Meta-Analysis of Age, Sex, and Race Disparities in the Era of Contemporary Urothelial Carcinoma Treatment
Abstract
Simple Summary
Abstract
1. Introduction
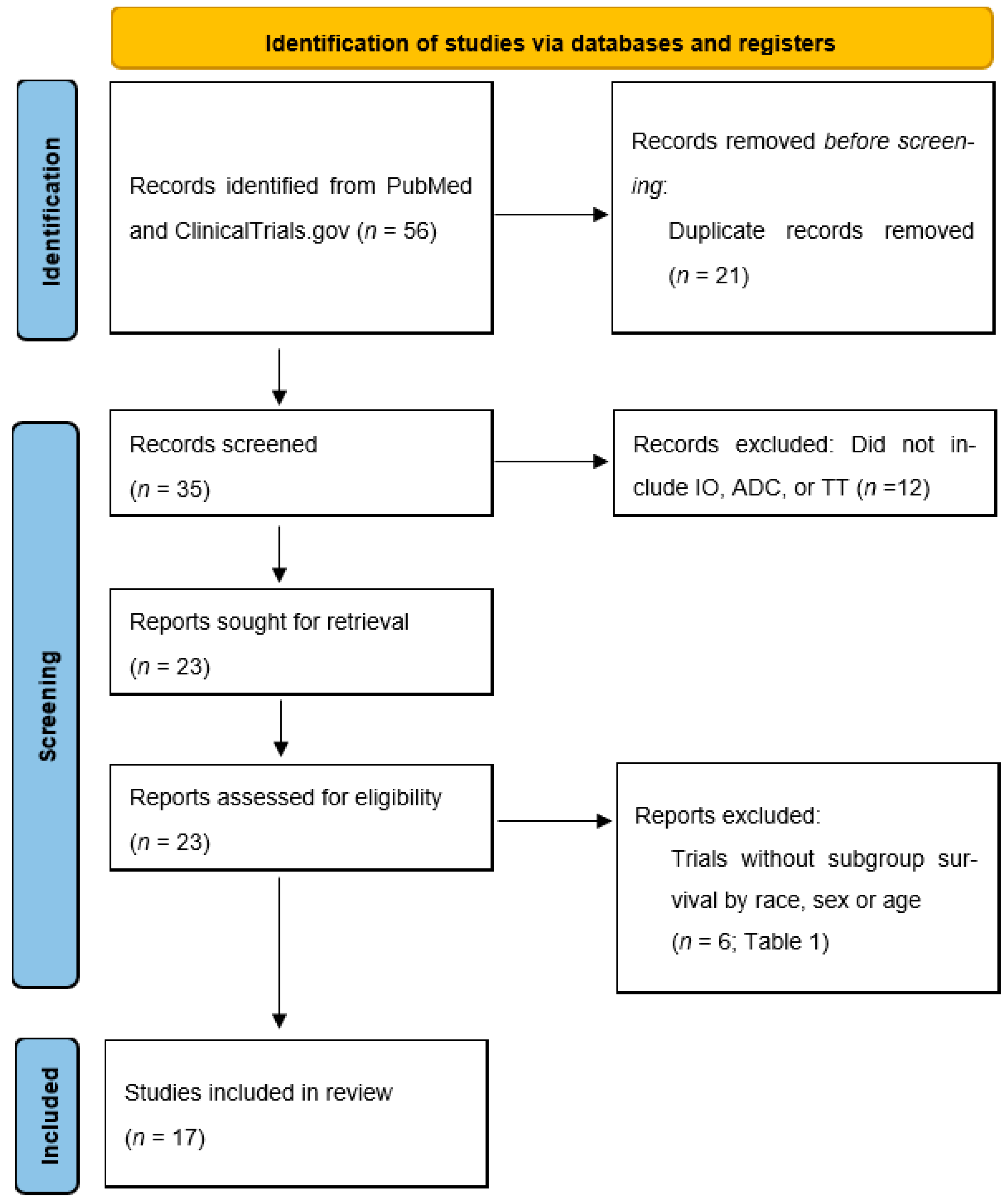
2. Methods
