Using Deauville Scoring to Guide Consolidative Radiotherapy in Diffuse Large B-Cell Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Cohort and Design
2.2. Variables and Outcome Measure
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Prognostic Factors for TTP
3.3. Predictive Value of DV for RT
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Armashi, A.R.; Al Zubaidi, A.; Mahmood, O.; Thierheimer, M.; Alkrekshi, A.; Abdulhaq, H. Trends in Diffuse Large B-Cell Lymphoma Mortality in the United States: A Comprehensive Review from 2000 to 2020. Blood 2023, 142 (Suppl. S1), 1765. [Google Scholar] [CrossRef]
- Chua, B.J.G.; Low, C.E.; Yau, C.E.; Tan, Y.H.; Chiang, J.; Chang, E.W.Y.; Chan, J.Y.; Poon, E.Y.L.; Somasundaram, N.; Rashid, M.F.B.H.; et al. Recent updates on central nervous system prophylaxis in patients with high-risk diffuse large B-cell lymphoma. Exp. Hematol. Oncol. 2024, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Ip, A.; Mutebi, A.; Wang, T.; Jun, M.; Kalsekar, A.; Navarro, F.R.; Wang, A.; Kamalakar, R.; Sacchi, M.; Elliott, B. Treatment Outcomes with Standard of Care in Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Real-World Data Analysis. Adv. Ther. 2024, 41, 1226–1244. [Google Scholar] [CrossRef]
- Spinner, M.A.; Advani, R.H. Current Frontline Treatment of Diffuse Large B-Cell Lymphoma. Oncology 2022, 36, 51–58. [Google Scholar] [CrossRef]
- Candelaria, M.; Dueñas-Gonzalez, A. Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in diffuse large B-cell lymphoma. Ther. Adv. Hematol. 2021, 12, 2040620721989579. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Terasawa, T.; Nihashi, T.; Hotta, T.; Nagai, H. 18F-FDG PET for posttherapy assessment of Hodgkin’s disease and aggressive Non-Hodgkin’s lymphoma: A systematic review. J. Nucl. Med. 2008, 49, 13–21. [Google Scholar] [CrossRef][Green Version]
- Zijlstra, J.M.; Lindauer-van der Werf, G.; Hoekstra, O.S.; Hooft, L.; Riphagen, I.I.; Huijgens, P.C. 18F-fluoro-deoxyglucose positron emission tomography for post-treatment evaluation of malignant lymphoma: A systematic review. Haematologica 2006, 91, 522–529. [Google Scholar]
- Han, S.; Choi, J.Y. Prognostic value of (18)F-FDG PET and PET/CT for assessment of treatment response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2020, 22, 119. [Google Scholar] [CrossRef]
- John, J.R.; Oommen, R.; Hephzibah, J.; Mathew, D.; Korula, A.; Shanthly, N.; Eapen, A. Validation of Deauville Score for Response Evaluation in Hodgkin’s Lymphoma. Indian J. Nucl. Med. 2023, 38, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Kluge, R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur. J. Nucl. Med. Mol. Imaging 2017, 44 (Suppl. S1), 97–110. [Google Scholar] [CrossRef] [PubMed]
