Simple Summary
Established in 2002, the Korean Gynecologic Oncology Group (KGOG) has presented improved clinical outcomes based on multi-center clinical trials. To date, KGOG has approved 156 studies and published 68 KGOG-led studies. The organization aims to advance gynecologic cancer research through sustained efforts and international collaboration.
Abstract
The Korean Gynecologic Oncology Group (KGOG) was established in 2002 and is the only organization in Korea conducting multi-center clinical trials for gynecologic cancers. Since its re-establishment as a non-profit organization in 2021, KGOG has grown significantly, now including 207 gynecologic oncology specialists from 76 hospitals. This growth is a testament to the dedication and hard work of all those involved in the organization. KGOG is committed to maximizing the activation of multi-center clinical research through policies that support patients with rare diseases and gynecologic cancer research, focusing on strengthening institutional capacity, equalizing participation opportunities, and enhancing information sharing. A significant milestone for KGOG was becoming a member of the US Gynecologic Oncology Group (GOG) in 2005, allowing participation in GOG clinical trials. KGOG later joined the Gynecologic Cancer InterGroup (GCIG) and strengthened its capabilities by hosting the first Endometrial Cancer Consensus Conference—Clinical Research (ECCC-CR) in 2023. KGOG holds biannual meetings and symposia, as well as 224 operating committee meetings annually to review the discussions of the Tumor Site Committee. KGOG has conducted 156 investigator-initiated trial (IIT) or sponsor-initiated trial (SIT) studies as KGOG-led or participated in research. Currently, 18 studies are registered, and 10 are in preparation. To date, 68 papers have been published. KGOG conducts six national projects and collaborates with external organizations such as the NRG Oncology Foundation, Gynecologic Oncology Group Partners (GOG-P), GCIG, East Asian Gynecologic Oncology Trial group (EAGOT), and the Japanese Gynecologic Oncology Group (JGOG). Through collaboration with renowned international research institutions, KGOG has significantly expanded the scope of its research, achieving noteworthy clinical outcomes. This report not only introduces the history and recent status of KGOG but also presents the exciting future direction of the organization, filled with potential breakthroughs and advancements in gynecologic oncology research.
1. Introduction
In the fall of 2002, the KGOG was established by leading figures in the field of gynecologic oncology in Korea, with the support of the Korean Society of Gynecologic Oncology (KSGO). This initiative aimed to model itself after advanced multi-institutional clinical trial organizations, such as the Gynecologic Oncology Group (GOG) in the United States, to conduct multi-institutional clinical trials. Over the past 20 years, KGOG has built upon the successes and failures of these trials to establish itself as a successful clinical research organization, continuing its efforts to make significant contributions to the field.
This paper aims to present KGOG’s history and research achievements and propose strategies and directions for its future development.
2. History of KGOG
2.1. The Beginning of KGOG
In October 2002, during the 9th International Gynecologic Cancer Society (IGCS) Meeting held in Seoul concurrently with the Korea–Japan Gynecologic Cancer Joint Meeting (KJGCJM), several professors from the KSGO recognized the need for a clinical research organization to lead clinical trials in gynecologic oncology in Korea. They made multifaceted efforts to overcome the surrounding opposition to data sharing and the immature era of clinical research. Consequently, the KGOG was established in October 2002 as an organization dedicated to clinical trials for gynecologic cancers in Korea [1,2].
In January 2003, the first operating committee meeting was held, where the KGOG preparatory committee was formed, drafting the KGOG bylaws, and discussions on clinical trial protocols began. Based on this, the first research committee was convened. The first KGOG workshop was held in August 2005. KGOG holds biannual meetings (spring and autumn) and two symposiums/workshops annually. In its initial stages, KGOG comprised the president, research committee, and operating committee, with advisory subcommittees for cervical cancer, ovarian cancer, endometrial cancer, pathology, radiation therapy, medical oncology, and translational research.
