Improvement in Central Neck Dissection Quality in Thyroid Cancer by Use of Tissue Autofluorescence
Abstract
Simple Summary
Abstract
1. Introduction
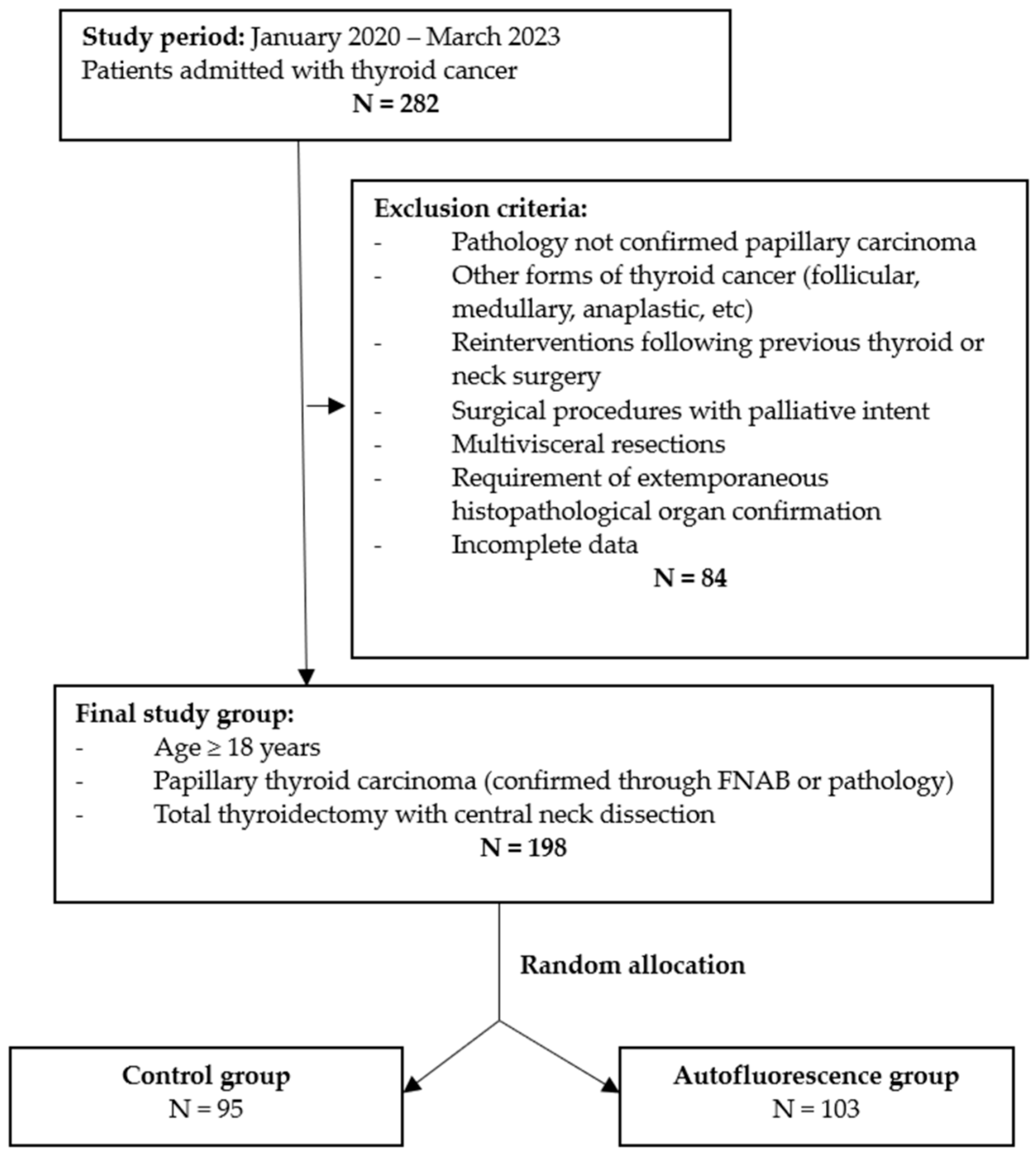
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khokhar, M.; Milas, M. Management of Nodal Disease in Thyroid Cancer. Surg. Clin. N. Am. 2019, 99, 611–632. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Hartl, D.; Leboulleux, S.; Baudin, E.; Lumbroso, J.D.; Al Ghuzlan, A.; Chami, L.; Schlumberger, M.; Travagli, J.P. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: Implications for radioiodine treatment. J. Clin. Endocrinol. Metab. 2009, 94, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Laird, A.M.; Gauger, P.G.; Miller, B.S.; Doherty, G.M. Evaluation of postoperative radioactive iodine scans in patients who underwent prophylactic central lymph node dissection. World J. Surg. 2012, 36, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Bergstralh, E.J.; Goellner, J.R.; Ebersold, J.R.; Grant, C.S. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993, 114, 1050–1057; discussion 1057–1058. [Google Scholar] [PubMed]
- Shah, M.D.; Hall, F.T.; Eski, S.J.; Witterick, I.J.; Walfish, P.G.; Freeman, J.L. Clinical course of thyroid carcinoma after neck dissection. Laryngoscope 2003, 113, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.S.; Dubner, S.; Sznyter, L.A.; Heller, K.S. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H.; Christodoulou, S.; Lappas, C.; Safioleas, M. Preoperative detection of cervical lymph node metastases in papillary thyroid cancer: A surgical perspective. Onkologie 2009, 32, 762–766. [Google Scholar] [CrossRef]
- Lorente-Poch, L.; Sancho, J.J.; Munoz-Nova, J.L.; Sanchez-Velazquez, P.; Sitges-Serra, A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg. 2015, 4, 82–90. [Google Scholar] [CrossRef]
- Sanabria, A.; Dominguez, L.C.; Vega, V.; Osorio, C.; Duarte, D. Cost-effectiveness analysis regarding postoperative administration of vitamin-D and calcium after thyroidectomy to prevent hypocalcaemia. Rev. Salud Publica 2011, 13, 804–813. [Google Scholar] [CrossRef][Green Version]
- Lang, B.H.; Ng, S.H.; Lau, L.L.; Cowling, B.J.; Wong, K.P.; Wan, K.Y. A systematic review and meta-analysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid 2013, 23, 1087–1098. [Google Scholar] [CrossRef]
- Teshima, M.; Otsuki, N.; Morita, N.; Furukawa, T.; Shinomiya, H.; Shinomiya, H.; Nibu, K.I. Postoperative hypoparathyroidism after total thyroidectomy for thyroid cancer. Auris Nasus Larynx 2018, 45, 1233–1238. [Google Scholar] [CrossRef]
- Sanabria, A.; Dominguez, L.C.; Vega, V.; Osorio, C.; Duarte, D. Routine postoperative administration of vitamin D and calcium after total thyroidectomy: A meta-analysis. Int. J. Surg. 2011, 9, 46–51. [Google Scholar] [CrossRef][Green Version]