- Kluge, R.; Chavdarova, L.; Hoffmann, M.; Kobe, C.; Malkowski, B.; Montravers, F.; Kurch, L.; Georgi, T.; Dietlein, M.; Wallace, W.H.; et al. Inter-Reader Reliability of Early FDG-PET/CT Response Assessment Using the Deauville Scale after 2 Cycles of Intensive Chemotherapy (OEPA) in Hodgkin’s Lymphoma. PLoS ONE 2016, 11, e0149072. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, R.T.; Advani, R.H.; Ai, W.Z.; Ambinder, R.F.; Aoun, P.; Bello, C.M.; Benitez, C.M.; Bernat, K.; Bierman, P.J.; Blum, K.A.; et al. Hodgkin Lymphoma Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 608–638. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Sauter, C.S.; Matasar, M.J.; Meikle, J.; Schoder, H.; Ulaner, G.A.; Migliacci, J.C.; Hilden, P.; Devlin, S.M.; Zelenetz, A.D.; Moskowitz, C.H. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood 2015, 125, 2579–2581. [Google Scholar] [CrossRef]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.L.; Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019, 9, 44. [Google Scholar] [CrossRef]
- Tilly, H.; da Silva, M.G.; Vitolo, U.; Jack, A.; Meignan, M.; Lopez-Guillermo, A.; Walewski, J.; André, M.; Johnson, P.W.; Pfreundschuh, M.; et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2015, 26, v116–v125. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, S.J.; Mun, S.H.; Song, J.H.; Choi, B.O. Consolidative Radiotherapy after Complete Remission following R-CHOP Immunochemotherapy in Stage III–IV Diffuse Large B-Cell Lymphoma Patients: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3940. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Freeman, C.L.; Savage, K.J.; Villa, D.R.; Scott, D.W.; Srour, L.; Gerrie, A.S.; Brown, M.J.; Slack, G.W.; Farinha, P.; Skinnider, B.; et al. Long-term results of PET-guided radiation in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP. Blood 2021, 137, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Mazloom, A.; Medeiros, L.J.; Zreik, T.G.; Wogan, C.; Shihadeh, F.; Rodriguez, M.A.; Fayad, L.; Fowler, N.; Reed, V.; et al. Benefit of Consolidative Radiation Therapy in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP Chemotherapy. J. Clin. Oncol. 2010, 28, 4170–4176. [Google Scholar] [CrossRef] [PubMed]
- Pregno, P.; Chiappella, A.; Bellò, M.; Botto, B.; Ferrero, S.; Franceschetti, S.; Giunta, F.; Ladetto, M.; Limerutti, G.; Menga, M.; et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 2012, 119, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Weiler-Sagie, M.; Bushelev, O.; Epelbaum, R.; Dann, E.J.; Haim, N.; Avivi, I.; Ben-Barak, A.; Ben-Arie, Y.; Bar-Shalom, R.; Israel, O. 18F-FDG Avidity in Lymphoma Readdressed: A Study of 766 Patients. J. Nucl. Med. 2010, 51, 25. [Google Scholar] [CrossRef]
