Systemic Therapy of Gastric Cancer—State of the Art and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
2. Systemic Therapy of Localized Gastric Cancer
2.1. Standard Perioperative Treatment
2.2. Immune Checkpoint Inhibition in Combination with Perioperative Chemotherapy
2.3. Perioperative HER2-Targeted Therapy in Combination with Perioperative Chemotherapy
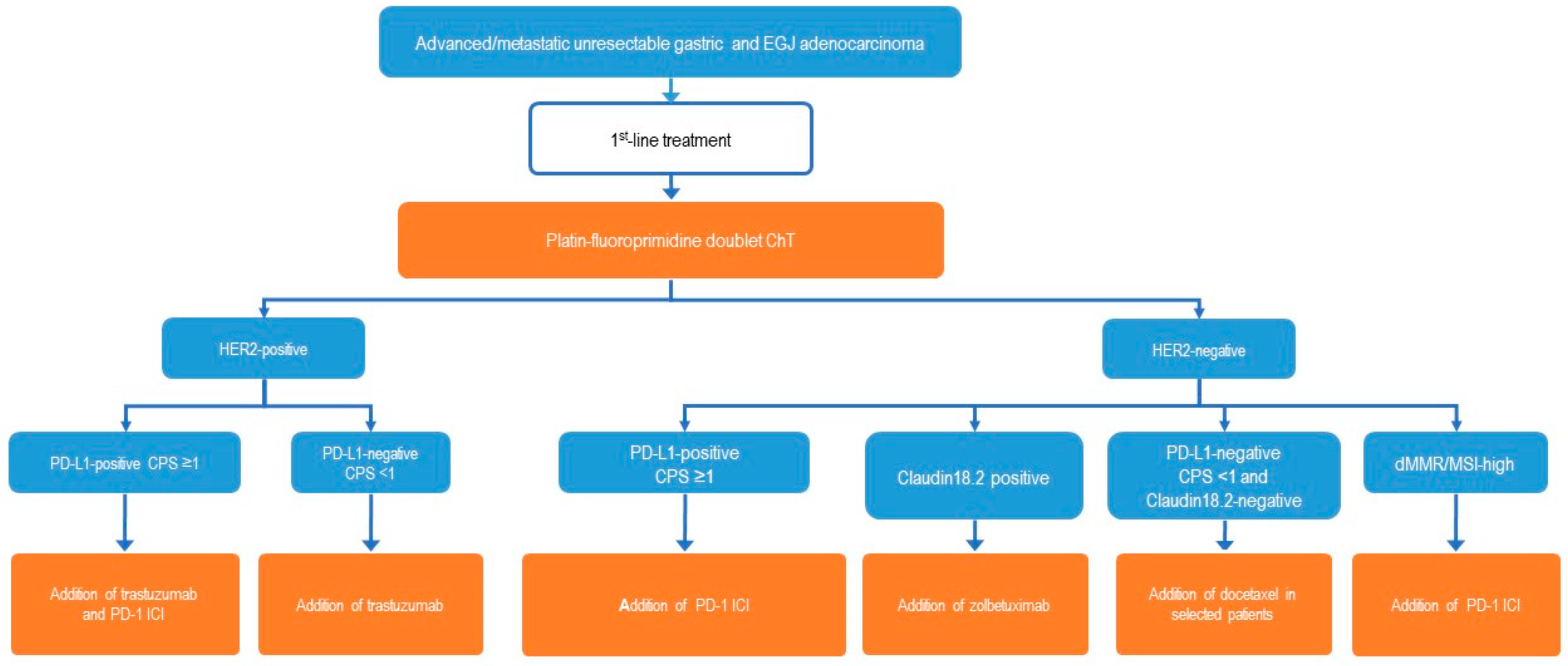
3. Systemic Therapy of Advanced Gastric Cancer
3.1. Current Status
3.2. Biomarkers
3.3. Principles of First-Line Chemotherapy
3.4. Microsatellite Stable (MSS) Tumors (Or Proficient Mismatch Repair, pMMR), HER2-Negative
3.5. HER2-Positive Tumors
3.6. dMMR/MSI-H Tumors
3.7. Claudin18.2 Positive Tumors
3.8. FGFR2b Positive Tumors
3.9. Second- and Third-Line Therapy of Gastric Cancer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Yeoh, K.G. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology 2015, 149, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Tramacere, I.; Negri, E.; Pelucchi, C.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; La Vecchia, C.; Boffetta, P. A meta-analysis on alcohol drinking and gastric cancer risk. Ann. Oncol. 2012, 23, 28–36. [Google Scholar] [CrossRef]
- Lu, L.; Mullins, C.S.; Schafmayer, C.; Zeißig, S.; Linnebacher, M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun. 2021, 41, 1137–1151. [Google Scholar] [CrossRef]
- Qiu, H.; Cao, S.; Xu, R. Cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021, 41, 1037–1048. [Google Scholar] [CrossRef]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 855–883. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021, 6th ed.; Japanese Gastric Cancer Association: Kyoto, Japan, 2023; Volume 26, pp. 1–25. [Google Scholar]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Yoshida, K.; Kodera, Y.; Kochi, M.; Ichikawa, W.; Kakeji, Y.; Sano, T.; Nagao, N.; Takahashi, M.; Takagane, A.; Watanabe, T.; et al. Addition of docetaxel to Oral Fluoropyrimidine improves efficacy in patients with stage III gastric cancer: Interim analysis of JACCRO GC-07, a randomized controlled trial. J. Clin. Oncol. 2019, 37, 1296–1304. [Google Scholar] [CrossRef]
- Park, S.H.; Lim, D.H.; Sohn, T.S.; Lee, J.; Zang, D.Y.; Kim, S.T.; Kang, J.H.; Oh, S.Y.; Hwang, I.G.; Ji, J.H.; et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: The ARTIST 2 trial. Ann. Oncol. 2021, 32, 368–374. [Google Scholar] [CrossRef]
- Shitara, K.; Rha, S.Y.; Wyrwicz, L.S.; Oshima, T.; Karaseva, N.; Osipov, M.; Yasui, H.; Yabusaki, H.; Afanasyev, S.; Park, Y.K.; et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): An interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024, 25, 212–224. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Al-Batran, S.E.; Wainberg, Z.A.; Van Cutsem, E.; Molena, D.; Muro, K.; Hyung, W.J.; Wyrwicz, L.S.; Oh, D.Y.; Omori, T.; et al. LBA73 Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase III MATTERHORN study. Ann. Oncol. 2023, 34, S1315–S1316. [Google Scholar] [CrossRef]