- Sousa Ade, A.; Salles, J.M.; Soares, J.M.; Moraes, G.M.; Carvalho, J.R.; Savassi-Rocha, P.R. Predictors factors for post-thyroidectomy hypocalcaemia. Rev. Col. Bras. Cir. 2012, 39, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E.; Abdelghani, S.; Friedlander, P.; Alrasheedi, S.; Tufano, R.P.; Bellows, C.F.; Slakey, D. Motor and sensory branching of the recurrent laryngeal nerve in thyroid surgery. Surgery 2011, 150, 1222–1227. [Google Scholar] [CrossRef]
- Kandil, E.; Abdel Khalek, M.; Aslam, R.; Friedlander, P.; Bellows, C.F.; Slakey, D. Recurrent laryngeal nerve: Significance of the anterior extralaryngeal branch. Surgery 2011, 149, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Benmiloud, F.; Rebaudet, S.; Varoquaux, A.; Penaranda, G.; Bannier, M.; Denizot, A. Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: A before and after controlled study. Surgery 2018, 163, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Benmiloud, F. Intraoperative Mapping Angiography of the Parathyroid Glands: Description, Advantages, and Limits of the Technique. VideoEndocrinology 2021, 8, ve.2021.0006. [Google Scholar] [CrossRef] [PubMed]
- Benmiloud, F.; Penaranda, G.; Chiche, L.; Rebaudet, S. Intraoperative Mapping Angiograms of the Parathyroid Glands Using Indocyanine Green during Thyroid Surgery: Results of the Fluogreen Study. World J. Surg. 2022, 46, 416–424. [Google Scholar] [CrossRef]
- Ladurner, R.; Sommerey, S.; Arabi, N.A.; Hallfeldt, K.K.J.; Stepp, H.; Gallwas, J.K.S. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg. Endosc. 2017, 31, 3140–3145. [Google Scholar] [CrossRef]
- Ladurner, R.; Al Arabi, N.; Guendogar, U.; Hallfeldt, K.; Stepp, H.; Gallwas, J. Near-infrared autofluorescence imaging to detect parathyroid glands in thyroid surgery. Ann. R. Coll. Surg. Engl. 2018, 100, 33–36. [Google Scholar] [CrossRef]
- Haugen, B.R.; Sawka, A.M.; Alexander, E.K.; Bible, K.C.; Caturegli, P.; Doherty, G.M.; Mandel, S.J.; Morris, J.C.; Nassar, A.; Pacini, F.; et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2017, 27, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.W.; Duh, Q.Y.; Heller, K.S.; LiVolsi, V.A.; Mandel, S.J.; Steward, D.L.; Tufano, R.P.; Tuttle, R.M.; American Thyroid Association Surgical Affairs Committee’s Taskforce on Thyroid Cancer Nodal Surgery. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012, 22, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Cranshaw, I.M.; Carnaille, B. Micrometastases in thyroid cancer. An important finding? Surg. Oncol. 2008, 17, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.J. Papillary and follicular thyroid carcinoma. N. Engl. J. Med. 1998, 338, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Murakami, N. The value of lymph-node dissection in patients with differentiated thyroid cancer. Surg. Clin. N. Am. 1987, 67, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Khafif, A.; Ben-Yosef, R.; Abergel, A.; Kesler, A.; Landsberg, R.; Fliss, D.M. Elective paratracheal neck dissection for lateral metastases from papillary carcinoma of the thyroid: Is it indicated? Head Neck 2008, 30, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Sancho, J.J.; Lennard, T.W.; Paunovic, I.; Triponez, F.; Sitges-Serra, A. Prophylactic central neck disection in papillary thyroid cancer: A consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch. Surg. 2014, 399, 155–163. [Google Scholar] [CrossRef]
