Personalized Brachytherapy: Applications and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
Rationale for Personalized Brachytherapy
- Proximity to tumor: BT allows for precise placement of radiation sources directly within or near the tumor, enabling highly conformal dose delivery.
- Steep dose gradient: A typical dose gradient of 5–20% per millimeter at the target volume periphery for a 192Ir source allows for rapid dose fall-off, sparing nearby OARs [3].
- Real-time visualization: Image guidance enables visualization of targets and OARs in real time, allowing for plan optimization based on current anatomy rather than pre-treatment imaging [4].
- Shorter treatment course: BT is typically delivered over a shorter duration than external beam radiotherapy, reducing intra-fraction motion considerations.
- Inter-fraction responsiveness: BT’s ability to adapt to tumor response and anatomical changes between fractions ensures appropriate target coverage and limits OAR dose.
2. Equipment Customization
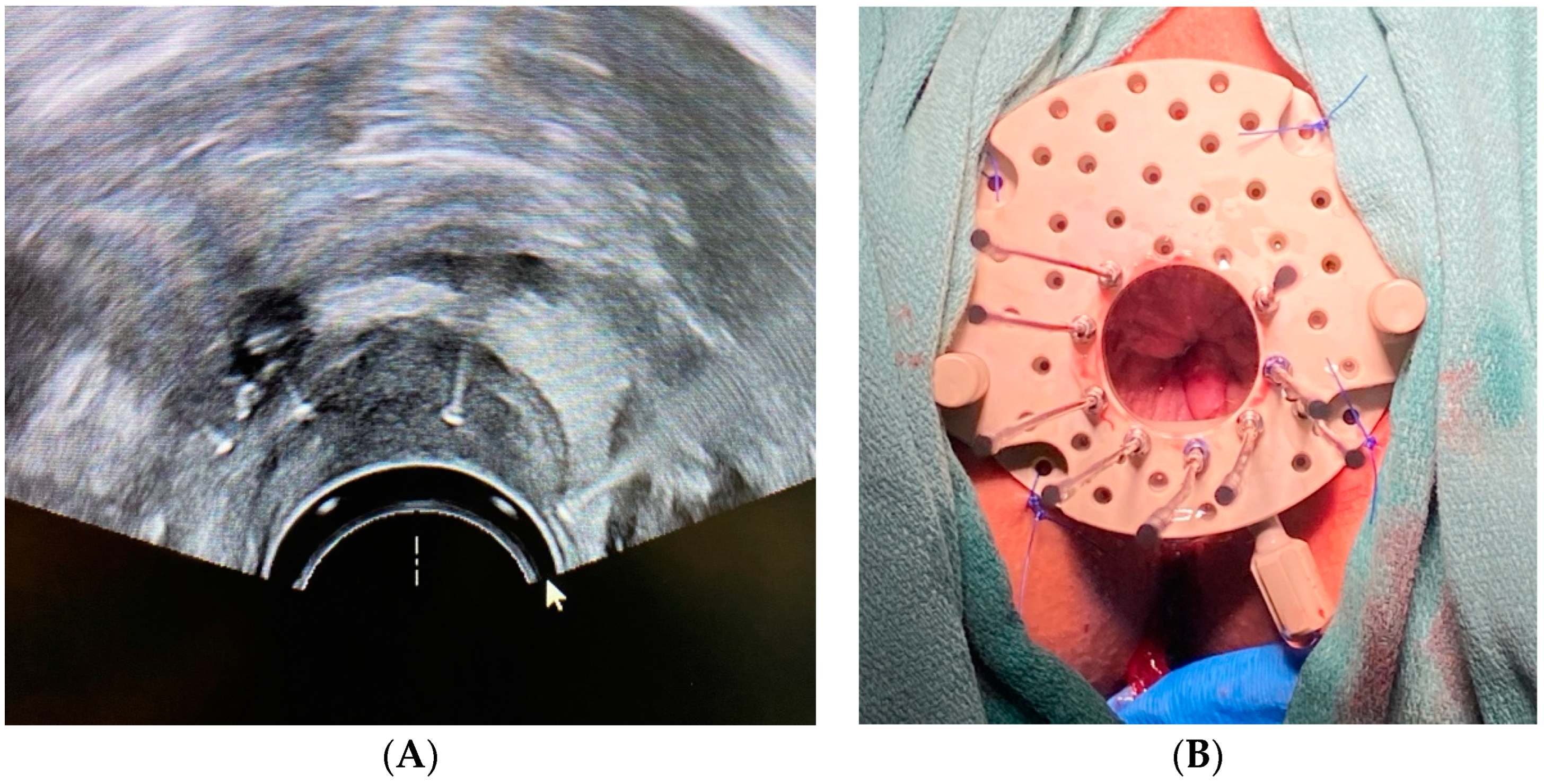
2.1. Patient 1
2.2. Patient 2
3. Treatment Planning and Image-Guidance
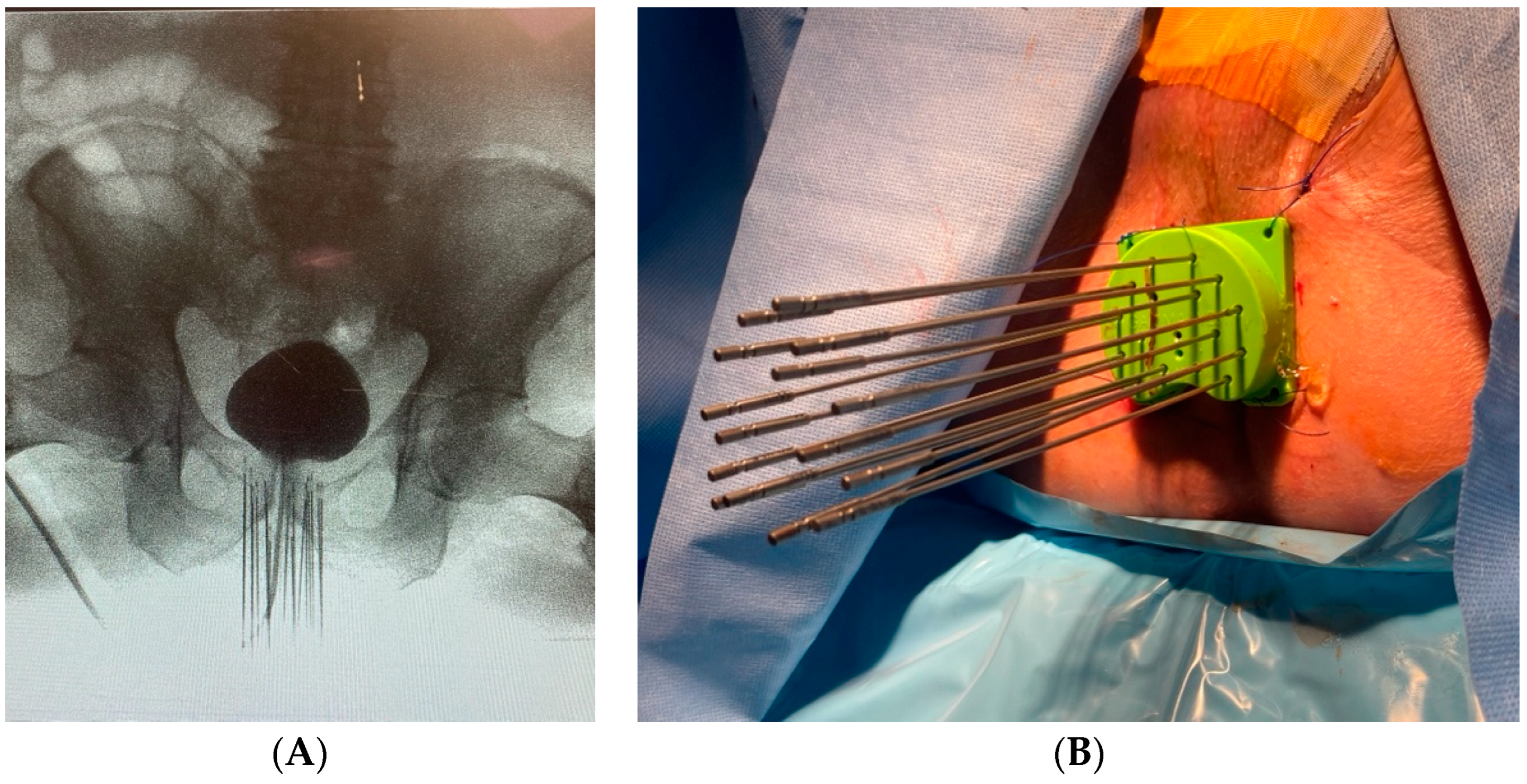
Patient 3
4. Target Volume Delineation, Dose, and Fractionation
Patient 4
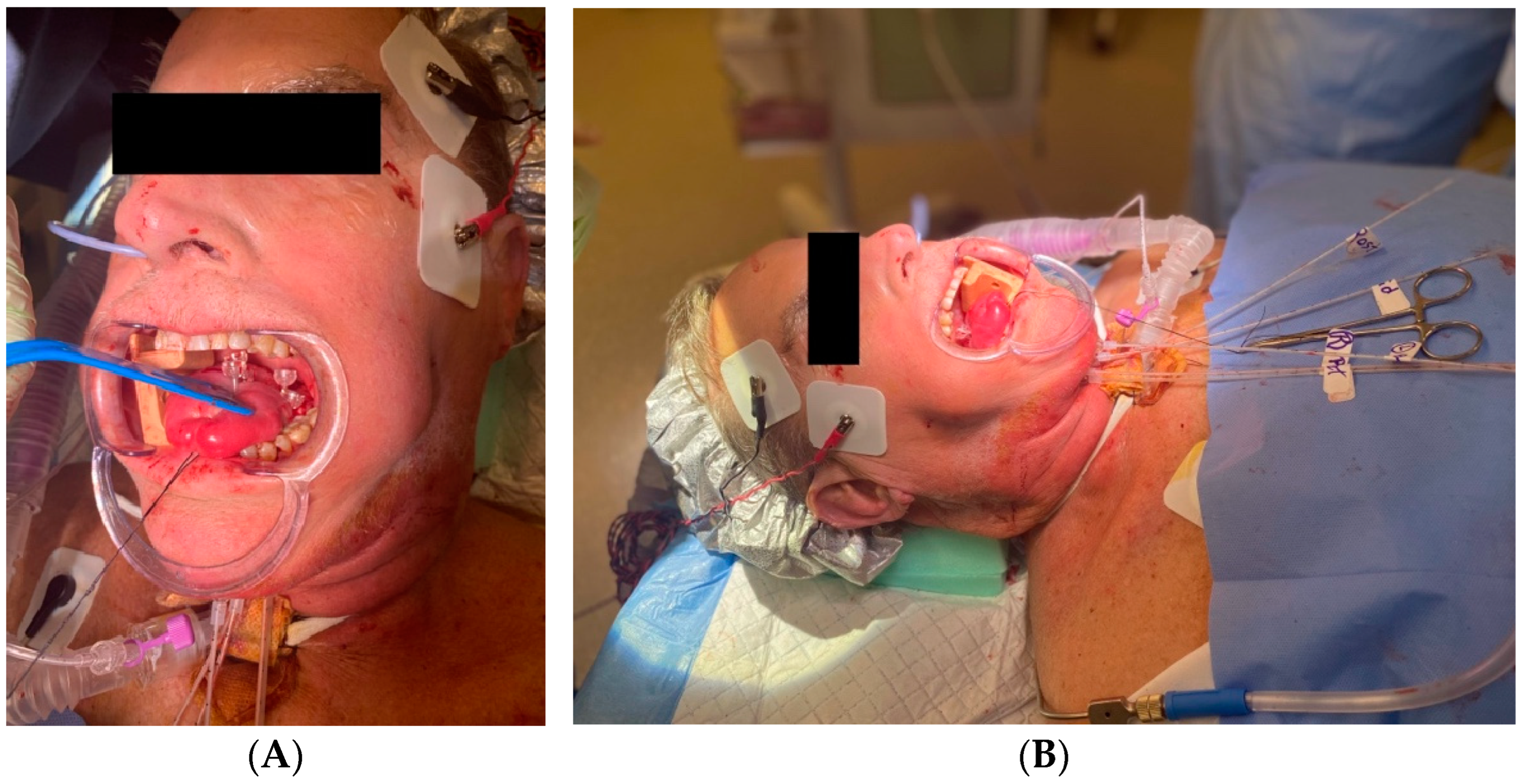
5. Anesthesia, Analgesia, and Antibiotics
6. Integration of Genomic Tests and Molecular Markers
7. Artificial Intelligence and Machine Learning
8. Challenges and Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ling, D.C.; Karukonda, P.; Smith, R.P.; Heron, D.E.; Beriwal, S. Declining Brachytherapy Utilization for High-Risk Prostate Cancer—Can Clinical Pathways Reverse the Trend? Brachytherapy 2018, 17, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sherwani, Z.; Lopez, M.; Vergalasova, I.; Zhang, X.; Eckroate, B.; Hollingsworth, J.; Girda, E.; Hathout, L. Disparities in Brachytherapy Utilization in Cervical Cancer in the United States: A Comprehensive Literature Review. Gynecol. Oncol. 2023, 179, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Tanderup, K.; Menard, C.; Polgar, C.; Lindegaard, J.C.; Kirisits, C.; Potter, R. Advancements in Brachytherapy. Adv. Drug Deliv. Rev. 2017, 109, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Dimopoulos, J.; Georg, P.; Lang, S.; Waldhäusl, C.; Wachter-Gerstner, N.; Weitmann, H.; Reinthaller, A.; Knocke, T.H.; Wachter, S.; et al. Clinical Impact of MRI Assisted Dose Volume Adaptation and Dose Escalation in Brachytherapy of Locally Advanced Cervix Cancer. Radiother. Oncol. 2007, 83, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.A.M.; Flynn, R.; Belanger, C.; Callaghan, C.; Kim, Y.; Jia, X.; Chen, Z.; Beaulieu, L. Brachytherapy Future Directions. Semin. Radiat. Oncol. 2020, 30, 94–106. [Google Scholar] [CrossRef]
- Skinner, L.B.; Niedermayr, T.; Prionas, N.; Perl, J.; Fahimian, B.; Kidd, E.A. Intensity Modulated Ir-192 Brachytherapy Using High-Z 3D Printed Applicators. Phys. Med. Biol. 2020, 65, 155018. [Google Scholar] [CrossRef]