- Lorenzen, S.; Götze, T.O.; Thuss-Patience, P.; Biebl, M.; Homann, N.; Schenk, M.; Lindig, U.; Heuer, V.; Kretzschmar, A.; Goekkurt, E.; et al. Perioperative Atezolizumab Plus Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel for Resectable Esophagogastric Cancer: Interim Results From the Randomized, Multicenter, Phase II/III DANTE/IKF-s633 Trial. J. Clin. Oncol. 2024, 42, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Terashima, M.; Kim, Y.W.; Boku, N.; Chung, H.C.; Chen, J.S.; Ji, J.; Yeh, T.S.; Chen, L.T.; Ryu, M.H.; et al. Adjuvant nivolumab plus chemotherapy versus placebo plus chemotherapy for stage III gastric or gastro-oesophageal junction cancer after gastrectomy with D2 or more extensive lymph-node dissection (ATTRACTION-5): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2024, 9, 705–717. [Google Scholar] [PubMed]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Randon, G.; Di Bartolomeo, M.; Luciani, A.; Chao, J.; Smyth, E.C.; Petrelli, F. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: A meta-analysis of randomized clinical trials. ESMO Open 2021, 6, 100036. [Google Scholar] [CrossRef]
- André, T.; Tougeron, D.; Piessen, G.; de la Fouchardière, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2023, 41, 255–265. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Raimondi, A.; Lonardi, S.; Murgioni, S.; Cardellino, G.G.; Tamberi, S.; Strippoli, A.; Palermo, F.; Prisciandaro, M.; Randon, G.; et al. INFINITY: A multicentre single-arm multi-cohort phase II trial of tremelimumab durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J. Clin. Oncol. 2023, 41 (Suppl. S4), 358. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Hegewisch-Becker, S.; Kunzmann, V.; Thuss-Patience, P.; Fuchs, M.; Homann, N.; Graeven, U.; Schulte, N.; Merx, K.; Pohl, M.; et al. Trastuzumab in combination with 5-fluorouracil leucovorin oxaliplatin docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int. J. Cancer 2021, 149, 1322–1331. [Google Scholar] [PubMed]
- Al-Batran, S.E.; Haag, G.M.; Ettrich, T.J.; Borchert, K.; Kretzschmar, A.; Teschendorf, C.; Siegler, G.M.; Ebert, M.; Goekkurt, E.; Welslau, M.K.; et al. 1421MO Final results and subgroup analysis of the PETRARCA randomized phase II AIO trial: Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma. Ann. Oncol. 2020, 31, S899. [Google Scholar] [CrossRef]
