Intraperitoneal Chemotherapy without Bevacizumab versus Intravenous Chemotherapy with Bevacizumab as the Frontline Adjuvant Therapy in Advanced Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Comprehensive Cancer Network Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 3.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 23 July 2024).
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Stegel, V.; Blatnik, A.; Škof, E.; Dragoš, V.Š.; Krajc, M.; Gregorič, B.; Škerl, P.; Strojnik, K.; Klančar, G.; Banjac, M.; et al. Real-World Data on Detection of Germline and Somatic Pathogenic/Likely Pathogenic Variants in BRCA1/2 and Other Susceptibility Genes in Ovarian Cancer Patients Using Next Generation Sequencing. Cancers 2022, 14, 1434. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gyne-cologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Liu, P.Y.; Hannigan, E.V.; O’Toole, R.; Williams, S.D.; Young, J.A.; Franklin, E.W.; Clarke-Pearson, D.L.; Malviya, V.K.; DuBeshter, B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N. Engl. J. Med. 1996, 335, 1950–1955. [Google Scholar] [CrossRef]
- Markman, M.; Bundy, B.N.; Alberts, D.S.; Fowler, J.M.; Clark-Pearson, D.L.; Carson, L.F.; Wadler, S.; Sickel, J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J. Clin. Oncol. 2001, 19, 1001–1007. [Google Scholar] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Walker, J.L.; Brady, M.F.; Wenzel, L.; Fleming, G.F.; Huang, H.Q.; DiSilvestro, P.A.; Fujiwara, K.; Alberts, D.S.; Zheng, W.; Tewari, K.S.; et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group study. J. Clin. Oncol. 2019, 37, 1380–1390. [Google Scholar] [CrossRef]
- Monk, B.J.; Chan, J.K. Is intraperitoneal chemotherapy still an acceptable option in primary adjuvant chemotherapy for ad-vanced ovarian cancer? Ann. Oncol. 2017, 28, viii40–viii45. [Google Scholar] [CrossRef] [PubMed]
- Calvert, A.H.; Newell, D.R.; Gumbrell, L.A.; O’Reilly, S.; Burnell, M.; Boxall, F.E.; Siddik, Z.H.; Judson, I.R.; Gore, M.E.; Wiltshaw, E. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J. Clin. Oncol. 1989, 7, 1748–1756. [Google Scholar] [CrossRef]