- Kudla, M.; Bachand, F.; Moore, J.; Batchelar, D. Patient-Specific Cylinder Templates for Hybrid Interstitial Vaginal Brachytherapy: Feasibility of Automated 3-D Design, 3D Printing, and Dosimetric Outlook. Brachytherapy 2023, 22, 468–476. [Google Scholar] [CrossRef]
- Yan, J.; Qin, X.; Zhang, F.; Hou, X.; Yu, L.; Qiu, J. Comparing Multichannel Cylinder and 3D-Printed Applicators for Vaginal Cuff Brachytherapy with Preliminary Exploration of Post-Hysterectomy Vaginal Morphology. J. Contemp. Brachyther. 2021, 13, 641–648. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Y.; Cui, M.; Miao, J.; Gao, L.; Hu, J.; Tian, D.; You, J. Dosimetry Comparison between a 3D Printed Minimally Invasive Guidance Template and Free Implantation in the Brachytherapy Treatment of Postoperative Recurrent Cervical Carcinoma. Cancer Manag. Res. 2019, 11, 5013–5018. [Google Scholar] [CrossRef]
- Towithelertkul, C.; Chugh, A.; Hattori, M.; Yoshimura, R.; Sumita, Y.I. A Custom-Made Brachytherapy Applicator for Recurrent Endometrial and Vaginal Cancer: A Dental Technique for Prosthesis Fabrication. J. Prosthet. Dent. 2021, 126, 711–714. [Google Scholar] [CrossRef]
- Lee, H.H.C.; Chiu, T.D.; Hrycushko, B.; Xiong, Z.; Hudak, S.; Woldu, S.; Mauck, R.; Corwin, T.; Meng, X.; Margulis, V.; et al. Organ Sparing Treatment for Penile Cancer Using a 3D-Printed High-Dose-Rate Brachytherapy Applicator. Brachytherapy 2023, 22, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Guix, B.; Finestres, F.; Tello, J.; Palma, C.; Martinez, A.; Guix, J.; Guix, R. Treatment of Skin Carcinomas of the Face by High-Dose-Rate Brachytherapy and Custom-Made Surface Molds. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 95–102. [Google Scholar] [CrossRef]
- Tardivon, A.A.; Kinkel, K.; Lartigau, E.; Masselot, J.; Gerbaulet, A.P.; Vanel, D. MR Imaging during Intracavitary Brachytherapy of Vaginal and Cervical Cancer: Preliminary Results. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 1996, 16, 1363–1370. [Google Scholar] [CrossRef]
- Haie-Meder, C.; Pötter, R.; Van Limbergen, E.; Briot, E.; De Brabandere, M.; Dimopoulos, J.; Dumas, I.; Hellebust, T.P.; Kirisits, C.; Lang, S.; et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group☆ (I): Concepts and Terms in 3D Image Based 3D Treatment Planning in Cervix Cancer Brachytherapy with Emphasis on MRI Assessment of GTV and CTV. Radiother. Oncol. 2005, 74, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, J.; Jiang, P. High-Dose-Rate Three-Dimensional Image-Guided Adaptive Brachytherapy (3D IGABT) for Locally Advanced Cervical Cancer (LACC): A Narrative Review on Imaging Modality and Clinical Evidence. Curr. Oncol. 2023, 31, 50–65. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-Guided Adaptive Brachytherapy in Locally Advanced Cervical Cancer (EMBRACE-I): A Multicentre Prospective Cohort Study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Perdrizet, J.; D’Souza, D.; Skliarenko, J.; Ang, M.; Barbera, L.; Gutierrez, E.; Ravi, A.; Tanderup, K.; Warde, P.; Chan, K.; et al. A Cost-Utility Analysis of Magnetic Resonance (MR) Guided Brachytherapy Versus Two-Dimensional and Computed Tomography (CT) Guided Brachytherapy for Locally Advanced Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 512–521. [Google Scholar] [CrossRef]