2.2. Research Achievements of KGOG
The classification number of the KGOG protocols was determined by organ to be a four-digit number starting with 1 for the cervix (KGOG 1XXX), 2 for the uterine body (KGOG 2XXX), and 3 for the ovary (KGOG 3XXX). The protocol for Surgery and Developmental Diagnostic and Therapeutics (DDT) was classified as 4 (KGOG 4XXX). In August 2004, the first KGOG protocol, KGOG 1001 (A Phase II Trial of Radiation Therapy with Concurrent Paclitaxel/Carboplatin Chemotherapy in High-risk Cervical Cancer Patients after Radical Hysterectomy), was initiated, and the study was conducted until 2010. The study was published in June 2013 [3]. Subsequently, the KGOG 3001 (An Open label, Single arm and Multi-center Phase II Clinical Trial of Gemcitabine Triplet [Paclitaxel + Carboplatin + Gemcitabine] as Consolidation Chemotherapy in Patients with Advanced Epithelial Ovarian Cancer) and KGOG 2001 (A Phase II Trial of Radiation Therapy with Concurrent Paclitaxel Chemotherapy in High-risk Endometrial Cancer Patients after Operation) protocols began in July and August 2005, respectively. The KGOG 2001 study was published in September 2014 [4]. The first study published by the Ovary–Fallopian tube Tumor Site Committees was KGOG 3003, a retrospective study of clear cell carcinoma of the ovary [5]. To date, 156 KGOG protocols have been developed and carried out. KGOG-led and -participated clinical trials are summarized in Table 1. Among them, the list of studies conducted and published by researchers affiliated with KGOG over the past five years is summarized in Table 2.

Table 1.
Summary of KGOG-led and -participated clinical trials.

Table 2.
Summary of studies conducted and published by KGOG.
2.3. Interaction with Other International Research Organizations
Since its establishment, the KGOG has collaborated with prominent international institutions leading multi-center research, such as the GOG Legacy and NRG in the United States, European Network for Gynaecological Oncological Trial group (ENGOT) in Europe, and the Gynecologic Cancer InterGroup (GCIG). As a member of these collaborative research efforts, KGOG has significantly contributed to international joint studies. KGOG became an associate member of the GOG in July 2005. After the KGOG-NCI-US Embassy Cooperation Meeting in April 2007, KGOG signed an agreement with GOG to conduct joint research and clinical trials. The first KGOG meeting was held in January 2008 during the GOG semi-annual meeting in San Diego, USA. Since then, KGOG has been participating in the GOG meetings twice a year as a full member and continues to cooperate closely with NRG, the successor of GOG.
KGOG became a full member of GCIG in October 2007 and has since participated in numerous studies. In November 2023, KGOG hosted the first Endometrial Cancer Consensus Conference—Clinical Research (ECCC-CR) in Songdo, Korea, alongside the KGOG semi-annual meeting, focusing on clinical trial guidelines for endometrial cancer [30].
Additionally, KGOG has maintained early interactions with Japan through the Japanese Gynecologic Oncology Group (JGOG) and KJGCJM. The first KGOG–JGOG collaboration meeting was held in April 2016. Since then, KGOG and JGOG have developed an advanced cooperative relationship, holding biannual face-to-face meetings to develop joint protocols [31]. Since 2012, KGOG has also been holding regular meetings with the Shanghai Gynecologic Oncology Group (SGOG) through the KGOG–SGOG meetings [32].
Recently, KGOG, along with JGOG, the Chinese Gynecological Cancer Society (CGCS), and the Taiwan Gynecologic Oncology Group (TGOG), established the East Asian Gynecologic Oncology Trial Group (EAGOT) in November 2021. EAGOT aims to develop clinical trial protocols for gynecologic cancers in East Asian women and expand research exchange among East Asian countries, with KGOG leading these efforts [33,34].