Study Population
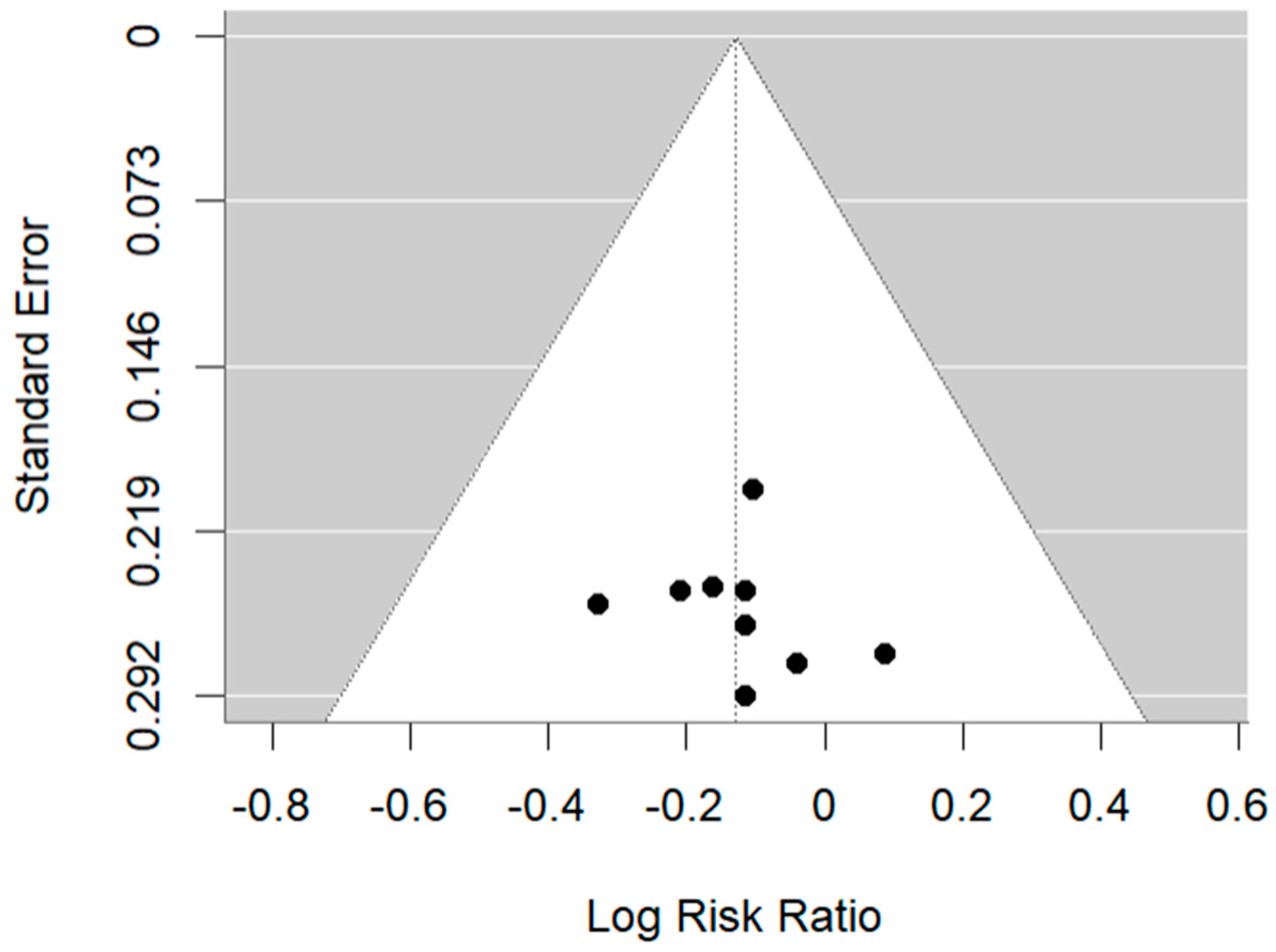
3. Results
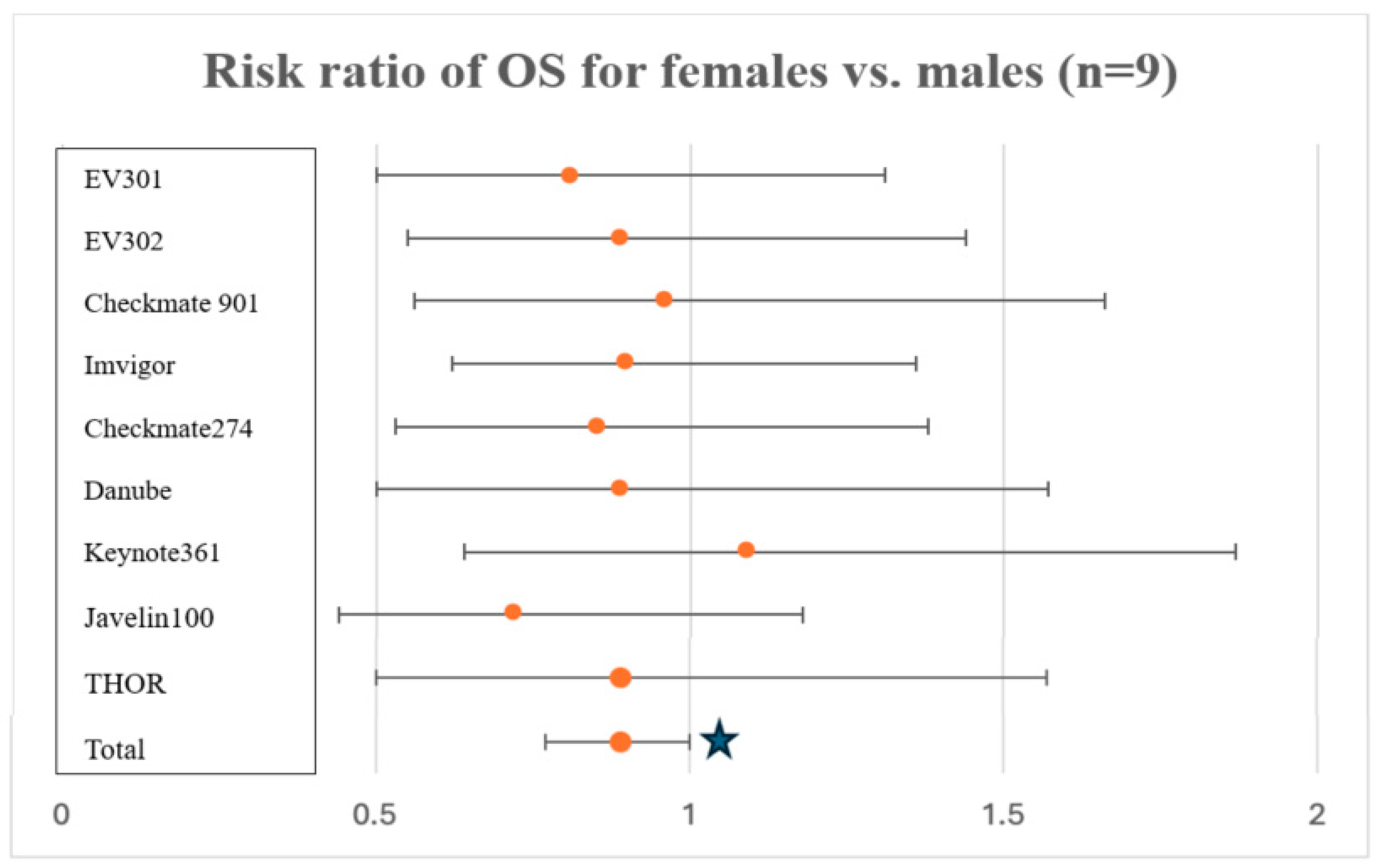
3.1. Sex
3.2. Race
3.3. Age
4. Discussion
4.1. Disparity in Survival by Sex
4.2. Disparity in Survival by Race
4.3. Disparity in Survival by Age
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review, 1975–2017. SEER. Available online: https://seer.cancer.gov/csr/1975_2017/index.html (accessed on 24 May 2024).