- Wagner, A.D.; Grabsch, H.I.; Mauer, M.; Fumagalli Romario, U.; Kang, Y.K.; Bouche, O.; Lorenzen, S.; Moehler, M.H.; Thuss-Patience, P.C.; Elme, A.; et al. Integration of trastuzumab (T), with or without pertuzumab (P), into perioperative chemotherapy (CT) of HER-2 positive gastric (GC) and esophagogastric junction cancer (EGJC): First results of the EORTC 1203 INNOVATION study, in collaboration with the Korean Cancer Study Group, and the Dutch Upper GI Cancer group. J. Clin. Oncol. 2023, 41 (Suppl. 16), 4057. [Google Scholar]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697, Erratum in Lancet 2010, 376, 1302. [Google Scholar] [CrossRef] [PubMed]
- Haffner, I.; Schierle, K.; Raimúndez, E.; Geier, B.; Maier, D.; Hasenauer, J.; Luber, B.; Walch, A.; Kolbe, K.; Riera Knorrenschild, J.; et al. HER2 Expression, Test Deviations, and Their Impact on Survival in Metastatic Gastric Cancer: Results From the Prospective Multicenter VARIANZ Study. J. Clin. Oncol. 2021, 39, 1468–1478. [Google Scholar] [CrossRef]
- Park, Y.; Nam, S.K.; Seo, S.H.; Park, K.U.; Oh, H.J.; Park, Y.S.; Suh, Y.S.; Ahn, S.H.; Park, D.J.; Kim, H.H.; et al. Comprehensive Study of Microsatellite Instability Testing and Its Comparison With Immunohistochemistry in Gastric Cancers. J. Gastric. Cancer 2023, 23, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Rüschoff, J.; Schildhaus, H.U.; Rüschoff, J.H.; Jöhrens, K.; Bocker Edmonston, T.; Dietmaier, W.; Bläker, H.; Baretton, G.; Horst, D.; Dietel, M.; et al. Testing for deficient mismatch repair and microsatellite instability: A focused update. Pathologie 2023, 44 (Suppl. S2), 61–70. [Google Scholar] [CrossRef] [PubMed]
- Baretton, G.B.; Lordick, F.; Interdisciplinary Expert Group; Gaiser, T.; Hofheinz, R.; Horst, D.; Lorenzen, S.; Moehler, M.; Röcken, C.; Schirmacher, P.; et al. Standardized and quality-assured predictive PD-L1 testing in the upper gastrointestinal tract. J. Cancer Res. Clin. Oncol. 2023, 149, 16231–16238. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Kubota, Y.; Kawazoe, A.; Mishima, S.; Nakamura, Y.; Kotani, D.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Yoshino, T.; et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open 2023, 8, 100762. [Google Scholar] [CrossRef]
- Hall, P.S.; Swinson, D.; Cairns, D.A.; Waters, J.S.; Petty, R.; Allmark, C.; Ruddock, S.; Falk, S.; Wadsley, J.; Roy, R.; et al. Efficacy of Reduced-Intensity Chemotherapy With Oxaliplatin and Capecitabine on Quality of Life and Cancer Control Among Older and Frail Patients With Advanced Gastroesophageal Cancer: The GO2 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 869–877. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Rha, S.Y.; Oh, D.Y.; Yañez, P.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- Yoon, H.H.; Jin, Z.; Kour, O.; Kankeu Fonkoua, L.A.; Shitara, K.; Gibson, M.K.; Prokop, L.J.; Moehler, M.; Kang, Y.K.; Shi, Q.; et al. Association of PD-L1 Expression and Other Variables With Benefit From Immune Checkpoint Inhibition in Advanced Gastroesophageal Cancer: Systematic Review and Meta-analysis of 17 Phase 3 Randomized Clinical Trials. JAMA Oncol. 