3. Current KGOG
3.1. Mission and Objectives of KGOG
The mission of the KGOG is three-fold as follows: First, to advance gynecologic oncology through collaborative multi-institutional research. This involves conducting and supporting domestic and international multi-center clinical trials related to the diagnosis, surgery, chemotherapy, and radiotherapy of gynecologic cancers. Second, to contribute to public health by developing new chemotherapy protocols and surgical techniques. This goal is furthered through the education and training of clinical trial experts in gynecologic cancer, enhancing the overall quality of care and treatment outcomes. Third, rational clinical trial policies should be proposed. By engaging in policy research, KGOG aims to shape effective clinical trial policies that reflect the latest advancements and best practices in gynecologic oncology. To achieve its mission, KGOG undertakes the following activities: (1) It develops, conducts, and exchanges information on multicenter clinical trial protocols for gynecologic cancer, ensuring standardized and collaborative research efforts across institutions. (2) It also participates in policy research to formulate and refine clinical trial policies, ensuring they are evidence-based and effective. (3) It is strengthening cooperation and exchanges with domestic and international institutions related to gynecologic cancer research. This fosters a global network of collaboration and information sharing. (4) Educating and training experts involved in gynecologic cancer research. This commitment ensures high expertise and competence among clinical trial professionals. (5) We promote and enlighten gynecologic cancer research to raise awareness and encourage investment in this critical field. (6) This engages in other activities deemed necessary by KGOG to fulfill its mission and achieve its objectives.
3.2. Structure of KGOG
KGOG has expanded to its current structure over approximately 20 years, passing through the leadership of five presidents: Professor Soon-Beom Kang (2002–2010), Professor Joo-Hyun Nam (2010–2014), Professor Byung-Gie Kim (2014–2016), Professor Yong-Man Kim (2016–2021), and Professor Jae-Hoon Kim (2021–present). The current organizational chart of KGOG is shown in Figure 1. In 2024, KGOG consisted of 4 Tumor Site Committees (Cervix, Vulva, Vagina; Uterine Corpus-GTT; Ovary–Fallopian tube; Rare Tumor), 3 Treatment Modality Committees (Surgery; Chemotherapy; Radiotherapy), and 14 other committees (Advisory; Operating; Steering; Protocol; Statistics and Data; International Collaboration; Publication; Audit; DSMB; Cancer Prevention and Control; Developmental Diagnostic and Therapeutic; Symptom Benefits; Pathology; Translational Research), and 2 supportive departments (SRB and Biobank). The operating Committee reviews the progress of KGOG research every month, while the Steering Committee is an ad hoc committee that primarily handles financial and administrative matters.
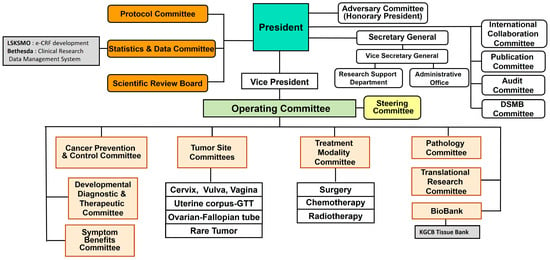
Figure 1.
Structure of KGOG.
3.3. Development Process of New Protocols
The researcher submits a summary-formatted research proposal to the Tumor Site Committee for initial review. If the study is deemed to have clinical value and is suitable for KGOG, the Tumor Site Committee chair submits the proposal to the Scientific Review Board (SRB) for a detailed secondary review. Comprising five internal and external reviewers plus one chair, the SRB assesses the rationality, feasibility, and ethical considerations of all clinical trials to be conducted by KGOG. Upon SRB approval, the principal investigator will present the proposal to the Operating Committee based on the discussions that took place within the Tumor Site Committee and SRB, and the Operating Committee attendees will discuss whether to proceed with the study. If more than two-thirds of attendees agree during the second review, the study is approved as a KGOG protocol. If not approved, decisions may include re-review, deferral, or rejection. Once the Protocol Committee approves the full protocol, the principal investigator drafts a research contract with KGOG and obtains a research number through the KGOG Secretariat to recruit participating institutions. The development process of new protocols is detailed in Figure 2.
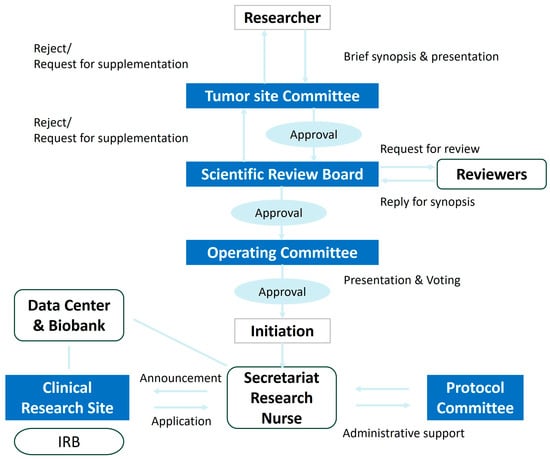
Figure 2.