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder cancer. Nat. Rev. Dis. Primer 2023, 9, 58. [Google Scholar] [CrossRef]
- Dobruch, J.; Daneshmand, S.; Fisch, M.; Lotan, Y.; Noon, A.P.; Resnick, M.J.; Shariat, S.F.; Zlotta, A.R.; Boorjian, S.A. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur. Urol. 2016, 69, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Koti, M.; Ingersoll, M.A.; Gupta, S.; Lam, C.M.; Li, X.; Kamat, A.M.; Black, P.C.; Siemens, D.R. Sex Differences in Bladder Cancer Immunobiology and Outcomes: A Collaborative Review with Implications for Treatment. Eur. Urol. Oncol. 2020, 3, 622–630. [Google Scholar] [CrossRef]
- Scosyrev, E.; Noyes, K.; Feng, C.; Messing, E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009, 115, 68–74. [Google Scholar] [CrossRef]
- Hasan, S.; Lazarev, S.; Garg, M.; Mehta, K.; Press, R.H.; Chhabra, A.; Choi, J.I.; Simone, C.B.; Gorovets, D. Racial inequity and other social disparities in the diagnosis and management of bladder cancer. Cancer Med. 2023, 12, 640–650. [Google Scholar] [CrossRef]
- Fang, W.; Yang, Z.-Y.; Chen, T.-Y.; Shen, X.-F.; Zhang, C. Ethnicity and survival in bladder cancer: A population-based study based on the SEER database. J. Transl. Med. 2020, 18, 145. [Google Scholar] [CrossRef]
- Lin, W.; Pan, X.; Zhang, C.; Ye, B.; Song, J. Impact of Age at Diagnosis of Bladder Cancer on Survival: A Surveillance, Epidemiology, and End Results-Based Study 2004–2015. Cancer Control J. Moffitt. Cancer Cent. 2023, 30, 10732748231152322. [Google Scholar] [CrossRef]
- Mamtani, R.; Zhang, H.; Parikh, R.B.; Patel, K.; Li, H.; Imai, K.; Hubbard, R.A. Uptake of Maintenance Immunotherapy and Changes in Upstream Treatment Selection Among Patients With Urothelial Cancer. JAMA Netw. Open 2023, 6, e238395. [Google Scholar] [CrossRef]
- Freudenburg, E.; Bagheri, I.; Srinivas, S.; Martinez, A.; Putluri, N.; Klaassen, Z.; Kamat, A.M.; Konety, B.R.; Kim, W.Y.; Dyrskjøt, L.; et al. Race reporting and disparities regarding clinical trials in bladder cancer: A systematic review. Cancer Causes Control. 2022, 33, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Radkiewicz, C.; Edgren, G.; Johansson, A.L.V.; Jahnson, S.; Häggström, C.; Akre, O.; Lambe, M.; Dickman, P.W. Sex Differences in Urothelial Bladder Cancer Survival. Clin. Genitourin. Cancer 2020, 18, 26–34.e6. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Ito, Y.; Hatano, K.; Nakai, Y.; Kakimoto, K.; Miyashiro, I.; Nishimura, K. Impact of sex difference on survival of bladder cancer: A population-based registry data in Japan. Int. J. Urol. 2019, 26, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Flammia, R.S.; Tufano, A.; Chierigo, F.; Würnschimmel, C.; Hoeh, B.; Sorce, G.; Tian, Z.; Anceschi, U.; Leonardo, C.; Del Giudice, F.; et al. The Effect of Sex on Disease Stage and Survival after Radical Cystectomy in Non-Urothelial Variant-Histology Bladder Cancer. J. Clin. Med. 2023, 12, 1776. [Google Scholar] [CrossRef]