2022, 8, 1456–1465. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Electronic address: Clinicalguidelines@esmo.org. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Van Cutsem, E.; di Bartolomeo, M.; Smyth, E.; Chau, I.; Park, H.; Siena, S.; Lonardi, S.; Wainberg, Z.A.; Ajani, J.; Chao, J.; et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): Primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023, 24, 744–756. [Google Scholar]
- Makiyama, A.; Sukawa, Y.; Kashiwada, T.; Kawada, J.; Hosokawa, A.; Horie, Y.; Tsuji, A.; Moriwaki, T.; Tanioka, H.; Shinozaki, K.; et al. Randomized, Phase II Study of Trastuzumab Beyond Progression in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT Study). J. Clin. Oncol. 2020, 38, 1919–1927. [Google Scholar] [CrossRef]
- Wekking, D.; Porcu, M.; Pellegrino, B.; Lai, E.; Mura, G.; Denaro, N.; Saba, L.; Musolino, A.; Scartozzi, M.; Solinas, C. Multidisciplinary clinical guidelines in proactive monitoring, early diagnosis, and effective management of trastuzumab deruxtecan (T-DXd)-induced interstitial lung disease (ILD) in breast cancer patients. ESMO Open 2023, 8, 102043. [Google Scholar] [CrossRef]
- Shimozaki, K.; Fukuoka, S.; Ooki, A.; Yamaguchi, K. HER2-low gastric cancer: Is the subgroup targetable? ESMO Open 2024, 9, 103679. [Google Scholar] [CrossRef]
- Kawakami, H.; Hironaka, S.; Esaki, T.; Chayama, K.; Tsuda, M.; Sugimoto, N.; Kadowaki, S.; Makiyama, A.; Machida, N.; Hirano, H.; et al. An Investigator-Initiated Phase 2 Study of Nivolumab Plus Low-Dose Ipilimumab as First-Line Therapy for Microsatellite Instability-High Advanced Gastric or Esophagogastric Junction Cancer (NO LIMIT, WJOG13320G/CA209-7W7). Cancers 2021, 13, 805. [Google Scholar] [CrossRef]
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Marabelle, A.; Cassier, P.A.; Fakih, M.; Kao, S.; Nielsen, D.; Italiano, A.; Guren, T.K.; van Dongen, M.G.J.; Spencer, K.; Bariani, G.M.; et al. Pembrolizumab for previously treated advanced anal squamous cell carcinoma: Results from the non-randomised, multicohort, multicentre, phase 2 KEYNOTE-158 study. Lancet Gastroenterol. Hepatol. 2022, 7, 446–454. [Google Scholar] [CrossRef]
- Lordick, F.; Van Cutsem, E.; Shitara, K.; Xu, R.H.; Ajani, J.A.; Shah, M.A.; Oh, M.; Ganguli, A.; Chang, L.; Rhoten, S.; et al. Health-related quality of life in patients with CLDN18.2-positive, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma: Results from the SPOTLIGHT and GLOW clinical trials. ESMO Open 2024, 9, 103663. [Google Scholar] [CrossRef]
- Klempner, S.J.; Lee, K.W.; Shitara, K.; Metges, J.P.; Lonardi, S.; Ilson, D.H.; Fazio, N.; Kim, T.Y.; Bai, L.Y.; Moran, D.; et al. ILUSTRO: Phase II Multicohort Trial of Zolbetuximab in Patients with Advanced or Metastatic Claudin 18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Clin. Cancer Res. 2023, 29, 3882–3891. [Google Scholar] [CrossRef]
