killer cells | Autologous NK cells | mUC, metastatic RCC | 1/2 | Not yet recruiting | NCT05108077 |
| Regimen | Drug Classes | Target | Inclusion Criteria | Phase | Status (March 2023) | Identifier | ORR | mPFS (Months) | mOS (Months) |
|---|---|---|---|---|---|---|---|---|---|
| PGV001 + atezolizumab | Personalized tumor neoantigen vaccine + anti-PDL1 | mUC | 1 | Completed | NCT03359239 | NA | NA | NA | |
| CV301 + atezolizumab | Poxviruses encoding CEA, MUC-1 + anti-PDL1 | CEA, MUC-1 | mUC | 2 | Completed | NCT03628716 | 9.4% | NA | NA |
| INO-5401 + INO-9012 + atezolizumab | Plasmids encoding WT1, PSMA, hTERT, IL12 + anti-PDL1 | WT1, PSMA, hTERT | mUC | 1/2a | Active, not recruiting | NCT03502785 | NA | NA | NA |
| Chimerical exosomal tumor vaccine | Exosomes in supernatant of chimeric APC-tumor cells | mUC | 1 | Recruiting | NCT05559177 | NA | NA | NA | |
| NEO-PV-01 + nivolumab | Personalized tumor neoantigen mRNA vaccine + anti-PD1 | mUC, NSCLC, melanoma | 1b | Completed | NCT02897765 | 27% | 5.8 | 20.7 | |
| NeoPepVac EVAX-01-CAF09b + anti-PD1/PDL1 | Personalized tumor neoantigen vaccine | mUC, NSCLC, melanoma | 1/2 | Active, not recruiting | NCT03715985 | NA | NA | NA | |
| IO102-IO103 + pembrolizumab | IDO + PD-L1 peptide vaccine + anti-PD1 | IDO, PD-L1 | 1L mUC, NSCLC, SCCHN | 2 | Recruiting | NCT05077709 | NA | NA | NA |
| OH2 | Oncolytic virus delivering GM-CSF gene | mUC | 2 | Recruiting | NCT05248789 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, D.M.; Mateen, R.; Qaddour, N.; Carrillo, A.; Verschraegen, C.; Yang, Y.; Li, Z.; Sundi, D.; Mortazavi, A.; Collier, K.A. A Comprehensive Review of Immunotherapy Clinical Trials for Metastatic Urothelial Carcinoma: Immune Checkpoint Inhibitors Alone or in Combination, Novel Antibodies, Cellular Therapies, and Vaccines. Cancers 2024, 16, 335. https://doi.org/10.3390/cancers16020335
Patel DM, Mateen R, Qaddour N, Carrillo A, Verschraegen C, Yang Y, Li Z, Sundi D, Mortazavi A, Collier KA. A Comprehensive Review of Immunotherapy Clinical Trials for Metastatic Urothelial Carcinoma: Immune Checkpoint Inhibitors Alone or in Combination, Novel Antibodies, Cellular Therapies, and Vaccines. Cancers. 2024; 16(2):335. https://doi.org/10.3390/cancers16020335
Chicago/Turabian StylePatel, Dixita M., Ruba Mateen, Noor Qaddour, Alessandra Carrillo, Claire Verschraegen, Yuanquan Yang, Zihai Li, Debasish Sundi, Amir Mortazavi, and Katharine A. Collier. 2024. "A Comprehensive Review of Immunotherapy Clinical Trials for Metastatic Urothelial Carcinoma: Immune Checkpoint Inhibitors Alone or in Combination, Novel Antibodies, Cellular Therapies, and Vaccines" Cancers 16, no. 2: 335. https://doi.org/10.3390/cancers16020335
APA StylePatel, D. M., Mateen, R., Qaddour, N., Carrillo, A., Verschraegen, C., Yang, Y., Li, Z., Sundi, D., Mortazavi, A., & Collier, K. A. (2024). A Comprehensive Review of Immunotherapy Clinical Trials for Metastatic Urothelial Carcinoma: Immune Checkpoint Inhibitors Alone or in Combination, Novel Antibodies, Cellular Therapies, and Vaccines. Cancers, 16(2), 335. https://doi.org/10.3390/cancers16020335