- Ting, W.H.; Hsiao, C.H.; Chen, H.H.; Wei, M.C.; Lin, H.H.; Hsiao, S.M. Comparisons of Clinical Outcomes in Women with Advanced Ovarian Cancer Treated with Frontline Intraperitoneal versus Dose-Dense Platinum/Paclitaxel Chemotherapy without Bevacizumab. Int. J. Environ. Res. Public. Health 2020, 17, 3603. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.; Nelstrop, A.E.; McClean, P.; Brady, M.F.; McGuire, W.P.; Hoskins, W.J.; Mitchell, H.; Lambert, H.E. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J. Clin. Oncol. 1996, 14, 1545–1551. [Google Scholar] [CrossRef]
- Vergote, I.; Rustin, G.J.; Eisenhauer, E.A.; Kristensen, G.B.; Pujade-Lauraine, E.; Parmar, M.K.; Friedlander, M.; Jakobsen, A.; Vermorken, J.B. Re: New guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J. Natl. Cancer Inst. 2000, 92, 1534–1535. [Google Scholar] [CrossRef]
- Krasner, C.N.; Castro, C.; Penson, R.T.; Roche, M.; Matulonis, U.A.; Morgan, M.A.; Drescher, C.; Armstrong, D.K.; Wolfe, J.K.; Lee, H.; et al. Final report on serial phase II trials of all-intraperitoneal chemotherapy with or without bevacizumab for women with newly diagnosed, optimally cytoreduced carcinoma of Müllerian origin. Gynecol. Oncol. 2019, 153, 223–229. [Google Scholar] [CrossRef]
- Nagao, S.; Fujiwara, K.; Yamamoto, K.; Tanabe, H.; Okamoto, A.; Takehara, K.; Saito, M.; Fujiwara, H.; Tan, D.S.P.; Yamaguchi, S.; et al. Intraperitoneal Carboplatin for Ovarian Cancer—A Phase 2/3 Trial. NEJM Evid. 2023, 2, EVIDoa2200225. [Google Scholar] [CrossRef]
- Derick, R.L.; Flessner, M.F. Pharmacokinetic Problems in Peritoneal Drug Administration: Tissue Penetration and Surface Exposure. J. Natl. Cancer Inst. 1997, 89, 480–487. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, J.; Righi, E.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef]