- Spaepen, K.; Stroobants, S.; Dupont, P.; Van Steenweghen, S.; Thomas, J.; Vandenberghe, P.; Vanuytsel, L.; Bormans, G.; Balzarini, J.; De Wolf-Peeters, C.; et al. Prognostic Value of Positron Emission Tomography (PET) With Fluorine-18 Fluorodeoxyglucose ([18F]FDG) After First-Line Chemotherapy in Non-Hodgkin’s Lymphoma: Is [18F]FDG-PET a Valid Alternative to Conventional Diagnostic Methods? J. Clin. Oncol. 2001, 19, 414–419. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Martelli, M.; Sehn, L.H.; Belada, D.; Carella, A.-M.; Chua, N.; Gonzalez-Barca, E.; Hong, X.; Pinto, A.; Shi, Y.; et al. End-of-treatment PET/CT predicts PFS and OS in DLBCL after first-line treatment: Results from GOYA. Blood Adv. 2021, 5, 1283–1290. [Google Scholar] [CrossRef]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef]
- Van Heertum, R.L.; Scarimbolo, R.; Wolodzko, J.G.; Klencke, B.; Messmann, R.; Tunc, F.; Sokol, L.; Agarwal, R.; Strafaci, J.A.; O’Neal, M. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: An operational approach for clinical trials. Drug Des. Devel. Ther. 2017, 11, 1719–1728. [Google Scholar] [CrossRef]
- Dabaja, B.S.; Phan, J.; Mawlawi, O.; Medeiros, L.J.; Etzel, C.; Liang, F.-W.; Podoloff, D.; Oki, Y.; Hagemeister, F.B.; Chuang, H.; et al. Clinical implications of positron emission tomography-negative residual computed tomography masses after chemotherapy for diffuse large B-cell lymphoma. Leuk. Lymphoma. 2013, 54, 2631–2638. [Google Scholar] [CrossRef][Green Version]
- Harrysson, S.; Eloranta, S.; Ekberg, S.; Enblad, G.; Jerkeman, M.; Wahlin, B.E.; Andersson, P.-O.; Smedby, K.E. Incidence of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) including CNS relapse in a population-based cohort of 4243 patients in Sweden. Blood Cancer J. 2021, 11, 9. [Google Scholar] [CrossRef]
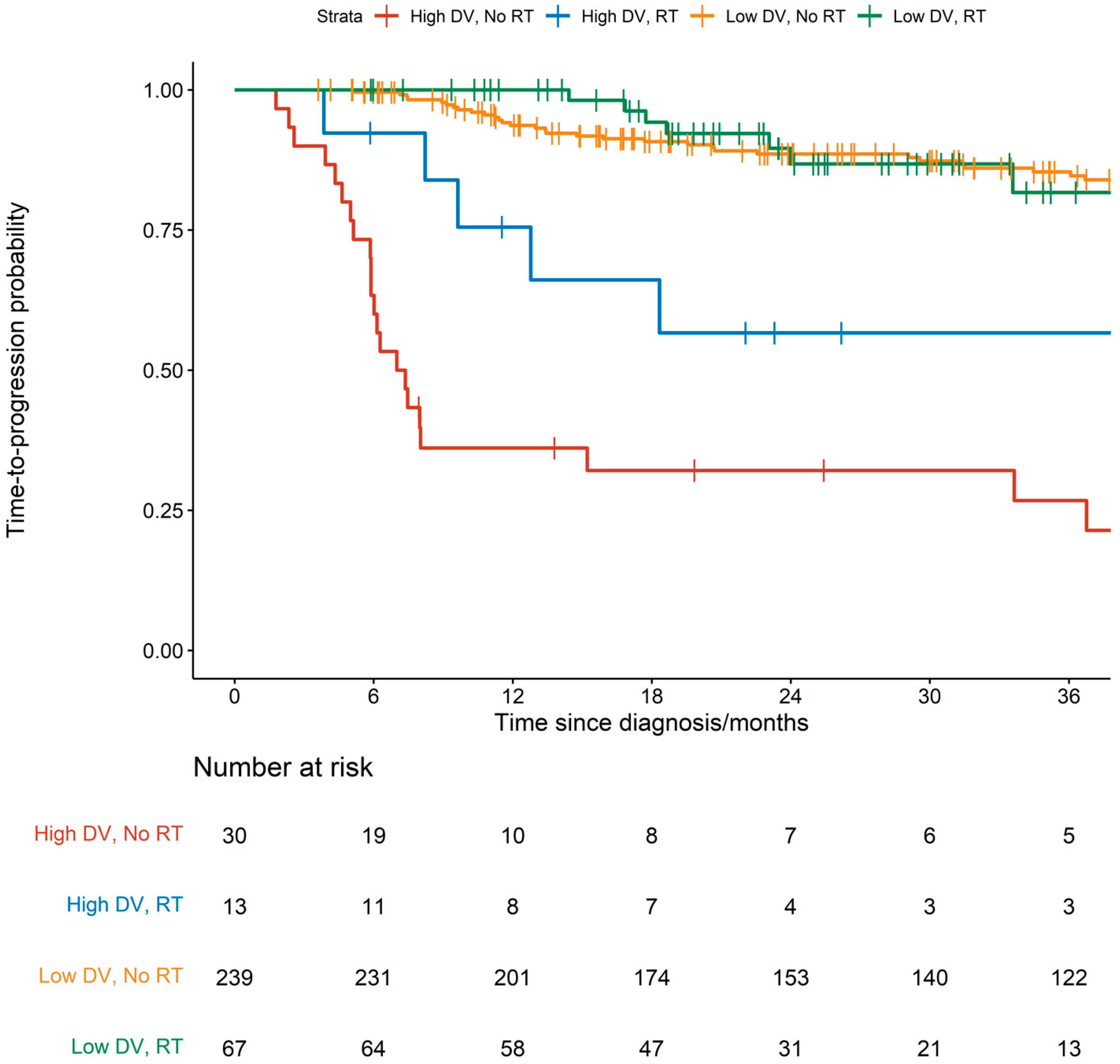
| Characteristic | RT-Omitted, N = 269 | RT-Treated, N = 80 | p-Value 2 |
|---|---|---|---|
| Gender | 0.616 | ||
| Female | 133/269 (49%) | 37/80 (46%) | |
| Male | 136/269 (51%) | 43/80 (54%) | |
| Age at Diagnosis 1 | 60.18 (15.18) | 58.33 (18.03) | 0.404 |
| Deauville Score | 0.008 | ||
| 1 | 145/269 (54%) | 30/80 (38%) | |
| 2 | 64/269 (24%) | 21/80 (26%) | |
| 3 | 30/269 (11%) | 16/80 (20%) | |
| 4 | 12/269 (4.5%) | 10/80 (13%) | |
| 5 | 18/269 (6.7%) | 3/80 (3.8%) | |
| Deauville Score | 0.223 | ||
| DV4-5 | 30/269 (11%) | 13/80 (16%) | |
| DV1-3 | 239/269 (89%) | 67/80 (84%) | |
| IPI Risk Group | 0.151 | ||
| 1 | 82/269 (30%) | 33/80 (41%) | |
| 2 | 77/269 (29%) | 20/80 (25%) | |