- Grover, S.; Harkenrider, M.M.; Cho, L.P.; Erickson, B.; Small, C.; Small, W.; Viswanathan, A.N. Image Guided Cervical Brachytherapy: 2014 Survey of the American Brachytherapy Society. Int. J. Radiat. Oncol. 2016, 94, 598–604. [Google Scholar] [CrossRef]
- Van Dyk, S.; Kondalsamy-Chennakesavan, S.; Schneider, M.; Bernshaw, D.; Narayan, K. Assessing Changes to the Brachytherapy Target for Cervical Cancer Using a Single MRI and Serial Ultrasound. Brachytherapy 2015, 14, 889–897. [Google Scholar] [CrossRef]
- Schmid, M.P.; Nesvacil, N.; Pötter, R.; Kronreif, G.; Kirisits, C. Transrectal Ultrasound for Image-Guided Adaptive Brachytherapy in Cervix Cancer—An Alternative to MRI for Target Definition? Radiother. Oncol. 2016, 120, 467–472. [Google Scholar] [CrossRef]
- Kim, N.; Park, W.; Cho, W.K.; Cho, Y.S. Clinical Outcomes after Positron Emission Tomography/Computed Tomography-based Image-guided Brachytherapy for Cervical Cancer. Asia Pac. J. Clin. Oncol. 2022, 18, 743–750. [Google Scholar] [CrossRef]
- Kissel, M.; Krhili, S.-L.; Minsat, M.; El Ayachy, R.; Bringer, S.; Lahmi, L.; Porte, J.; Labib, A.; Graff, P.; Crehange, G. Dose-Escalation in Prostate Cancer: Results of Randomized Trials. Cancer/Radiothérapie 2022, 26, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.; Rae, W.I.D.; Alber, M.L. Image-Guided Adaptive Brachytherapy Dose Escalation for Cervix Cancer via Fractionation Compensation. Brachytherapy 2017, 16, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Lucia, F.; Miranda, O.; Bourbonne, V.; Martin, E.; Pradier, O.; Schick, U. Integration of Functional Imaging in Brachytherapy. Cancer/Radiothérapie 2022, 26, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image Guided Brachytherapy in Locally Advanced Cervical Cancer: Improved Pelvic Control and Survival in RetroEMBRACE, a Multicenter Cohort Study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Schernberg, A.; Bockel, S.; Annede, P.; Fumagalli, I.; Escande, A.; Mignot, F.; Kissel, M.; Morice, P.; Bentivegna, E.; Gouy, S.; et al. Tumor Shrinkage During Chemoradiation in Locally Advanced Cervical Cancer Patients: Prognostic Significance, and Impact for Image-Guided Adaptive Brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Van Den Bergh, R.C.N.; Briers, E.; Van Den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer—2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Cervical Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Budrukkar, A.; Murthy, V.; Kashid, S.; Swain, M.; Rangarajan, V.; Laskar, S.G.; Kannan, S.; Kale, S.; Upreti, R.; Pai, P.; et al. Intensity-Modulated Radiation Therapy Alone Versus Intensity-Modulated Radiation Therapy and Brachytherapy for Early-Stage Oropharyngeal Cancers: Results From a Randomized Controlled Trial. Int. J. Radiat. Oncol. 2024, 118, 1541–1551. [Google Scholar] [CrossRef]