- Popadich, A.; Levin, O.; Lee, J.C.; Smooke-Praw, S.; Ro, K.; Fazel, M.; Arora, A.; Tolley, N.S.; Palazzo, F.; Learoyd, D.L.; et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery 2011, 150, 1048–1057. [Google Scholar] [CrossRef]
- Sywak, M.; Cornford, L.; Roach, P.; Stalberg, P.; Sidhu, S.; Delbridge, L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery 2006, 140, 1000–1005; discussion 1005–1007. [Google Scholar] [CrossRef]
- Qubain, S.W.; Nakano, S.; Baba, M.; Takao, S.; Aikou, T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery 2002, 131, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Arturi, F.; Russo, D.; Giuffrida, D.; Ippolito, A.; Perrotti, N.; Vigneri, R.; Filetti, S. Early diagnosis by genetic analysis of differentiated thyroid cancer metastases in small lymph nodes. J. Clin. Endocrinol. Metab. 1997, 82, 1638–1641. [Google Scholar] [CrossRef]
- Roh, J.L.; Koch, W.M. Role of sentinel lymph node biopsy in thyroid cancer. Expert Rev. Anticancer Ther. 2010, 10, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.M.; Leboulleux, S.; Al Ghuzlan, A.; Baudin, E.; Chami, L.; Schlumberger, M.; Travagli, J.P. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann. Surg. 2012, 255, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.S.; Evans, D.B.; Fareau, G.G.; Carroll, T.; Yen, T.W. Effect of prophylactic central compartment neck dissection on serum thyroglobulin and recommendations for adjuvant radioactive iodine in patients with differentiated thyroid cancer. Ann. Surg. Oncol. 2012, 19, 4217–4222. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, X.Y.; Zhao, T.T.; Zheng, X.Y.; Jin, F.; Li, J.G. Study on the skip metastasis of axillary lymph nodes in breast cancer and their relation with Gli1 expression. Tumour Biol. 2012, 33, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Giugliano, G.; Santoro, L.; Ywata De Carvalho, A.; Massaro, M.A.; Gibelli, B.; De Fiori, E.; Grosso, E.; Ansarin, M.; Calabrese, L. Role of prophylactic central neck dissection in cN0 papillary thyroid cancer. Acta Otorhinolaryngol. Ital. 2009, 29, 61–69. [Google Scholar] [PubMed]
- Ryu, I.S.; Song, C.I.; Choi, S.H.; Roh, J.L.; Nam, S.Y.; Kim, S.Y. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann. Surg. Oncol. 2014, 21, 277–283. [Google Scholar] [CrossRef]
- Barczynski, M.; Konturek, A.; Stopa, M.; Nowak, W. Prophylactic central neck dissection for papillary thyroid cancer. Br. J. Surg. 2013, 100, 410–418. [Google Scholar] [CrossRef]
- Eltelety, A.M.; Terris, D.J. Neck Dissection in the Surgical Treatment of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 143–151. [Google Scholar] [CrossRef]