- Nakayama, I.; Qi, C.; Chen, Y.; Nakamura, Y.; Shen, L.; Shitara, K. Claudin 18.2 as a novel therapeutic target. Nat. Rev. Clin. Oncol. 2024, 21, 354–369. [Google Scholar] [CrossRef]
- Gordon, A.; Johnston, E.; Lau, D.K.; Starling, N. Targeting FGFR2 Positive Gastroesophageal Cancer: Current and Clinical Developments. Onco. Targets Ther. 2022, 15, 1183–1196. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.K.; Qin, S.; Yamaguchi, K.; Kim, I.H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022, 23, 1430–1440. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Lorenzen, S.; Thuss-Patience, P.; Pauligk, C.; Gökkurt, E.; Ettrich, T.; Lordick, F.; Stahl, M.; Reichardt, P.; Sökler, M.; Pink, D.; et al. FOLFIRI plus ramucirumab versus paclitaxel plus ramucirumab as second-line therapy for patients with advanced or metastatic gastroesophageal adenocarcinoma with or without prior docetaxel–results from the phase II RAMIRIS Study of the German Gastric Cancer Study Group at AIO. Eur. J. Cancer 2022, 165, 48–57. [Google Scholar]
- Lam, L.L.; Pavlakis, N.; Shitara, K.; Sjoquist, K.M.; Martin, A.J.; Yip, S.; Kang, Y.K.; Bang, Y.J.; Chen, L.T.; Moehler, M.; et al. INTEGRATE II: Randomised phase III controlled trials of regorafenib containing regimens versus standard of care in refractory Advanced Gastro-Oesophageal Cancer (AGOC): A study by the Australasian Gastro-Intestinal Trials Group (AGITG). BMC Cancer 2023, 23, 180. [Google Scholar] [CrossRef]
- Ambrosini, M.; Del Re, M.; Manca, P.; Hendifar, A.; Drilon, A.; Harada, G.; Ree, A.H.; Klempner, S.; Mælandsmo, G.M.; Flatmark, K.; et al. ALK Inhibitors in Patients With ALK Fusion-Positive GI Cancers: An International Data Set and a Molecular Case Series. JCO Precis. Oncol. 2022, 6, e2200015. [Google Scholar] [CrossRef]
- Chon, H.J.; Kim, H.R.; Shin, E.; Kim, C.; Heo, S.J.; Lee, C.K.; Park, J.K.; Noh, S.H.; Chung, H.C.; Rha, S.Y. The Clinicopathologic Features and Prognostic Impact of ALK Positivity in Patients with Resected Gastric Cancer. Ann. Surg. Oncol. 2015, 22, 3938–3945. [Google Scholar] [CrossRef]
- Deng, N.; Goh, L.K.; Wang, H.; Das, K.; Tao, J.; Tan, I.B.; Zhang, S.; Lee, M.; Wu, J.; Lim, K.H.; et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012, 61, 673–684. [Google Scholar] [CrossRef]
- Catenacci, D.V.; Moya, S.; Lomnicki, S.; Chase, L.M.; Peterson, B.F.; Reizine, N.; Alpert, L.; Setia, N.; Xiao, S.Y.; Hart, J.; et al. Personalized Antibodies for Gas-troesophageal Adenocarcinoma (PANGEA): A Phase II Study Evaluating an Individualized Treatment Strategy for Meta-static Disease. Cancer Discov. 2021, 11, 308–325. [Google Scholar] [CrossRef]