| 3 | 66/269 (25%) | 13/80 (16%) | |
| 4 | 37/269 (14%) | 14/80 (18%) | |
| Unavailable | 7/269 (2.6%) | 0/80 (0%) | |
| Stratified IPI Risk Group | 0.276 | ||
| High ≥ 3 | 103/269 (38%) | 27/80 (34%) | |
| Low < 3 | 159/269 (59%) | 53/80 (66%) | |
| Unavailable | 7/269 (2.6%) | 0/80 (0%) | |
| Bulky Status | 0.003 | ||
| Bulky | 28/269 (10%) | 20/80 (25%) | |
| Not Bulky | 216/269 (80%) | 52/80 (65%) | |
| Unavailable | 25/269 (9.3%) | 8/80 (10%) | |
| ECOG Status | 0.571 | ||
| 0 | 162/269 (60%) | 53/80 (66%) | |
| 1 | 89/269 (33%) | 24/80 (30%) | |
| 2 | 8/269 (3.0%) | 3/80 (3.8%) | |
| 3 | 3/269 (1.1%) | 0/80 (0%) | |
| Unavailable | 7/269 (2.6%) | 0/80 (0%) | |
| Stratified ECOG Status | 0.526 | ||
| High ≥ 2 | 11/269 (4.1%) | 3/80 (3.8%) | |
| Low < 2 | 251/269 (93%) | 77/80 (96%) | |
| Unavailable | 7/269 (2.6%) | 0/80 (0%) | |
| B Symptoms | 0.117 | ||
| No | 150/269 (56%) | 35/80 (44%) | |
| Yes | 74/269 (28%) | 31/80 (39%) | |
| Unavailable | 45/269 (17%) | 14/80 (18%) | |
| Elevated LDH | 0.039 | ||
| No | 72/269 (27%) | 11/80 (14%) | |
| Yes | 192/269 (71%) | 68/80 (85%) | |
| Unavailable | 5/269 (1.9%) | 1/80 (1.3%) | |
| Extranodal Involvement | 0.105 | ||
| No | 109/269 (41%) | 23/80 (29%) | |
| Yes | 159/269 (59%) | 57/80 (71%) | |
| Unavailable | 1/269 (0.4%) | 0/80 (0%) | |
| Hemoglobin (g/dL) | 12.18 (2.03) | 11.67 (2.00) | 0.047 |
| Unknown | 1 | 0 | |
| Hemoglobin (g/dL) | 0.127 | ||
| Hb < 11 | 71/269 (26%) | 30/80 (38%) | |
| Hb ≥ 11 | 197/269 (73%) | 50/80 (63%) | |
| Unavailable | 1/269 (0.4%) | 0/80 (0%) | |
| Bone Marrow Involvement | 0.293 | ||
| No | 243/269 (90%) | 77/80 (96%) | |
| Yes | 21/269 (7.8%) | 3/80 (3.8%) | |
| Unavailable | 5/269 (1.9%) | 0/80 (0%) | |
| Extranodal Involvement | 0.619 | ||
| Extranodal sites = 0/1 | 199/269 (74%) | 57/80 (71%) | |
| Extranodal sites ≥ 2 | 67/269 (25%) | 23/80 (29%) | |
| Unavailable | 3/269 (1.1%) | 0/80 (0%) | |
| Ann Arbor Staging | 0.007 | ||
| Ann Stage I/II | 115/269 (43%) | 49/80 (61%) | |
| Ann Stage III/IV | 153/269 (57%) | 31/80 (39%) | |
| Unavailable | 1/269 (0.4%) | 0/80 (0%) | |
| Chemotherapy | 0.004 | ||
| CVP-R | 2/269 (0.7%) | 2/80 (2.5%) | |
| EPOCH-R | 34/269 (13%) | 17/80 (21%) | |
| De-Angelis-R | 0/269 (0%) | 2/80 (2.5%) | |
| CHOEP-R | 1/269 (0.4%) | 0/80 (0%) | |
| RCEPP | 1/269 (0.4%) | 0/80 (0%) | |
| MR-CHOP | 17/269 (6.3%) | 10/80 (13%) | |
| CHOP | 3/269 (1.1%) | 1/80 (1.3%) | |
| R-CHOP | 193/269 (72%) | 40/80 (50%) | |
| Others | 18/269 (6.7%) | 8/80 (10%) |
| Events/Patients | HR (95% CI. p-Value) | ||
|---|---|---|---|
| Age | Per year increase | 78/349 | 1.02 (1.00–1.04, p = 0.010) |
| Deauville Score | DV1-3 | 51/306 | 1.00 (ref) |