- Varela Cagetti, L.; Moureau-Zabotto, L.; Zemmour, C.; Ferré, M.; Giovaninni, M.; Poizat, F.; Lelong, B.; De Chaisemartin, C.; Mitry, E.; Tyran, M.; et al. The Impact of Brachytherapy Boost for Anal Canal Cancers in the Era of De-Escalation Treatments. Brachytherapy 2023, 22, 531–541. [Google Scholar] [CrossRef]
- Campbell, S.R.; Shah, C.; Scott, J.G.; Mesko, N.; Nystrom, L.; Kolar, M.; Largo, A.C.; Kamrava, M.; Mourtada, F.; Naghavi, A.O.; et al. American Brachytherapy Society (ABS) Consensus Statement for Soft-Tissue Sarcoma Brachytherapy. Brachytherapy 2021, 20, 1200–1218. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Ouhib, Z.; Kamrava, M.; Koyfman, S.A.; Campbell, S.R.; Bhatnagar, A.; Canavan, J.; Husain, Z.; Barker, C.A.; Cohen, G.N.; et al. The American Brachytherapy Society Consensus Statement for Skin Brachytherapy. Brachytherapy 2020, 19, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Small, W.; Beriwal, S.; Demanes, D.J.; Dusenbery, K.E.; Eifel, P.; Erickson, B.; Jones, E.; Rownd, J.J.; De Los Santos, J.F.; Viswanathan, A.N.; et al. American Brachytherapy Society Consensus Guidelines for Adjuvant Vaginal Cuff Brachytherapy after Hysterectomy. Brachytherapy 2012, 11, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Mulani, J.; Mittal, P.; Singh, M.; Shinde, A.; Gurram, L.; Scaria, L.; Aravindakshan, D.; Kohle, S.; Rane, P.; et al. Early Outcomes of Abbreviated Multi-Fractionated Brachytherapy Schedule for Cervix Cancer during COVID-19 Pandemic. Brachytherapy 2023, 22, 125–131. [Google Scholar] [CrossRef]
- Stevens, M.J.; Ko, F.; Martland, J.; Brown, R.; Bell, L.; Atyeo, J.; Yim, J. Safety and Efficacy of Single Insertion Accelerated MR-Image Guided Brachytherapy Following Chemo–Radiation in Locally Advanced Cervix Cancer: Modifying Our EMBRACE during the COVID Pandemic. Radiat. Oncol. 2023, 18, 54. [Google Scholar] [CrossRef]
- Hanania, A.N.; Myers, P.; Yoder, A.K.; Bulut, A.; Henry Yu, Z.; Eraj, S.; Bowers, J.; Bonnen, M.D.; Echeverria, A.; Hall, T.R.; et al. Inversely and Adaptively Planned Interstitial Brachytherapy: A Single Implant Approach. Gynecol. Oncol. 2019, 152, 353–360. [Google Scholar] [CrossRef]
- Smith, M.D.; Todd, J.G.; Symonds, R.P. Analgesia for Pelvic Brachytherapy. Br. J. Anaesth. 2002, 88, 270–276. [Google Scholar] [CrossRef]
- Locke, G.E.; Mendez, L.C.; Martell, K.; Weiss, Y.; Choi, S.; D’Alimonte, L.; Barnes, E.; Taggar, A.; Leung, E. Opioid Consumption and Pain in Patients with Gynecological Cancer Who Underwent Spinal Anesthesia vs. General Anesthesia for Interstitial Brachytherapy. Brachytherapy 2022, 21, 806–813. [Google Scholar] [CrossRef]