- Mancuso, M.R.; Davis, R.; Norberg, S.M.; O’Brien, S.; Sennino, B.; Nakahara, T.; Yao, V.J.; Inai, T.; Brooks, P.; Freimark, B.; et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Investig. 2006, 116, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Lambrechts, D.; Prenen, H.; Jain, R.K.; Carmeliet, P. Lessons from the adjuvant bevacizumab trial on colon cancer: What next? J. Clin. Oncol. 2011, 29, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.S.; Twu, N.F.; Lai, C.R.; Horng, H.C.; Chao, K.C.; Juang, C.M. Importance of delivered cycles and nomo-gram for intraperitoneal chemotherapy in ovarian cancer. Gynecol. Oncol. 2009, 114, 415–419. [Google Scholar] [CrossRef]
- Kim, J.; Chang, Y.; Kim, T.J.; Lee, J.W.; Kim, B.G.; Bae, D.S.; Choi, C.H. Optimal cutoff age for predicting prognosis associated with serous epithelial ovarian cancer: What is the best age cutoff? J. Gynecol. Oncol. 2019, 30, e11. [Google Scholar] [CrossRef]
- Chan, J.K.; Urban, R.; Cheung, M.K.; Osann, K.; Shin, J.Y.; Husain, A.; Teng, N.N.; Kapp, D.S.; Berek, J.S.; Leiserowitz, G.S. Ovarian cancer in younger vs older women: A population-based analysis. Br. J. Cancer 2006, 95, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Y.; Liao, S.B.; Li, L. Preoperative serum levels of HE4 and CA125 predict primary optimal cytoreduction in advanced epithelial ovarian cancer: A preliminary model study. J. Ovarian Res. 2020, 13, 17. [Google Scholar] [CrossRef]
- Obeidat, B.; Latimer, J.; Crawford, R. Can optimal primary cytoreduction be predicted in advanced stage epithelial ovarian cancer? Role of preoperative serum CA-125 level. Gynecol. Obstet. Investig. 2004, 57, 153–156. [Google Scholar] [CrossRef]
- Chi, D.S.; Zivanovic, O.; Palayekar, M.J.; Eisenhauer, E.L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Leitao, M.M.; Brown, C.L.; Barakat, R.R. A contemporary analysis of the ability of preoperative serum CA-125 to predict primary cytoreductive outcome in patients with advanced ovarian, tubal and peritoneal carcinoma. Gynecol. Oncol. 2009, 112, 6–10. [Google Scholar] [CrossRef]
- Zorn, K.K.; Tian, C.; McGuire, W.P.; Hoskins, W.J.; Markman, M.; Muggia, F.M.; Rose, P.G.; Ozols, R.F.; Spriggs, D.; Armstrong, D.K. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: A Gynecologic Oncology Group study. Cancer 2009, 115, 1028–1035. [Google Scholar] [CrossRef]
| Variable | Intravenous + Bevacizumab (n = 23) | Intravenous (n = 63) | Intraperitoneal (n = 57) | p a |