- Ebert, K.; Haffner, I.; Zwingenberger, G.; Keller, S.; Raimúndez, E.; Geffers, R.; Wirtz, R.; Barbaria, E.; Hollerieth, V.; Arnold, R.; et al. Combining gene expression analysis of gastric cancer cell lines and tumor specimens to identify biomarkers for anti-HER therapies—The role of HAS2, SHB and HBEGF. BMC Cancer 2022, 22, 254. [Google Scholar] [CrossRef]
- Lordick, F.; Kang, Y.K.; Chung, H.C.; Salman, P.; Oh, S.C.; Bodoky, G.; Kurteva, G.; Volovat, C.; Moiseyenko, V.M.; Gorbunova, V.; et al. Capecitabine and cisplatin with or without cetuxi-mab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 490–499. [Google Scholar] [CrossRef]
- Moehler, M.; Mueller, A.; Trarbach, T.; Lordick, F.; Seufferlein, T.; Kubicka, S.; Geissler, M.; Schwarz, S.; Galle, P.R.; Kanzler, S.; et al. Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: A prospective multi-center biomarker-oriented phase II study. Ann. Oncol. 2011, 22, 1358–1366. [Google Scholar] [CrossRef]
- Lennerz, J.K.; Kwak, E.L.; Ackerman, A.; Michael, M.; Fox, S.B.; Bergethon, K.; Lauwers, G.Y.; Christensen, J.G.; Wilner, K.D.; Haber, D.A.; et al. MET Amplification Identifies a Small and Aggressive Subgroup of Esophagogastric Adenocarcinoma With Evidence of Responsiveness to Crizotinib. JCO 2011, 29, 4803–4810. [Google Scholar] [CrossRef]
- Bahleda, R.; Italiano, A.; Hierro, C.; Mita, A.; Cervantes, A.; Chan, N.; Awad, M.; Calvo, E.; Moreno, V.; Govindan, R.; et al. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors. Clin. Cancer Res. 2019, 25, 4888–4897. [Google Scholar] [CrossRef]
- Cha, Y.; Kim, H.-P.; Lim, Y.; Han, S.-W.; Song, S.-H.; Kim, T.-Y. FGFR2 amplification is predictive of sensitivity to regorafenib in gastric and colorectal cancers in vitro. Mol. Oncol. 2018, 12, 993–1003. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-line pemigatinib vs gem-citabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef]
- Betts, G.; Valentine, H.; Pritchard, S.; Swindell, R.; Williams, V.; Morgan, S.; Griffiths, E.A.; Welch, I.; West, C.; Womack, C. FGFR2, HER2 and cMet in gastric adenocar-cinoma: Detection, prognostic significance and assessment of downstream pathway activation. Virchows Arch. 2014, 464, 145–156. [Google Scholar] [CrossRef]
- Weaver, A.; Bossaer, J.B. Fibroblast growth factor receptor (FGFR) inhibitors: A review of a novel therapeutic class. J. Oncol. Pharm Pr. 2021, 27, 702–710. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef]
- Merz, V.; Zecchetto, C.; Simionato, F.; Cavaliere, A.; Casalino, S.; Pavarana, M.; Giacopuzzi, S.; Bencivenga, M.; Tomezzoli, A.; Santoro, R.; et al. A phase II trial of the FGFR inhibitor pem-igatinib in patients with metastatic esophageal-gastric junction/gastric cancer trastuzumab resistant: The FiGhTeR trial. Ther. Adv. Med. Oncol. 2020, 12, 1758835920937889. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Siu, H.C.; Leung, S.Y.; Stratton, M.R. A mutational signature in gastric cancer suggests therapeu-tic strategies. Nat. Commun. 2015, 6, 8683. [Google Scholar] [CrossRef]