- Roessler, B.; Six, L.M.; Gustorff, B. Anaesthesia for Brachytherapy. Curr. Opin. Anaesthesiol. 2008, 21, 514–518. [Google Scholar] [CrossRef]
- Dicker, A.P.; Figura, A.T.; Waterman, F.M.; Valicenti, R.K.; Strup, S.E.; Gomella, L.G. Is There a Role for Antibiotic Prophylaxis in Transperineal Interstitial Permanent Prostate Brachytherapy? Tech. Urol. 2000, 6, 104–108. [Google Scholar]
- Hoffelt, S.C.; Wallner, K.; Merrick, G. Epididymitis after Prostate Brachytherapy. Urology 2004, 63, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, T.; Ohkubo, Y.; Mochida, K.; Kondo, S.; Oguchi, O.; Yoshida, D. Are Prophylactic Antibiotics Required for Combined Intracavitary and Interstitial Brachytherapy of Gynecologic Cancers? J. Radiat. Res. 2024, 65, 387–392. [Google Scholar] [CrossRef]
- Razmjoo, S.; Bagheri, A.; Shahbazian, H.; Hosseini, S.-M.; Ebrahimian-Tabrizi, F. Role of Non-Absorbable Oral Antibiotics in Bowel Preparation for Intracavitary Brachytherapy: Effects of Rifaximin on Rectal Dosimetric Parameters during Vaginal Cuff Brachytherapy. J. Contemp. Brachyther. 2021, 13, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Chen, R. The DecisionDx-UM Gene Expression Profile Test Provides Risk Stratification and Individualized Patient Care in Uveal Melanoma. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Chevli, N.; Zuhour, R.J.; Messer, J.A.; Haque, W.; Schefler, A.C.; Bernicker, E.H.; Chevez-Barrios, P.; Farach, A.M.; Butler, E.B.; Teh, B.S. Contemporary Trends in Management of Uveal Melanoma. J. Contemp. Brachytherapy 2022, 14, 123–129. [Google Scholar] [CrossRef]
- Messer, J.A.; Zuhour, R.J.; Haque, W.; Lewis, G.D.; Schefler, A.C.; Wong, A.; Bernicker, E.H.; Chevez-Barrios, P.; Quan, E.M.; Farach, A.; et al. Eye Plaque Brachytherapy versus Enucleation for Ocular Melanoma: An Analysis from the National Cancer Database. J. Contemp. Brachyther. 2020, 12, 303–310. [Google Scholar] [CrossRef]
- Vu, C.C.; Blas, K.G.; Lanni, T.B.; Gustafson, G.S.; Krauss, D.J. Cost-Effectiveness of Prostate Boost with High-Dose-Rate Brachytherapy versus Intensity-Modulated Radiation Therapy in the Treatment of Intermediate-High Risk Prostate Cancer. Brachytherapy 2018, 17, 852–857. [Google Scholar] [CrossRef]
- Andring, L.M.; Teh, B.S.; Butler, E.B.; Farach, A.M. Focal versus Whole Gland Salvage Brachytherapy for Recurrent Prostate Cancer in the Prostate Specific Membrane Antigen Era: A Narrative Review. Chin. Clin. Oncol. 2023, 12, 26. [Google Scholar] [CrossRef]
- Larionova, I.; Rakina, M.; Ivanyuk, E.; Trushchuk, Y.; Chernyshova, A.; Denisov, E. Radiotherapy Resistance: Identifying Universal Biomarkers for Various Human Cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 1015–1031. [Google Scholar] [CrossRef]
- Escande, A.; Haie-Meder, C.; Maroun, P.; Gouy, S.; Mazeron, R.; Leroy, T.; Bentivegna, E.; Morice, P.; Deutsch, E.; Chargari, C. Neutrophilia in Locally Advanced Cervical Cancer: A Novel Biomarker for Image-Guided Adaptive Brachytherapy? Oncotarget 2016, 7, 74886–74894. [Google Scholar] [CrossRef]