|---|---|---|---|---|
| Age (years) | 54.7 ± 12.5 | 60.0 ± 10.2 | 58.2 ± 9.9 | 0.108 |
| Body mass index (kg/m2) | 23.4 ± 2.4 | 24.2 ± 4.1 | 24.9 ± 5.3 | 0.762 |
| Baseline CA-125 (U/mL) | 3012 ± 3994 | 1697 ± 2602 | 2058 ± 3070 | 0.241 |
| ECOG Score | ||||
| 0 | 6 (26) | 21 (33) | 3 (5) | 0.001 |
| 1 | 11 (48) | 28 (44) | 41 (72) | |
| 2 | 6 (26) | 12 (19) | 13 (23) | |
| 3 | 0 (0) | 2 (3) | 0 (0) | |
| FIGO Stage | ||||
| 2 | 1 (4) | 9 (14) | 6 (11) | 0.341 |
| 3 | 12 (52) | 40 (63) | 37 (65) | |
| 4 | 10 (43) | 14 (22) | 14 (25) | |
| Site | ||||
| Ovary | 22 (96) | 58 (92) | 52 (83) | 0.312 |
| Fallopian tube | 1 (4) | 0 (0) | 2 (4) | |
| Peritoneum | 0 (0) | 5 (8) | 3 (5) | |
| Histologic subtype | ||||
| Serous | 13 (57) | 45 (71) | 41 (72) | 0.622 |
| Clear cell | 4 (17) | 7 (11) | 5 (9) | |
| Others | 6 (26) | 11 (17) | 11 (19) | |
| Cell grade | ||||
| 1 | 0 (0) | 2 (3) | 3 (5) | 0.660 |
| 2 | 1 (4) | 4 (6) | 1 (2) | |
| 3 | 22 (96) | 55 (87) | 51 (89) | |
| Not available | 0 (0) | 2 (3) | 2 (4) | |
| Tumor HRD status | ||||
| Negative HRD | 4 (17) | 5 (8) | 10 (18) | 0.594 |
| Positive HRD and no tumor BRCA mutation | 1 (4) | 2 (3) | 5 (9) | |
| Tumor BRCA mutation | 0 (0) | 1 (2) | 7 (12) | |
| Unknown | 18 (78) | 55 (87) | 33 (57) | |
| Neoadjuvant chemotherapy | 1 (4) | 5 (8) | 1 (2) | 0.325 |
| Debulking surgery | ||||
| No residual (R0) | 5 (22) | 13 (21) | 21 (37) | 0.070 |
| Optimal (residual < 1 cm) | 3 (13) | 15 (24) | 16 (28) | |
| Suboptimal (residual > 1 cm) | 15 (65) | 35 (56) | 20 (35) | |
| Lymph node metastasis | 11 (48) | 21 (33) | 24 (42) | 0.171 |
| Number of chemotherapy cycles | 5.8 ± 1.9 | 6.1 ± 1.5 | 5.5 ± 1.5 | 0.125 |
| PARP inhibitor | 0.481 | |||
| Olaparib | 2 (9) | 5 (8) | 5 (9) | |
| Niraparib | 2 (9) | 1 (2) | 1 (2) | |
| IP-preferring physicians (n = 2) | 5 (22) | 33 (52) | 54 (95) | <0.001 |
| Other physicians (n = 4) | 18 (78) | 30 (48) | 3 (5) | |
| Follow-up interval | 29.8 (18.6, 46.4) | 30.5 (14.7, 47.1) | 39.3 (14.6, 67.5) | 0.181 |
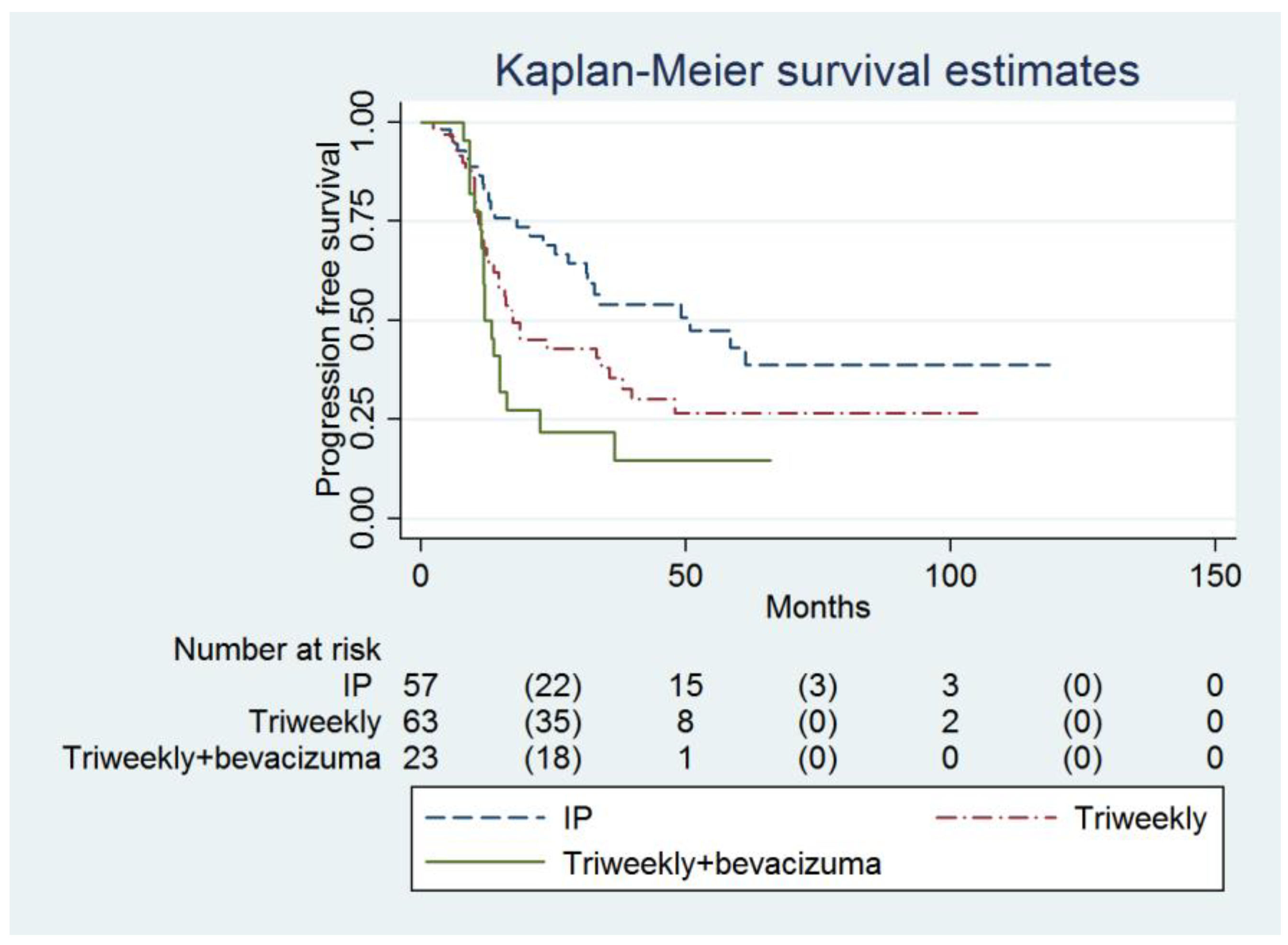
| Progression-free survival (months) | 11.9 (11.2 to 16.3) | 17.4 (12.8 to 35.5) | 49.1 (27.8 to infinity) | 0.007 |