- Uhlig, A.; Hosseini, A.S.A.; Simon, J.; Lotz, J.; Trojan, L.; Schmid, M.; Uhlig, J. Gender Specific Differences in Disease-Free, Cancer Specific and Overall Survival after Radical Cystectomy for Bladder Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2018, 200, 48–60. [Google Scholar] [CrossRef]
- Johnson, A.M.; O’Connell, M.J.; Miyamoto, H.; Huang, J.; Yao, J.L.; Messing, E.M.; E Reeder, J. Androgenic dependence of exophytic tumor growth in a transgenic mouse model of bladder cancer: A role for thrombospondin-1. BMC Urol. 2008, 8, 7. [Google Scholar] [CrossRef]
- Miyamoto, H.; Yang, Z.; Chen, Y.-T.; Ishiguro, H.; Uemura, H.; Kubota, Y.; Nagashima, Y.; Chang, Y.-J.; Hu, Y.-C.; Tsai, M.-Y.; et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl. Cancer Inst. 2007, 99, 558–568. [Google Scholar] [CrossRef]
- Doshi, B.; Athans, S.R.; Woloszynska, A. Biological differences underlying sex and gender disparities in bladder cancer: Current synopsis and future directions. Oncogenesis 2023, 12, 1–11. [Google Scholar] [CrossRef]
- Klein, S.L.; Morgan, R. The impact of sex and gender on immunotherapy outcomes. Biol. Sex Differ. 2020, 11, 24. [Google Scholar] [CrossRef]
- Wang, S.; Cowley, L.A.; Liu, X.-S. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules 2019, 24, 3214–3225. [Google Scholar] [CrossRef]
- Jacobs, E.A.; Rolle, I.; Ferrans, C.E.; Whitaker, E.E.; Warnecke, R.B. Understanding African Americans’ Views of the Trustworthiness of Physicians. J. Gen. Intern. Med. 2006, 21, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.N.; Wells, K.M.; Joy-Gaba, J.A.; Hawkins, C.B.; Nosek, B.A. Do physicians’ implicit views of African Americans affect clinical decision making? J. Am. Board Fam. Med. 2014, 27, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.; Canchola, A.J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J. Clin. Oncol. 2018, 36, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mayer, W.J.; McWhorter, W.P. Black/white differences in non-treatment of bladder cancer patients and implications for survival. Am. J. Public Health 1989, 79, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, U.; Fedewa, S.A.; Ward, E.M. Treatment of muscle invasive bladder cancer: Evidence from the National Cancer Database, 2003 to 2007. J. Urol. 2011, 185, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, J.; Zeymo, A.; Egan, J.; Carvalho, F.; Krasnow, R.; Stamatakis, L.; Lynch, J.; Hwang, J.; Williams, S.; Kowalczyk, K. Sex and Racial Disparities in the Treatment and Outcomes of Muscle-invasive Bladder Cancer. Urology 2021, 151, 154–162. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Q.; Li, Y. Racial differences in Urinary Bladder Cancer in the United States. Sci. Rep. 2018, 8, 12521. [Google Scholar] [CrossRef]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef]