- Moreno-Acosta, P.; Vallard, A.; Carrillo, S.; Gamboa, O.; Romero-Rojas, A.; Molano, M.; Acosta, J.; Mayorga, D.; Rancoule, C.; Garcia, M.A.; et al. Biomarkers of Resistance to Radiation Therapy: A Prospective Study in Cervical Carcinoma. Radiat. Oncol. 2017, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Ecke, T.; Huang-Tiel, H.-J.; Golka, K.; Selinski, S.; Geis, B.; Koswig, S.; Bathe, K.; Hallmann, S.; Gerullis, H. Prostate Specific Antigen (PSA) as Predicting Marker for Clinical Outcome and Evaluation of Early Toxicity Rate after High-Dose Rate Brachytherapy (HDR-BT) in Combination with Additional External Beam Radiation Therapy (EBRT) for High Risk Prostate Cancer. Int. J. Mol. Sci. 2016, 17, 1879. [Google Scholar] [CrossRef] [PubMed]
- Fijuth, J. Educational Corner Brachytherapy in Paediatric Malignancies—Review of Indications. J. Contemp. Brachyther. 2010, 2, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, N.; Musunuru, H.B.; Keller, A.; Beriwal, S. Feasibility of Breast Radiation Therapy in a Fanconi Anemia Patient Diagnosed with Breast Cancer: A Case Report and Review of Literature. Clin. Transl. Radiat. Oncol. 2021, 28, 129–132. [Google Scholar] [CrossRef]
- Eschrich, S.A.; Pramana, J.; Zhang, H.; Zhao, H.; Boulware, D.; Lee, J.-H.; Bloom, G.; Rocha-Lima, C.; Kelley, S.; Calvin, D.P.; et al. A Gene Expression Model of Intrinsic Tumor Radiosensitivity: Prediction of Response and Prognosis After Chemoradiation. Int. J. Radiat. Oncol. 2009, 75, 489–496. [Google Scholar] [CrossRef]
- Scott, J.G.; Sedor, G.; Ellsworth, P.; Scarborough, J.A.; Ahmed, K.A.; Oliver, D.E.; Eschrich, S.A.; Kattan, M.W.; Torres-Roca, J.F. Pan-Cancer Prediction of Radiotherapy Benefit Using Genomic-Adjusted Radiation Dose (GARD): A Cohort-Based Pooled Analysis. Lancet Oncol. 2021, 22, 1221–1229. [Google Scholar] [CrossRef]
- Chiang, C.L.; Chan, K.S.K.; Li, H.; Ng, W.T.; Chow, J.C.H.; Choi, H.C.W.; Lam, K.O.; Lee, V.H.F.; Ngan, R.K.C.; Lee, A.W.M.; et al. Using the Genomic Adjusted Radiation Dose (GARD) to Personalize the Radiation Dose in Nasopharyngeal Cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2024, 196, 110287. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.O.S.; Horn, S.; Brady, D.; McMahon, S.J.; Yoosuf, A.B.M.; Mitchell, D.; Crowther, K.; Lyons, C.A.; Hounsell, A.R.; Prise, K.M.; et al. Prostate Cancer Treated with Brachytherapy; an Exploratory Study of Dose-Dependent Biomarkers and Quality of Life. Radiat. Oncol. 2017, 12, 53. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Ni, R.; Chow, R.; Rink, A.; Weersink, R.; Croke, J.; Raman, S. Artificial Intelligence Applications in Brachytherapy: A Literature Review. Brachytherapy 2023, 22, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Albuquerque, K. Artificial Intelligence and Deep Learning for Brachytherapy. Semin. Radiat. Oncol. 2022, 32, 389–399. [Google Scholar] [CrossRef]