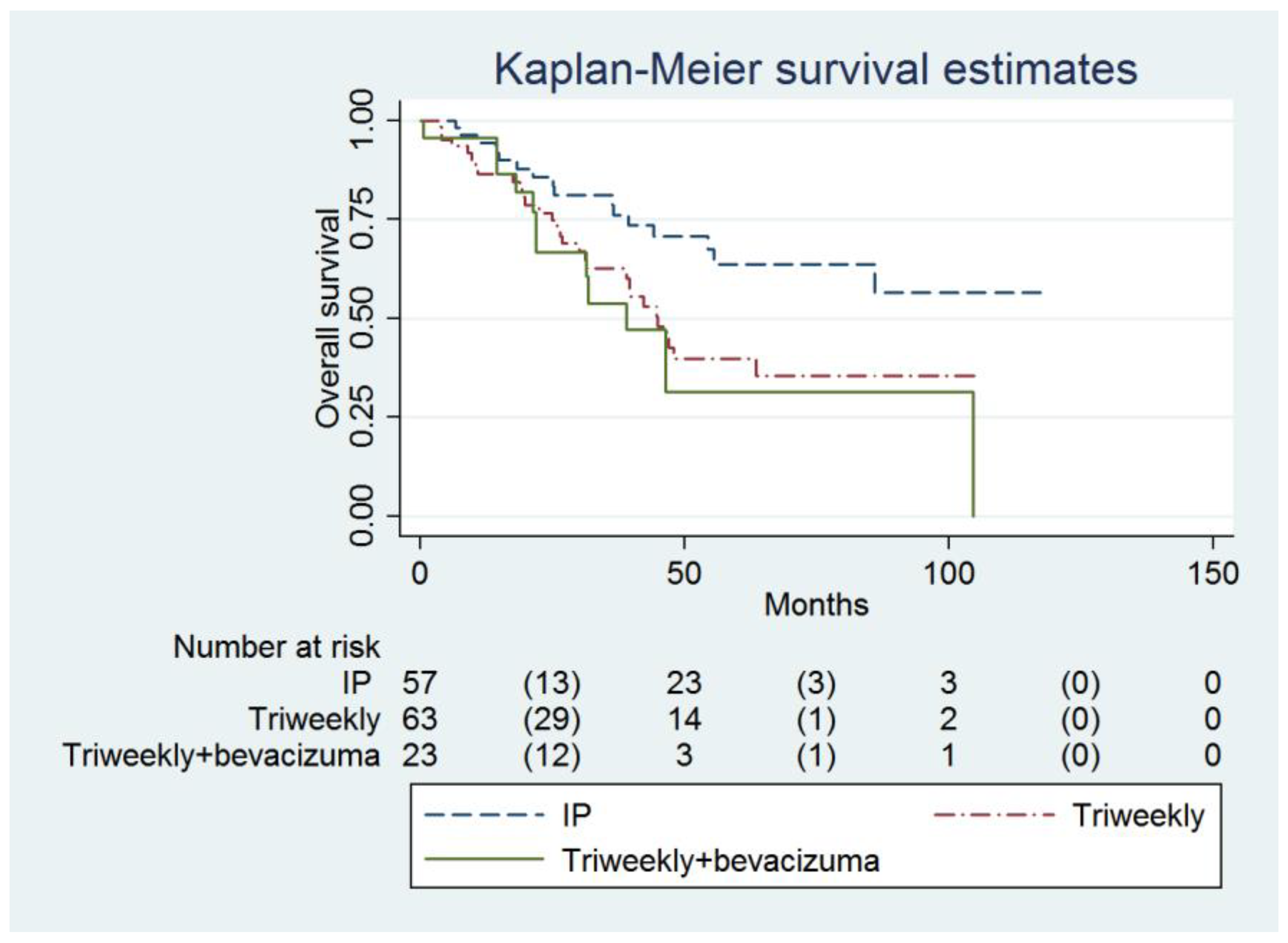
| Overall survival (months) | 38.9 (21.9 to infinity) | 46.4 (31.5 to infinity) | Not reached (55.6 to infinity) | 0.018 |
| Clinical response | ||||
| CR | 13 (57) | 49 (78) | 45 (79) | 0.232 |
| PR | 8 (35) | 8 (13) | 9 (16) | |
| SD | 1 (4) | 1 (2) | 1 (2) | |
| PD | 1 (4) | 4 (6) | 2 (4) |
| Variable | Hazard Ratio | Univariate 95% CI | p a | Hazard Ratio | Multivariable 95% CI | p b |
|---|---|---|---|---|---|---|
| Regimen | ||||||
| Intravenous + bevacizumab | 1.00 | - | - | 1.00 | - | - |
| Intravenous | 0.60 | 0.34 to 1.07 | 0.087 | 0.79 | 0.43 to 1.44 | 0.432 |
| Intraperitoneal | 0.38 | 0.21 to 0.71 | 0.002 | 0.45 | 0.24 to 0.87 | 0.017 |
| Age (years) | 1.007 | 0.986 to 1.030 | 0.505 | - | - | - |
| Body mass index (kg/m2) | 0.960 | 0.915 to 1.007 | 0.093 | - | - | - |
| Baseline CA-125 (U/mL) | 1.00008 | 1.00003 to 1.00014 | 0.006 | 1.00007 | 1.00001 to 100,014 | 0.031 |
| ECOG Score | ||||||
| 0 | 1.00 | - | - | - | - | - |
| 1 | 0.80 | 0.46 to 1.40 | 0.432 | - | - | - |
| 2 | 1.38 | 0.72 to 2.63 | 0.335 | - | - | - |
| 3 | 1.70 | 0.39 to 7.43 | 0.479 | - | - | - |
| FIGO Stage | ||||||
| 2 | 1.00 | - | - | 1.00 | - | - |
| 3 | 5.96 | 1.85 o 19.18 | 0.003 | 5.33 | 1.61 to 17.68 | 0.006 |
| 4 | 8.75 | 2.61 to 29.33 | <0.001 | 6.35 | 1.81 to 22.22 | 0.004 |
| Site | ||||||
| Ovary | 1.00 | - | - | - | - | - |
| Fallopian tube | 0.35 | 0.05 to 2.54 | 0.300 | - | - | - |
| Peritoneum | 1.92 | 0.83 to 4.43 | 0.126 | - | - | - |
| Histologic subtype | ||||||
| Serous | 1.00 | - | - | - | - | - |
| Clear cell | 1.51 | 0.79 to 2.89 | 0.218 | - | - | - |
| Others | 0.67 | 0.37 to 1.21 | 0.189 | - | - | - |
| Cell grade | ||||||
| 1 | 1.00 | - | - | - | - | - |
| 2 | 1.12 | 0.19 to 6.68 | 0.905 | - | - | - |
| 3 | 2.15 | 0.53 to 8.76 | 0.288 | - | - | - |
| Tumor HRD status | ||||||
| Negative HRD | 1.00 | - | - | - | - | - |
| Positive HRD and no tumor BRCA mutation | 1.09 | 0.23 to 5.14 | 0.911 | - | - | - |
| Tumor BRCA mutation | 0.49 | 0.16 to 1.52 | 0.217 | - | - | - |