Flow-chart of development of a new protocol in KGOG.
As of January 2024, KGOG is composed of a comprehensive national network of 76 institutions and hospitals, with a total of 207 gynecological oncologists, statisticians, radiation oncologists, pathologists, and medical oncologists as members (Supplementary Figure S1). In proportion to the population and number of patients, most hospitals are located in the metropolitan area, but for this reason, patients have high access to hospitals and can be centrally concentrated, so KGOG covers not only large-scale studies such as Phase III but also small-scale studies. The clinical trials actively conducted by KGOG in 2024 are summarized in Table 3.

Table 3.
Ongoing clinical trials by KGOG in 2024.
3.4. Partnership between GOG-P and KGOG
In 2014, GOG integrated with the National Surgical Adjuvant Breast and Bowel Project (NSABP) and the Radiation Therapy Oncology Group (RTOG) to form NRG Oncology. As a result, since 2011, Phase 3 studies on gynecologic oncology have significantly decreased in the United States, and KGOG has faced many limitations in newly participating in NRG studies since the mid-2010s. To overcome these challenges, GOG established the GOG Foundation in 2017, consisting of GOG Partners and the NRG Oncology gynecologic program [35]. After much discussion, the KGOG–GOG collaboration agreement was signed in October 2023, allowing KGOG to participate in GOG Partners studies. This has expanded opportunities for KGOG to participate in major international clinical trials, focusing on SIT. The new study initiation process is displayed in Figure 3, with a legal review conducted by both institutions.
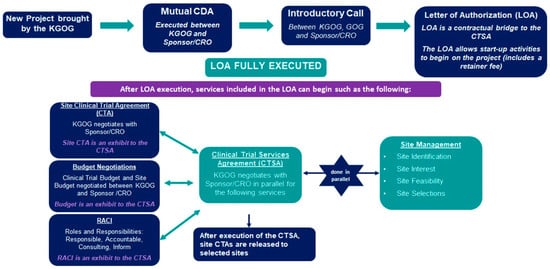
Figure 3.
New study initiation process between GOG-P and KGOG. CDA: Confidential Disclosure Agreement; CRO: Contract Research Organization; CTSA: Clinical Trial Services Agreement; GOG: Gynecologic Oncology Group; KGOG: Korean Gynecologic Oncology Group; RACI: Responsible, Accountable, Consulting, Inform.
Upon receiving the Letter of Authorization (LOA), start-up activities for the project begin, acting as a contractual bridge to the CTSA (Clinical Trial Site Agreement). After LOA execution, negotiations for the Site Clinical Trial Agreement (CTA), budget, and RACI (Roles and Responsibilities) are conducted in parallel with KGOG and the sponsor/CRO through the CTSA. Currently, five study protocols are in the new study initiation process. By establishing a partnership with GOG Partners, information about Asian gynecologic cancer patients will be reflected in new drug information, enabling the selection of more appropriate treatment options. Furthermore, through continuous development, KGOG will be able to keep pace with global trends in the field of gynecologic cancer clinical research.
The KGOG is dedicated to advancing gynecologic cancer research and treatment through core services such as site selection assistance, financial management, investigator meetings, and effective communication. These services ensure efficient study execution, transparent financial transactions, and robust collaboration among researchers. KGOG aims to transform the standard of care in gynecologic oncology through close collaboration with researchers and research organizations both in Korea and worldwide. We are committed to advancing investigator-initiated trials based on national grants and strengthening our partnership with research organizations, led by GOG-P to expand sponsor-initiated studies. By scientifically conducting clinical trials proposed by individual researchers or sponsors, we aim to elevate the standards of gynecologic cancer care.