- Agrawal, N.; Evasovich, M.R.; Kandil, E.; Noureldine, S.I.; Felger, E.A.; Tufano, R.P.; Kraus, D.H.; Orloff, L.A.; Grogan, R.; Angelos, P.; et al. Indications and extent of central neck dissection for papillary thyroid cancer: An American Head and Neck Society Consensus Statement. Head Neck 2017, 39, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Jimeno, J.; Miquel, J.; Iglesias, M.; Munne, A.; Sancho, J.J.; Sitges-Serra, A. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery 2005, 138, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Merdad, M.; Eskander, A.; Kroeker, T.; Freeman, J.L. Predictors of level II and Vb neck disease in metastatic papillary thyroid cancer. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 1030–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eskander, A.; Merdad, M.; Freeman, J.L.; Witterick, I.J. Pattern of spread to the lateral neck in metastatic well-differentiated thyroid cancer: A systematic review and meta-analysis. Thyroid 2013, 23, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Heaton, C.M.; Chang, J.L.; Orloff, L.A. Prognostic Implications of Lymph Node Yield in Central and Lateral Neck Dissections for Well-Differentiated Papillary Thyroid Carcinoma. Thyroid 2016, 26, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.; McHenry, C.R. Selective lateral compartment neck dissection for thyroid cancer. J. Surg. Res. 2013, 184, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.S.; Marvel, J.; Smith, P.; Hankins, P.; Wolf, P.; Goepfert, H. Paratracheal lymph node dissection for carcinoma of the larynx, hypopharynx, and cervical esophagus. Otolaryngol. Head Neck Surg. 1993, 108, 11–17. [Google Scholar] [CrossRef]
- Paras, C.; Keller, M.; White, L.; Phay, J.; Mahadevan-Jansen, A. Near-infrared autofluorescence for the detection of parathyroid glands. J. Biomed. Opt. 2011, 16, 067012. [Google Scholar] [CrossRef]
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Solorzano, C.C.; Broome, J.T.; Mahadevan-Jansen, A. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J. Clin. Endocrinol. Metab. 2014, 99, 4574–4580. [Google Scholar] [CrossRef]
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Mahadevan-Jansen, A.; Broome, J.T. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013, 154, 1371–1377; discussion 1377. [Google Scholar] [CrossRef]
- De Leeuw, F.; Breuskin, I.; Abbaci, M.; Casiraghi, O.; Mirghani, H.; Ben Lakhdar, A.; Laplace-Builhe, C.; Hartl, D. Intraoperative Near-infrared Imaging for Parathyroid Gland Identification by Auto-fluorescence: A Feasibility Study. World J. Surg. 2016, 40, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Falco, J.; Dip, F.; Quadri, P.; de la Fuente, M.; Rosenthal, R. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. J. Am. Coll. Surg. 2016, 223, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Kahramangil, B.; Berber, E. The use of near-infrared fluorescence imaging in endocrine surgical procedures. J. Surg. Oncol. 