- Orlando, N.; Gyacskov, I.; Gillies, D.J.; Guo, F.; Romagnoli, C.; D’Souza, D.; Cool, D.W.; Hoover, D.A.; Fenster, A. Effect of Dataset Size, Image Quality, and Image Type on Deep Learning-Based Automatic Prostate Segmentation in 3D Ultrasound. Phys. Med. Biol. 2022, 67, 074002. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Gonzalez, Y.; Shen, C.; Klages, P.; Albuquerque, K.; Jia, X. Deep-Learning-Assisted Automatic Digitization of Applicators in 3D CT Image-Based High-Dose-Rate Brachytherapy of Gynecological Cancer. Brachytherapy 2019, 18, 841–851. [Google Scholar] [CrossRef]
- Akhavanallaf, A.; Mohammadi, R.; Shiri, I.; Salimi, Y.; Arabi, H.; Zaidi, H. Personalized Brachytherapy Dose Reconstruction Using Deep Learning. Comput. Biol. Med. 2021, 136, 104755. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Pineau, J.; Keyes, R.; Enger, S.A. RapidBrachyDL: Rapid Radiation Dose Calculations in Brachytherapy Via Deep Learning. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 802–812. [Google Scholar] [CrossRef]
- Shen, C.; Gonzalez, Y.; Klages, P.; Qin, N.; Jung, H.; Chen, L.; Nguyen, D.; Jiang, S.B.; Jia, X. Intelligent Inverse Treatment Planning via Deep Reinforcement Learning, a Proof-of-Principle Study in High Dose-Rate Brachytherapy for Cervical Cancer. Phys. Med. Biol. 2019, 64, 115013. [Google Scholar] [CrossRef]
- Fan, J.; Xing, L.; Yang, Y. Independent Verification of Brachytherapy Treatment Plan by Using Deep Learning Inference Modeling. Phys. Med. Biol. 2021, 66, 125014. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Chen, J.; Zhong, Z.; Hrycushko, B.; Zhou, L.; Jiang, S.; Albuquerque, K.; Gu, X. Deep Convolutional Neural Network with Transfer Learning for Rectum Toxicity Prediction in Cervical Cancer Radiotherapy: A Feasibility Study. Phys. Med. Biol. 2017, 62, 8246–8263. [Google Scholar] [CrossRef]
- Han, D.Y.; Webster, M.J.; Scanderbeg, D.J.; Yashar, C.; Choi, D.; Song, B.; Devic, S.; Ravi, A.; Song, W.Y. Direction-Modulated Brachytherapy for High-Dose-Rate Treatment of Cervical Cancer. I: Theoretical Design. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 666–673. [Google Scholar] [CrossRef]
- Callaghan, C.M.; Adams, Q.; Flynn, R.T.; Wu, X.; Xu, W.; Kim, Y. Systematic Review of Intensity-Modulated Brachytherapy (IMBT): Static and Dynamic Techniques. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 206–221. [Google Scholar] [CrossRef]
- Poon, E.; Williamson, J.F.; Vuong, T.; Verhaegen, F. Patient-Specific Monte Carlo Dose Calculations for High-Dose-Rate Endorectal Brachytherapy with Shielded Intracavitary Applicator. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1259–1266. [Google Scholar] [CrossRef]
- Yang, W.; Kim, Y.; Wu, X.; Song, Q.; Liu, Y.; Bhatia, S.K.; Sun, W.; Flynn, R.T. Rotating-Shield Brachytherapy for Cervical Cancer. Phys. Med. Biol. 2013, 58, 3931–3941. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, H.; Kim, Y.; Wu, X.; Flynn, R.T. Multihelix Rotating Shield Brachytherapy for Cervical Cancer. Med. Phys. 2015, 42, 6579–6588. [Google Scholar] [CrossRef] [PubMed]