3.5. Challenges of KGOG in the Future
Over the past 20 years, KGOG has achieved many accomplishments in the field of clinical trials and treatments for gynecologic cancers. However, it is true that there are still several challenges to overcome. First, while the government is streamlining regulatory processes to stimulate the medical market, it has not yet fully met the enthusiasm of researchers and patients for clinical trials. Second, although the patient recruitment rate is higher in Korea compared to the U.S. or Western Europe where clinical trials are more active, the relatively smaller population in Korea can make it difficult for multi-national pharmaceutical companies or contract research organizations (CROs) to prioritize Korea for large-scale, multi-center trials. Lastly, since Korea is a non-English-speaking country, language and cultural barriers must be addressed. All clinical trial documents, including patient consent forms, must be translated into Korean, and professional translators need to be hired to ensure the accuracy and quality of these translations. Therefore, to successfully conduct clinical research in Korea, it is crucial to gain knowledge about the factors that influence clinical research and the methods that can be used to overcome these challenges.
4. Conclusions
As mentioned above, KGOG has grown and developed significantly on its own and with international exchanges over the past 20 years. In the future, KGOG plans to develop KGOG-led investigator-initiated trials in quantity and quality to lead the clinical trials for new drug discovery and development, which have been rapidly growing recently. Additionally, based on solid relationships with international research organizations, KGOG will establish “Global Standards” for gynecologic oncology research and strive to promote gynecologic cancer research beyond East Asia to a global scale through exchanges with GOG-P, ENGOT, and EAGOT.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16193422/s1, Figure S1: Distribution of KGOG-Participant Institutions.
Author Contributions
Conceptualization, K.-J.M., N.K.K. and J.-H.K.; methodology, J.-Y.S., M.C.C., S.W.L., K.H.L., M.K.K., S.K., C.H.C., J.-W.L., E.-J.L., K.-Y.E., S.W.K., H.C., S.J.L., M.C.L., J.B., C.W.Y., K.K. and D.-Y.K.; validation, C.L., S.Y.R., S.J., J.-W.K., B.-H.N., S.-B.K., K.T.K., J.-H.N., B.-G.K. and Y.-M.K.; formal analysis, K.-J.M., N.K.K. and J.-H.K.; resources, J.-Y.S. and J.-H.K.; data curation, K.-J.M., N.K.K. and J.-H.K.; writing—original draft preparation, K.-J.M., N.K.K. and J.-H.K.; writing—review and editing, K.-J.M., N.K.K., J.-Y.S., M.C.C., S.W.L., K.H.L., M.K.K., S.K., C.H.C., J.-W.L., E.-J.L., K.-Y.E., S.W.K., H.C., S.J.L., M.C.L., J.B., C.W.Y., K.K., D.-Y.K. and J.-H.K.; visualization, K.-J.M., N.K.K. and J.-H.K.; supervision, S.-B.K., K.T.K., J.-H.N., B.-G.K. and Y.-M.K.; project administration, J.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant under the National Cancer Center of the Republic of Korea (No.: RS-2024-00360954).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author Byung-Ho Nam is the founder and CEO of company Herings. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Chang, K.H.; Kim, J.W. The Journal of Gynecologic Oncology and the Asian Society of Gynecologic Oncology: The history, the present and the future. J. Gynecol. Oncol. 2012, 23, 1–2. [Google Scholar] [CrossRef][Green Version]