2017, 115, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Kahramangil, B.; Berber, E. ASO Author Reflections: Parathyroid Autofluorescence and Near-Infrared Imaging. Ann. Surg. Oncol. 2018, 25, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; McWade, M.A.; Paras, C.; Mannoh, E.A.; Sanders, M.E.; White, L.M.; Broome, J.T.; Phay, J.E.; Baregamian, N.; Solorzano, C.C.; et al. Developing a Clinical Prototype to Guide Surgeons for Intraoperative Label-Free Identification of Parathyroid Glands in Real Time. Thyroid 2018, 28, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.M.; Jain, A.; Randeva, H.; Watkinson, J.; Shaha, A. Postoperative hypocalcemia--the difference a definition makes. Head Neck 2010, 32, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Rajinikanth, J.; Paul, M.J.; Abraham, D.T.; Ben Selvan, C.K.; Nair, A. Surgical audit of inadvertent parathyroidectomy during total thyroidectomy: Incidence, risk factors, and outcome. Medscape J. Med. 2009, 11, 29. [Google Scholar] [PubMed]
- Falco, J.; Dip, F.; Quadri, P.; de la Fuente, M.; Prunello, M.; Rosenthal, R.J. Increased identification of parathyroid glands using near infrared light during thyroid and parathyroid surgery. Surg. Endosc. 2017, 31, 3737–3742. [Google Scholar] [CrossRef]
- Phitayakorn, R.; McHenry, C.R. Incidence and location of ectopic abnormal parathyroid glands. Am. J. Surg. 2006, 191, 418–423. [Google Scholar] [CrossRef]
- Pattou, F.N.; Pellissier, L.C.; Noel, C.; Wambergue, F.; Huglo, D.G.; Proye, C.A. Supernumerary parathyroid glands: Frequency and surgical significance in treatment of renal hyperparathyroidism. World J. Surg. 2000, 24, 1330–1334. [Google Scholar] [CrossRef]
- Van Slycke, S.; Van Den Heede, K.; Brusselaers, N.; Vermeersch, H. Feasibility of Autofluorescence for Parathyroid Glands during Thyroid Surgery and the Risk of Hypocalcemia: First Results in Belgium and Review of the Literature. Surg. Innov. 2021, 28, 409–418. [Google Scholar] [CrossRef] [PubMed]
- McWade, M.A.; Sanders, M.E.; Broome, J.T.; Solorzano, C.C.; Mahadevan-Jansen, A. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016, 159, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; MacAulay, C.; McLean, D.I.; Palcic, B. Reconstruction of in vivo skin autofluorescence spectrum from microscopic properties by Monte Carlo simulation. J. Photochem. Photobiol. B 1997, 38, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Sauer, L.; Vitale, A.S.; Milliken, C.M.; Modersitzki, N.K.; Blount, J.D.; Bernstein, P.S. Autofluorescence Lifetimes Measured with Fluorescence Lifetime Imaging Ophthalmoscopy (FLIO) Are Affected by Age, but Not by Pigmentation or Gender. Transl. Vis. Sci. Technol. 2020, 9, 2. [Google Scholar] [CrossRef]