| Neoadjuvant chemotherapy | 1.57 | 0.63 to 3.89 | 0.334 | - | - | - |
| Debulking surgery | ||||||
| No residual (R0) | 1.00 | - | - | 1.00 | - | - |
| Optimal (residual < 1 cm) | 2.43 | 1.23 to 4.80 | 0.011 | 1.67 | 0.82 to 3.38 | 0.158 |
| Suboptimal (residual > 1 cm) | 3.04 | 1.68 to 5.51 | <0.001 | 1.59 | 0.84 to 3.02 | 0.156 |
| Lymph node metastasis | 1.42 | 0.89 to 2.29 | 0.144 | - | - | - |
| Number of chemotherapy cycles | 1.11 | 0.92 to 1.34 | 0.289 | - | - | - |
| PARP inhibitor | ||||||
| No | 1.00 | - | - | - | - | - |
| Niraparib | 4.57 × 1015 | 0 to infinity | 1.000 | - | - | - |
| Olaparib | 1.31 | 0.60 to 2.86 | 0.494 | - | - | - |
| Variable | Hazard Ratio | Univariate 95% CI | p a | Hazard Ratio | Multivariable 95% CI | p b |
|---|---|---|---|---|---|---|
| Regimen | ||||||
| Intravenous + bevacizumab | 1.00 | - | - | 1.00 | - | - |
| Intravenous | 0.78 | 0.41 to 1.51 | 0.469 | 0.79 | 0.38 to 1.67 | 0.530 |
| Intraperitoneal | 0.38 | 0.18 to 0.81 | 0.011 | 0.34 | 0.15 to 0.79 | 0.012 |
| Age (years) | 1.04 | 1.01 to 1.07 | 0.002 | 1.03 | 1.00 to 1.06 | 0.022 |
| Body mass index (kg/m2) | 0.97 | 0.91 to 1.03 | 0.377 | - | - | - |
| Baseline CA-125 (U/mL) | 1.00007 | 0.9999 to 1.00015 | 0.098 | - | - | - |
| ECOG Score | ||||||
| 0 | 1.00 | - | - | - | - | - |
| 1 | 0.72 | 0.37 to 1.39 | 0.329 | - | - | - |
| 2 | 1.78 | 0.89 to 3.57 | 0.104 | - | - | - |
| 3 | 1.03 | 0.13 to 7.88 | 0.977 | - | - | - |
| FIGO Stage | ||||||
| 2 | 1.00 | - | - | 1.00 | - | - |
| 3 | 11.29 | 1.54 to 82.73 | 0.017 | 8.68 | 1.15 to 65.44 | 0.036 |
| 4 | 19.95 | 2.68 to148.57 | 0.003 | 14.40 | 1.87 to 111.11 | 0.010 |
| Site | ||||||
| Ovary | 1.00 | - | - | - | - | - |
| Fallopian tube | 4.11 × 10−15 | 0 to infinity | 1.00 | 2.10 × 10−20 | - | - |
| Peritoneum | 2.94 | 1.33 to 6.51 | 0.008 | 1.87 | 0.75 to 4.66 | 0.180 |
| Histologic subtype | ||||||
| Serous | 1.00 | - | - | - | - | - |
| Clear cell | 0.81 | 0.32 to 2.05 | 0.654 | - | - | - |
| Others | 0.66 | 0.34 to 1.26 | 0.205 | - | - | - |
| Cell grade | ||||||
| 1 | 1.00 | - | - | - | - | - |
| 2 | 0.53 | 0.03 to 8.64 | 0.656 | - | - | - |
| 3 | 3.25 | 0.45 to 23.54 | 0.243 | - | - | - |