- Ryu, H.S. Asian Society of Gynecologic Oncology (ASGO): A new society for doctors working against gynecologic cancers in Asia. Gynecol. Oncol. 2009, 112, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Kang, S.B.; Kim, Y.T.; Park, B.J.; Kim, Y.M.; Lee, J.M.; Kim, S.M.; Kim, Y.T.; Kim, J.H.; Kim, K.T. Chemoradiation with paclitaxel and carboplatin in high-risk cervical cancer patients after radical hysterectomy: A Korean Gynecologic Oncology Group study. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Nam, B.H.; Kim, S.M.; Cho, C.H.; Kim, B.G.; Ryu, H.S.; Kang, S.B.; Kim, J.H. A phase 2 trial of radiation therapy with concurrent paclitaxel chemotherapy after surgery in patients with high-risk endometrial cancer: A Korean Gynecologic Oncologic Group study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 140–146. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Park, S.I.; Nam, B.H.; Kim, I.; Yoo, C.W.; Nam, J.H.; Lee, K.H.; Cho, C.H.; Kim, J.H.; Park, S.Y.; et al. Prognostic significance of histological grade in clear-cell carcinoma of the ovary: A retrospective study of Korean Gynecologic Oncology Group. Ann. Oncol. 2009, 20, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Chung, Y.S.; Lee, J.W.; Kwon, B.S.; Park, B.K.; Kim, S.I.; Shim, S.H.; Lee, K.B.; Seong, S.J.; Lee, S.J.; et al. Feasibility and efficacy of gonadotropin-releasing hormone agonists for the prevention of chemotherapy-induced ovarian insufficiency in patients with malignant ovarian germ cell tumours (KGOG 3048R). Eur. J. Cancer 2020, 133, 56–65. [Google Scholar] [CrossRef]
- Kim, M.K.; Seong, S.J.; Park, D.C.; Hong, J.H.; Roh, J.W.; Kang, S.B. Comparison of diagnostic accuracy between endometrial curettage and aspiration biopsy in patients treated with progestin for endometrial hyperplasia: A Korean Gynecologic Oncology Group study. J. Gynecol. Oncol. 2020, 31, e51. [Google Scholar] [CrossRef]
- Paik, E.S.; Lim, M.C.; Kim, M.H.; Kim, Y.H.; Song, E.S.; Seong, S.J.; Suh, D.H.; Lee, J.M.; Lee, C.; Choi, C.H. Prognostic Model for Survival and Recurrence in Patients with Early-Stage Cervical Cancer: A Korean Gynecologic Oncology Group Study (KGOG 1028). Cancer Res. Treat. 2020, 52, 320–333. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, J.W.; Kim, K.; Lee, M.; Yoo, J.; Choi, M.C.; Hwangbo, S.; Kwak, Y.H.; Lee, J.M.; Shin, S.J.; et al. Comparisons of survival outcomes between bevacizumab and olaparib in BRCA-mutated, platinum-sensitive relapsed ovarian cancer: A Korean Gynecologic Oncology Group study (KGOG 3052). J. Gynecol. Oncol. 2021, 32, e90. [Google Scholar] [CrossRef]
- Ouh, Y.T.; Park, J.J.; Kang, M.; Kim, M.; Song, J.Y.; Shin, S.J.; Shim, S.H.; Yoo, H.J.; Lee, M.; Lee, S.J.; et al. Discrepancy between Cytology and Histology in Cervical Cancer Screening: A Multicenter Retrospective Study (KGOG 1040). J. Korean Med. Sci. 2021, 36, e164. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Kim, S.C.; Kim, Y.B.; Nam, B.H.; No, J.H.; Cho, H.; Ju, W.; Suh, D.H.; Kim, Y.H. Modulated electro-hyperthermia with weekly paclitaxel or cisplatin in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: The KGOG 3030 trial. Exp. Ther. Med. 2021, 22, 787. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Seol, A.; Lee, M.; Kim, J.W.; Kim, H.S.; Kim, K.; Suh, D.H.; Kim, S.; Kim, S.W.; Lee, J.Y. A Phase II Trial to Evaluate the Efficacy of Bortezomib and Liposomal Doxorubicin in Patients With BRCA Wild-type Platinum-resistant Recurrent Ovarian Cancer (KGOG 3044/EBLIN). In Vivo 2022, 36, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Sa, J.K.; Kim, J.; Kang, S.; Kim, S.W.; Song, T.; Shim, S.H.; Choi, M.C.; No, J.H.; Song, J.Y.; Kim, D.; et al. Somatic genomic landscape of East Asian epithelial ovarian carcinoma and its clinical implications from prospective clinical sequencing: A Korean Gynecologic Oncology Group study (KGOG 3047). Int. J. Cancer 2022, 151, 1086–1097. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, B.G.; Kim, J.W.; Lee, J.B.; Park, E.; Joung, J.G.; Kim, S.; Choi, C.H.; Kim, H.S.; Korean Gynecologic Oncology Group, i. Biomarker-guided targeted therapy in platinum-resistant ovarian cancer (AMBITION.; KGOG 3045): A multicentre, open-label, five-arm, uncontrolled, umbrella trial. J. Gynecol. Oncol. 2022, 33, e45. [Google Scholar] [CrossRef]