- Lichtman, S.M.; Wildiers, H.; Chatelut, E.; Steer, C.; Budman, D.; Morrison, V.A.; Tranchand, B.; Shapira, I.; Aapro, M. International Society of Geriatric Oncology Chemotherapy Taskforce: Evaluation of chemotherapy in older patients--an analysis of the medical literature. J. Clin. Oncol. Off J. Am. Soc. Clin. Oncol. 2007, 25, 1832–1843. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet Lond. Engl. 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.-T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Sattar, J.; Kartolo, A.; Hopman, W.M.; Lakoff, J.M.; Baetz, T. The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population. J. Geriatr. Oncol. 2019, 10, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Jodon, G.; Fischer, S.M.; Kessler, E.R. Treatment of urothelial cancer in elderly patients: Focus on immune checkpoint inhibitors. Drugs Aging 2018, 35, 409–421. [Google Scholar] [CrossRef]
| Trial | Agent | Indication | % Female | OS Female | % Black | % Asian | OS Race | % >75 | OS >75 |
|---|---|---|---|---|---|---|---|---|---|
| EV-301 | EV | 1L Cis-ineligible | 22.7 | NSB * | NR | NR | NR | 17.3 | NSB * |
| EV-302 | Pembro + EV | 1L Cis-ineligible | 22.2 | ND vs. men | 0.7 | 22.4 | NR | 23.1 | ND |
| Checkmate 901 | Nivo+ GC | 1L | 22.4 | NSB * | 0 | 24.7 | All races NSB | 11.2 | NSB * |
| KEYNOTE 052 | Pembro | 1L Cis-ineligible | 23 | NR | 2.2 | 7 | NR | 49 | ND |
| IMVigor211 | Atezo | 2L | 30 | NR | 0.3 | 12.7 | NR | NR | NR |
| IMVigor 130 | Atezo + GC | 1L | 25 | NSB * | 1 | 22.6 | Asian: NSB * | NR | <65 NSB |
| Javelin Bladder 100 | Avelumab | 1L maintenance | 24 | NSB * | 0.8 | 17.9 | Asian/Other: NSB * | NR | <65 NSB |
| DANUBE | Durvalumab +/− Tremilimumab | 1L | 26 | NB all | 0.6 | 21.5 | NB all | NR | NB all |
| KEYNOTE 361 | Pembro +/− chemo | 1L | 24.5 | NB all | 0.8 | 17.9 | NR | NR | NR |
| THOR | Erdafitinib | 2L FGFR2/3-mt | 29.4 | NSB * | 0 | 27.2 | NR | NR | NR |
| Checkmate 274 | Nivo | Adjuvant | 24.9 | NSB * | 0.6 | 22.7 | Asian: NSB * | 17.3 | NSB * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barsouk, A.; Elghawy, O.; Yang, A.; Sussman, J.H.; Mamtani, R.; Mei, L. Meta-Analysis of Age, Sex, and Race Disparities in the Era of Contemporary Urothelial Carcinoma Treatment. Cancers 2024, 16, 3338. https://doi.org/10.3390/cancers16193338
Barsouk A, Elghawy O, Yang A, Sussman JH, Mamtani R, Mei L. Meta-Analysis of Age, Sex, and Race Disparities in the Era of Contemporary Urothelial Carcinoma Treatment. Cancers. 2024; 16(19):3338. https://doi.org/10.3390/cancers16193338
Chicago/Turabian StyleBarsouk, Adam, Omar Elghawy, Austin Yang, Jonathan H. Sussman, Ronac Mamtani, and Lin Mei. 2024. "Meta-Analysis of Age, Sex, and Race Disparities in the Era of Contemporary Urothelial Carcinoma Treatment" Cancers 16, no. 19: 3338. https://doi.org/10.3390/cancers16193338
APA StyleBarsouk, A., Elghawy, O., Yang, A., Sussman, J. H., Mamtani, R., & Mei, L. (2024). Meta-Analysis of Age, Sex, and Race Disparities in the Era of Contemporary Urothelial Carcinoma Treatment. Cancers, 16(19), 3338. https://doi.org/10.3390/cancers16193338