| Tumor HRD status | ||||||
| Negative HRD | 1.00 | - | - | - | - | - |
| Positive HRD and no tumor BRCA mutation | 2.48 | 0.24 to 25.74 | 0.446 | - | - | |
| Tumor BRCA mutation | 1.50 | 0.42 to 5.31 | 0.533 | - | - | - |
| Neoadjuvant chemotherapy | 1.35 | 0.49 to 3.76 | 0.562 | - | - | - |
| Debulking surgery | ||||||
| No residual (R0) | 1.00 | - | - | 1.00 | - | - |
| Optimal (residual < 1 cm) | 2.32 | 1.02 to 5.32 | 0.046 | 2.10 | 0.89 to 4.96 | 0.091 |
| Suboptimal (residual > 1 cm) | 3.47 | 1.71 to 7.01 | 0.001 | 2.08 | 0.97 to 4.47 | 0.061 |
| Lymph node metastasis | 1.54 | 0.89 to 2.67 | 0.123 | - | - | - |
| Number of chemotherapy cycles | 0.83 | 0.69 to 0.99 | 0.035 | 0.70 | 0.58 to 0.84 | <0.001 |
| PARP inhibitor | ||||||
| No | 1.00 | - | - | - | - | - |
| Niraparib | 1.10 | 0.15 to 8.00 | 0.922 | - | - | - |
| Olaparib | 0.77 | 0.24 to 2.47 | 0.660 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, W.-H.; Chen, H.-H.; Wei, M.-C.; Sun, H.-D.; Hsiao, S.-M. Intraperitoneal Chemotherapy without Bevacizumab versus Intravenous Chemotherapy with Bevacizumab as the Frontline Adjuvant Therapy in Advanced Ovarian Cancer. Cancers 2024, 16, 3382. https://doi.org/10.3390/cancers16193382
Ting W-H, Chen H-H, Wei M-C, Sun H-D, Hsiao S-M. Intraperitoneal Chemotherapy without Bevacizumab versus Intravenous Chemotherapy with Bevacizumab as the Frontline Adjuvant Therapy in Advanced Ovarian Cancer. Cancers. 2024; 16(19):3382. https://doi.org/10.3390/cancers16193382
Chicago/Turabian StyleTing, Wan-Hua, Hui-Hua Chen, Ming-Chow Wei, Hsu-Dong Sun, and Sheng-Mou Hsiao. 2024. "Intraperitoneal Chemotherapy without Bevacizumab versus Intravenous Chemotherapy with Bevacizumab as the Frontline Adjuvant Therapy in Advanced Ovarian Cancer" Cancers 16, no. 19: 3382. https://doi.org/10.3390/cancers16193382
APA StyleTing, W.-H., Chen, H.-H., Wei, M.-C., Sun, H.-D., & Hsiao, S.-M. (2024). Intraperitoneal Chemotherapy without Bevacizumab versus Intravenous Chemotherapy with Bevacizumab as the Frontline Adjuvant Therapy in Advanced Ovarian Cancer. Cancers, 16(19), 3382. https://doi.org/10.3390/cancers16193382