- Park, J.; Lim, M.C.; Lee, J.K.; Jeong, D.H.; Kim, S.I.; Choi, M.C.; Kim, B.G.; Lee, J.Y. A single-arm, phase II study of niraparib and bevacizumab maintenance therapy in platinum-sensitive, recurrent ovarian cancer patients previously treated with a PARP inhibitor: Korean Gynecologic Oncology Group (KGOG 3056)/NIRVANA-R trial. J. Gynecol. Oncol. 2022, 33, e12. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.H.; Baek, M.H.; Park, E.; Kim, S.W. Randomized comparison between sentinel lymph node mapping using indocyanine green plus a fluorescent camera versus lymph node dissection in clinical stage I-II endometrial cancer: A Korean Gynecologic Oncology Group trial (KGOG2029/SELYE). J. Gynecol. Oncol. 2022, 33, e73. [Google Scholar] [CrossRef]
- Shin, W.; Park, S.Y.; Seo, S.S.; Lim, M.C.; Kim, J.Y.; Kang, S. Predicting the risk of the distant recurrence of cervical cancer after concurrent chemoradiation: A validation study of the Korean Gynecologic Oncologic Group (KGOG)-1024 model. Gynecol. Oncol. 2022, 164, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Aiob, A.; Kim, K.; Lee, N.W. Rate of occult atypical hyperplasia or endometrial cancer in women of older age groups with nonatypical endometrial hyperplasia (KGOG 2026). J. Obstet. Gynaecol. Res. 2023, 49, 2979–2980. [Google Scholar] [CrossRef]
- Lee, S.U.; Kim, J.Y.; Kim, M.K.; Kim, Y.S.; Kim, Y.J.; Eom, K.Y.; Wee, C.W. Pattern of practice for postoperative management of endometrial cancer in Korea: A survey by the Korean Gynecologic Oncology Group and the Korean Radiation Oncology Group (KGOG 2028-KROG 2104). J. Gynecol. Oncol. 2023, 34, e54. [Google Scholar] [CrossRef]
- Kim, Y.N.; Joung, J.G.; Park, E.; Kim, J.W.; Lee, J.B.; Lim, J.; Kim, S.; Choi, C.H.; Kim, H.S.; Chung, J.; et al. Randomized, two-arm, noncomparative phase 2 study of olaparib plus cediranib or durvalumab in HRR-mutated, platinum-resistant ovarian cancer: A substudy of KGOG 3045. Int. J. Cancer 2023, 153, 2032–2044. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.B.; Lim, M.C.; Kim, B.G.; Kim, J.W.; Kim, S.; Choi, C.H.; Kim, H.S.; Park, S.Y.; Lee, J.Y.; et al. Phase II study of durvalumab and tremelimumab with front-line neoadjuvant chemotherapy in patients with advanced-stage ovarian cancer: Primary analysis in the original cohort of KGOG3046/TRU-D. J. Immunother. Cancer 2023, 11, e007444. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kang, O.J.; Lee, Y.Y.; Kim, Y.S. A prospective randomized controlled trial evaluating the safety and efficacy of patient blood management program in patients with gynecologic cancer (KGOG 4011/PBM). Int. J. Gynecol. Cancer 2023, 33, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Choi, C.H.; Kim, K.; Kim, M.H.; Lim, M.C.; Lee, B.; Kim, M.; Kim, Y.H.; Seong, S.J.; Lee, J.M. Determination of ovarian transposition through prediction of postoperative adjuvant therapy in young patients with early stage cervical cancer undergoing surgery: A Korean multicenter retrospective study (KGOG 1042). Obstet. Gynecol. Sci. 2024, 67, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.S.; Lee, K.B.; Lee, K.H.; Chang, H.K.; Kim, J.Y.; Lim, M.C.; Choi, C.H.; Cho, H.; Kim, D.Y.; Kim, Y.H.; et al. Therapeutic effects of surgical debulking of metastatic lymph nodes in cervical cancer IIICr: A trial protocol for a phase III, multicenter, randomized controlled study (KGOG1047/DEBULK trial). J. Gynecol. Oncol. 