- Adams, Q.E.; Xu, J.; Breitbach, E.K.; Li, X.; Enger, S.A.; Rockey, W.R.; Kim, Y.; Wu, X.; Flynn, R.T. Interstitial Rotating Shield Brachytherapy for Prostate Cancer. Med. Phys. 2014, 41, 051703. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Baniel, C.C.; Sriramaneni, R.N.; Bradley, K.; Markovina, S.; Morris, Z.S. Combining Brachytherapy and Immunotherapy to Achieve in Situ Tumor Vaccination: A Review of Cooperative Mechanisms and Clinical Opportunities. Brachytherapy 2018, 17, 995–1003. [Google Scholar] [CrossRef]
- Hodge, J.W.; Sharp, H.J.; Gameiro, S.R. Abscopal Regression of Antigen Disparate Tumors by Antigen Cascade after Systemic Tumor Vaccination in Combination with Local Tumor Radiation. Cancer Biother. Radiopharm. 2012, 27, 12–22. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Barbes, B.; Mayorga, L.; Sanchez-Paulete, A.R.; Ponz-Sarvise, M.; Pérez-Gracia, J.L.; Melero, I. Brachytherapy Attains Abscopal Effects When Combined with Immunostimulatory Monoclonal Antibodies. Brachytherapy 2017, 16, 1246–1251. [Google Scholar] [CrossRef]
- Patel, A.; Orakwue, C.W.; Olek, D.; Guzman, J.C.A.; Lim, K.; Pino, R.; Teh, B.S.; Butler, B.; Satkunasivam, R.; Farach, A. A Feasibility Study of Utilizing a Cadaveric Training Model for Novel Robotic Bladder Cancer Brachytherapy Techniques. Brachytherapy 2023, 22, 195–198. [Google Scholar] [CrossRef]
- Tan, L.-T.; Tanderup, K.; Kirisits, C.; Mahantshetty, U.; Swamidas, J.; Jürgenliemk-Schulz, I.; Lindegaard, J.; de Leeuw, A.; Nesvacil, N.; Assenholt, M.; et al. Education and Training for Image-Guided Adaptive Brachytherapy for Cervix Cancer-The (GEC)-ESTRO/EMBRACE Perspective. Brachytherapy 2020, 19, 827–836. [Google Scholar] [CrossRef]
- Petereit, D.G. Increasing Global Access to Brachytherapy: The ABS 300 in 10 Initiative and Ongoing International Efforts. Brachytherapy 2022, 21, 1–3. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathak, P.; Thomas, J.J.; Baghwala, A.; Li, C.; Teh, B.S.; Butler, E.B.; Farach, A.M. Personalized Brachytherapy: Applications and Future Directions. Cancers 2024, 16, 3424. https://doi.org/10.3390/cancers16193424
Pathak P, Thomas JJ, Baghwala A, Li C, Teh BS, Butler EB, Farach AM. Personalized Brachytherapy: Applications and Future Directions. Cancers. 2024; 16(19):3424. https://doi.org/10.3390/cancers16193424
Chicago/Turabian StylePathak, Piyush, Justin J. Thomas, Arjit Baghwala, Chengfeng Li, Bin S. Teh, Edward B. Butler, and Andrew M. Farach. 2024. "Personalized Brachytherapy: Applications and Future Directions" Cancers 16, no. 19: 3424. https://doi.org/10.3390/cancers16193424
APA StylePathak, P., Thomas, J. J., Baghwala, A., Li, C., Teh, B. S., Butler, E. B., & Farach, A. M. (2024). Personalized Brachytherapy: Applications and Future Directions. Cancers, 16(19), 3424. https://doi.org/10.3390/cancers16193424