- Bang, Y.J.; Xu, R.H.; Chin, K.; Lee, K.W.; Park, S.H.; Rha, S.Y.; Shen, L.; Qin, S.; Xu, N.; Im, S.A.; et al. Olaparib in combination with paclitaxel in patients with ad-vanced gastric cancer who have progressed following first-line therapy (GOLD): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1637–1651. [Google Scholar] [CrossRef]
- Petrelli, A.; Rizzolio, S.; Pietrantonio, F.; Bellomo, S.E.; Benelli, M.; De Cecco, L.; Romagnoli, D.; Berrino, E.; Orrù, C.; Ribisi, S.; et al. BRCA2 Germline Mutations Identify Gastric Cancers Responsive to PARP Inhibitors. Cancer Res. 2023, 83, 1699–1710. [Google Scholar] [CrossRef]
- Secrier, M.; Li, X.; De Silva, N.; Eldridge, M.D.; Contino, G.; Bornschein, J.; MacRae, S.; Grehan, N.; O’Donovan, M.; Miremadi, A.; et al. Mutational signatures in esophageal adeno-carcinoma define etiologically distinct subgroups with therapeutic relevance. Nat. Genet. 2016, 48, 1131–1141. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Yaeger, R.; Spira, A.I.; Pelster, M.S.; Sabari, J.K.; Hafez, N.; Barve, M.; Velastegui, K.; Yan, X.; Shetty, A.; et al. Adagrasib in Advanced Solid Tumors Harboring a KRASG12C Mutation. J. Clin. Oncol. 2023, 41, 4097–4106. [Google Scholar] [CrossRef]
- Salem, M.E.; El-Refai, S.M.; Sha, W.; Puccini, A.; Grothey, A.; George, T.J.; Hwang, J.J.; O’Neil, B.; Barrett, A.S.; Kadakia, K.C.; et al. Landscape of KRASG12C, Associated Ge-nomic Alterations, and Interrelation With Immuno-Oncology Biomarkers in KRAS-Mutated Cancers. JCO Precis. Oncol. 2022, 6, e2100245. [Google Scholar] [CrossRef]
- Aparicio, T.; Cozic, N.; de La Fouchardière, C.; Meriaux, E.; Plaza, J.; Mineur, L.; Guimbaud, R.; Samalin, E.; Mary, F.; Lecomte, T.; et al. The Activity of Crizotinib in Chemo-Refractory MET-Amplified Esophageal and Gastric Adenocarcinomas: Results from the AcSé-Crizotinib Program. Target Oncol. 2021, 16, 381–388. [Google Scholar] [CrossRef]
- Lee, J.; Tran, P.; Klempner, S.J. Targeting the MET Pathway in Gastric and Oesophageal Cancers: Refining the Optimal Approach. Clin. Oncol. 2016, 28, e35–e44. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.T.; Kim, K.; Lee, H.; Kozarewa, I.; Mortimer, P.G.; Odegaard, J.I.; Harrington, E.A.; Lee, J.; Lee, T.; et al. Tumor Genomic Profiling Guides Patients with Metastat-ic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov. 2019, 9, 1388–1405. [Google Scholar] [CrossRef]
- Akimoto, E.; Kuwata, T.; Shitara, K.; Kawazoe, A.; Sakamoto, N.; Ishii, G.; Ochiai, A.; Kinoshita, T. Impact of Programmed Death-Ligand 1 Expres-sion on Mismatch Repair Deficiency and Epstein-Barr Virus Status on Survival Outcomes in Patients with Stage II/III Gas-tric Cancer After Surgery. Ann. Surg. Oncol. 2023, 30, 5227–5236. [Google Scholar] [CrossRef]