2024, 35, e57. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.H.; Lee, H.G.; Lee, B.; Kang, S.; Kim, J.H.; Kim, B.G.; Kim, J.W.; Kim, M.H.; Chen, X.; No, J.H.; et al. Prediction of final pathology depending on preoperative myometrial invasion and grade assessment in low-risk endometrial cancer patients: A Korean Gynecologic Oncology Group ancillary study. PLoS ONE 2024, 19, e0305360. [Google Scholar] [CrossRef]
- Kim, S.I.; Joung, J.G.; Kim, Y.N.; Park, J.; Park, E.; Kim, J.W.; Lee, S.; Lee, J.B.; Kim, S.; Choi, C.H.; et al. Durvalumab with or without tremelimumab plus chemotherapy in HRR non-mutated, platinum-resistant ovarian cancer (KGOG 3045): A phase II umbrella trial. Gynecol. Oncol. 2024, 182, 7–14. [Google Scholar] [CrossRef]
- Park, J.; Cho, H.W.; Lim, M.C.; Choi, C.H.; Lee, J.Y. OPERA: A phase II trial of oregovomab plus non-platinum chemotherapy in PARP inhibitor/platinum-resistant ovarian cancer. Future Oncol. 2024; in press. [Google Scholar] [CrossRef]
- Kim, N.K.; Choi, C.H.; Seong, S.J.; Lee, J.M.; Lee, B.; Kim, K. Treatment outcomes according to various progestin treatment strategies in patients with atypical hyperplasia/endometrial intraepithelial neoplasia—Multicenter retrospective study (KGOG2033). Gynecol. Oncol. 2024, 183, 68–73. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, N.K.; Kim, K.; Lee, Y.J.; Lee, K.H.; Lee, J.M.; Lee, K.B.; Suh, D.H.; Kim, S.; Kim, M.K.; et al. Effects of subcutaneous drain on wound dehiscence and infection in gynecological midline laparotomy: Secondary analysis of a Korean Gynecologic Oncology Group study (KGOG 4001). Eur. J. Surg. Oncol. 2024, 50, 108484. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; Kim, J.W.; Eminowicz, G.; Allanson, E.; Eberst, L.; Kim, S.I.; Nout, R.A.; Park, J.Y.; Lorusso, D.; Mileshkin, L.; et al. Clinical research in endometrial cancer: Consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol. 2024, 25, e420–e431. [Google Scholar] [CrossRef]
- Yoshihara, K.; Sekine, M.; Nishino, K.; Enomoto, T. The 61st Annual Meeting of the Japanese Society for Gynecologic Oncology (JSGO). J. Gynecol. Oncol. 2019, 30, e114. [Google Scholar] [CrossRef]
- Chang, H.K.; Kim, B.G.; Shi, T.Y.; Zang, R. The 5th Shanghai Gynecologic Oncology Group (SGOG)-Korean Gynecologic Oncology Group (KGOG) joint meeting and 2016 Asia-Pacific Ovarian cancer Laparotomy and Laparoscopic Operation (APOLLO) symposium in Shanghai. J. Gynecol. Oncol. 2016, 27, e64. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Okamoto, A.; Kim, J.H.; Lai, C.H.; Wu, X.; Kim, Y.M. East Asian Gynecologic Oncology Trial Group (EAGOT): Founding history and future perspective. J. Gynecol. Oncol. 2023, 34, e86. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Shimada, M.; Tamate, M.; Cho, H.W.; Zhu, J.; Chou, H.H.; Kajiyama, H.; Okamoto, A.; Aoki, D.; Kang, S.; et al. Current treatment strategies for ovarian cancer in the East Asian Gynecologic Oncology Trial Group (EAGOT). J. Gynecol. Oncol. 2024, 35, e87. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Coleman, R.L.; Pignata, S.; Bookman, M.A.; Marth, C.; Herzog, T.J.; Gonzalez-Martin, A.; Copeland, L.J.; European Network of Gynaecological Oncological Trial Groups; GOG Foundation Inc. Joint ENGOT and GOG Foundation requirements for trials with industry partners. Gynecol. Oncol. 2019, 154, 255–258. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).