- Serra, C.; Silveira, L.; Canudo, A. Identification of inadvertently removed parathyroid glands during thyroid surgery using autofluorescence. Gland Surg. 2020, 9, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Silveira, L. Evaluation of structural and ultrastructural changes in thyroid and parathyroid glands after near infrared irradiation: Study on an animal model. PeerJ 2021, 9, e11891. [Google Scholar] [CrossRef] [PubMed]
- Makovac, P.; Muradbegovic, M.; Mathieson, T.; Demarchi, M.S.; Triponez, F. Preliminary experience with the EleVision IR system in detection of parathyroid glands autofluorescence and perfusion assessment with ICG. Front. Endocrinol. 2022, 13, 1030007. [Google Scholar] [CrossRef]
- Ladurner, R.; Lerchenberger, M.; Al Arabi, N.; Gallwas, J.K.S.; Stepp, H.; Hallfeldt, K.K.J. Parathyroid Autofluorescence-How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience. Molecules 2019, 24, 2560. [Google Scholar] [CrossRef]
| Patient Characteristics | NAF Group N = 95 | AF Group N = 103 | p Value |
|---|---|---|---|
| Age (years) | 49.7 ± 18.2 | 50.1 ± 19.1 | n.s. |
| Gender | n.s. | ||
| Male | 17 (17.9%) | 21 (20.4%) | |
| Female | 78 (82.1%) | 82 (79.6%) | |
| Multifocal thyroid papillary carcinoma | n.s. | ||
| Yes | 18 (18.9%) | 11 (10.7%) | |
| No | 77 (81.1%) | 92 (89.3%) | |
| Tumor size | n.s. | ||
| T1 | 47 (49.5%) | 38 (36.9%) | |
| T2 | 40 (42.1%) | 56 (54.4%) | |
| T3 | 8 (8.4%) | 9 (8.7%) | |
| No. of excised lymph nodes | 9.2 ± 4.1 | 21.3 ± 4.8 | <0.01 |
| Nodal metastasis | <0.01 | ||
| Yes | 34 (35.8%) | 59 (57.3%) | |
| No | 61 (64.2%) | 44 (42.7%) | |
| No. of metastatic lymph nodes | 3.7 ± 1.5 | 8.2 ± 4.4 | <0.01 |
| Parathyroid Gland Identification Characteristics | NAF Group N = 95 | AF Group N = 103 | p Value |
|---|---|---|---|
| No. of identified parathyroid glands | <0.01 | ||
| 1 | 5 (5.3%) | 2 (1.9%) | |
| 2 | 16 (16.8%) | 5 (4.9%) | |
| 3 | 28 (29.5%) | 21 (20.3%) | |
| 4 | 44 (46.3%) | 65 (63.1%) | |
| ≥5 | 2 (2.1%) | 10 (9.7%) | |
| Accidentally excised parathyroid glands | |||
| Patients | 17 (17.9%) | 8 (7.8%) | <0.04 |
| Mean no. | 1.2 ± 0.4 | 1.1 ± 0.3 | n.s. |
| Intentionally excised parathyroid glands | |||
| Patients | 7 (7.4%) | 6 (5.8%) | n.s. |
| Mean no. | 1 ± 0 | 1 ± 0 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagoe, O.C.; Ionică, M. Improvement in Central Neck Dissection Quality in Thyroid Cancer by Use of Tissue Autofluorescence. Cancers 2024, 16, 258. https://doi.org/10.3390/cancers16020258
Neagoe OC, Ionică M. Improvement in Central Neck Dissection Quality in Thyroid Cancer by Use of Tissue Autofluorescence. Cancers. 2024; 16(2):258. https://doi.org/10.3390/cancers16020258
Chicago/Turabian StyleNeagoe, Octavian Constantin, and Mihaela Ionică. 2024. "Improvement in Central Neck Dissection Quality in Thyroid Cancer by Use of Tissue Autofluorescence" Cancers 16, no. 2: 258. https://doi.org/10.3390/cancers16020258
APA StyleNeagoe, O. C., & Ionică, M. (2024). Improvement in Central Neck Dissection Quality in Thyroid Cancer by Use of Tissue Autofluorescence. Cancers, 16(2), 258. https://doi.org/10.3390/cancers16020258