- Derks, S.; Liao, X.; Chiaravalli, A.M.; Xu, X.; Camargo, M.C.; Solcia, E.; Sessa, F.; Fleitas, T.; Freeman, G.J.; Rodig, S.J.; et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget 2016, 7, 32925–32932. [Google Scholar] [CrossRef]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clini-cal responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- van Der Sluis, K.; van Sandick, J.W.; van Dieren, J.M.; Vollebergh, M.A.; Grootscholten, C.; van den Berg, J.G.; Snaebjornsson, P.; Hartemink, K.J.; Veenhof, A.A.; Chalabi, M.; et al. The clinical impact of testing for biomarkers in gastric cancer patients: A real-world cohort. Histopathology 2023, 82, 826–836. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.H.; Caglevic, C.; Chung, H.C.; Muro, K.; Van Cutsem, E.; et al. Molecular determinants of clinical outcomes with pembrolizumab versus paclitaxel in a randomized, open-label, phase III trial in patients with gastroesoph-ageal adenocarcinoma. Ann. Oncol. 2021, 32, 1127–1136. [Google Scholar] [CrossRef]
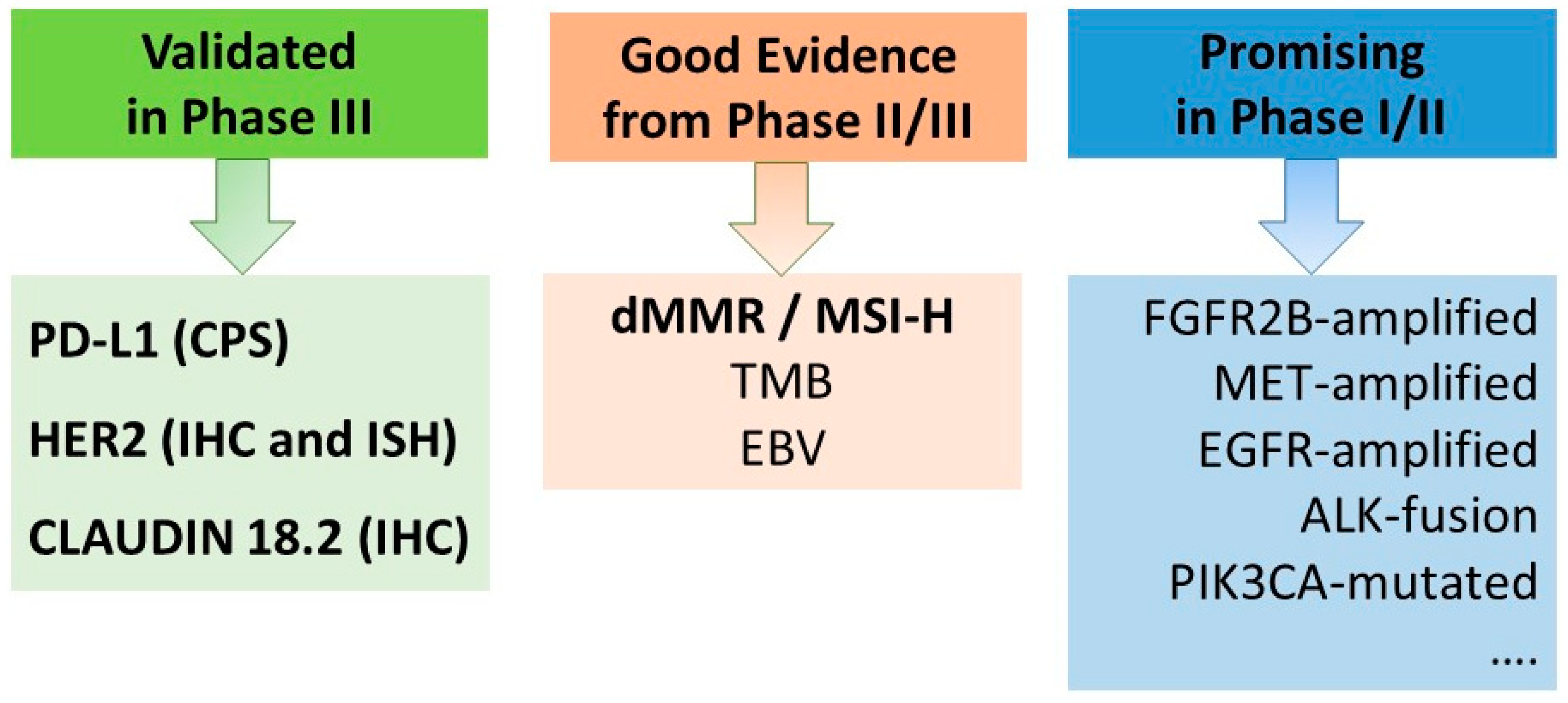
| Molecular Alteration | Frequency | Drug | Reference |
|---|---|---|---|
| ALK fusion/expression | 1%/8% | Alectinib, Lorlatinib | [64,65] |
| EGFR amp | 6–10% | Afatinib, Cetuximab | [65,66,67,68,69,70,71] |
| FGFR2 amp | 2–11% | Erdafitinib, Pemigatinib, Regorafenib, Futibatinib | [60,67,72,73,74,75,76,77] |
| FGFR1 amp | 2% | Erdafitinib, Pemigatinib, Regorafenib | [60,66,67,72,73,74,75] |
| FGFR3 amp | 2% | Erdafitinib, Pemigatinib, Regorafenib | [60,66,72,73,74,75,76,77,78] |
| HRD | 7–12% | Olaparib | [79,80,81,82] |
| KRAS G12C mut | 1% | Sotorasib, Adagrasib | [83,84] |
| MET amp | 2–11% | Crizotinib, Cabozantinib, Savolitinib | [67,82,85,86,87] |
| PIK3CA mut/amp | 3.5% | Alpelisib | [86] |
| Immuno markers | |||
| EBV+ in PD-L1- | <1–2% | ICI | [67,87,88,89,90,91] |
| TMB-high in MSS GC | 20% | ICI | [92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lordick, F.; Rha, S.Y.; Muro, K.; Yong, W.P.; Lordick Obermannová, R. Systemic Therapy of Gastric Cancer—State of the Art and Future Perspectives. Cancers 2024, 16, 3337. https://doi.org/10.3390/cancers16193337
Lordick F, Rha SY, Muro K, Yong WP, Lordick Obermannová R. Systemic Therapy of Gastric Cancer—State of the Art and Future Perspectives. Cancers. 2024; 16(19):3337. https://doi.org/10.3390/cancers16193337
Chicago/Turabian StyleLordick, Florian, Sun Young Rha, Kei Muro, Wei Peng Yong, and Radka Lordick Obermannová. 2024. "Systemic Therapy of Gastric Cancer—State of the Art and Future Perspectives" Cancers 16, no. 19: 3337. https://doi.org/10.3390/cancers16193337
APA StyleLordick, F., Rha, S. Y., Muro, K., Yong, W. P., & Lordick Obermannová, R. (2024). Systemic Therapy of Gastric Cancer—State of the Art and Future Perspectives. Cancers, 16(19), 3337. https://doi.org/10.3390/cancers16193337