| DV4-5 | 27/43 | 6.74 (4.21–10.79, p < 0.001) | |
| International Prognostic Index | High ≥ 3 | 44/130 | 1.00 (ref) |
| Low < 3 | 34/212 | 0.41 (0.26–0.64, p < 0.001) | |
| Unavailable | 0/7 | 0.00 (0.00–Inf, p = 0.995) | |
| Overall Bulky Status | bulky | 13/48 | 1.00 (ref) |
| not bulky | 59/268 | 0.72 (0.39–1.31, p = 0.280) | |
| Unavailable | 6/33 | 0.60 (0.23–1.58, p = 0.303) | |
| ECOG Score | High ≥ 2 | 8/14 | 1.00 (ref) |
| Low < 2 | 68/328 | 0.26 (0.13–0.55, p < 0.001) | |
| Unavailable | 2/7 | 0.33 (0.07–1.59, p = 0.168) | |
| Extranodal Involvement | No | 26/132 | 1.00 (ref) |
| Yes | 52/216 | 1.41 (0.88–2.25, p = 0.157) | |
| Unavailable | 0/1 | 0.00 (0.00–Inf, p = 0.996) | |
| Presence of B Symptoms | No | 32/185 | 1.00 (ref) |
| Yes | 33/105 | 2.10 (1.29–3.41, p = 0.003) | |
| Unavailable | 13/59 | 1.32 (0.69–2.51, p = 0.404) | |
| Receipt of Consolidative RT | RT-omitted | 65/269 | 1.00 (ref) |
| RT-treated | 13/80 | 0.81 (0.45–1.48, p = 0.501) | |
| Elevated LDH | No | 12/83 | 1.00 (ref) |
| Yes | 65/260 | 1.89 (1.02–3.50, p = 0.043) | |
| Unavailable | 1/6 | 1.23 (0.16–9.46, p = 0.843) | |
| Hemoglobin Levels | Mean (SD) | 78/348 | 0.84 (0.75–0.93, p = 0.001) |
| Marrow Involvement | No | 64/320 | 1.00 (ref) |
| Yes | 13/24 | 2.76 (1.52–5.01, p = 0.001) | |
| Unavailable | 1/5 | 2.06 (0.29–14.93, p = 0.473) | |
| Ann Arbor Staging | Ann Stage I/IE | 3/65 | 1.00 (ref) |
| Ann Stage II/IIE | 14/99 | 3.50 (1.01–12.18, p = 0.049) | |
| Ann Stage III/IIIE | 22/52 | 13.06 (3.91–43.67, p < 0.001) | |
| Ann Stage IV | 39/132 | 8.31 (2.57–26.89, p < 0.001) | |
| Unavailable | 0/1 | 0.00 (0.00–Inf, p = 0.997) | |
| Ann Arbor Staging | Ann Stage I/II | 17/164 | 1.00 (ref) |
| Ann Stage III/IV | 61/184 | 3.94 (2.30–6.74, p < 0.001) | |
| Unavailable | 0/1 | 0.00 (0.00–Inf, p = 0.997) |
| Characteristic | HR | 95% CI | p-Value |
|---|---|---|---|
| Receipt of Consolidative RT | 0.009 | ||
| RT-omitted | 1.00 (ref) | — | |
| RT-treated | 0.290 | 0.105, 0.801 | |
| Deauville Score | 0.000 | ||
| DV4-5 | 1.00 (ref) | — | |
| DV1-3 | 0.108 | 0.060, 0.192 | |
| Marrow Involvement | 0.111 | ||
| No | 1.00 (ref) | — | |
| Yes | 1.902 | 0.992, 3.646 | |
| Unavailable | 3.684 | 0.493, 27.52 | |
| Hemoglobin Levels | 0.967 | 0.851, 1.099 | 0.608 |
| Presence of B Symptoms | 0.044 | ||
| No | 1.00 (ref) | — | |
| Yes | 1.797 | 1.037, 3.113 | |
| Unavailable | 0.844 | 0.427, 1.668 | |
| International Prognostic Index | 0.064 | ||
| High ≥ 3 | 1.00 (ref) | — | |
| Low < 3 | 0.743 | 0.447, 1.236 | |
| Unavailable | 0.000 | 0.000, Inf | |
| Interaction term between Receipt of RT and Deauville Score | 2.977 | 0.840, 10.55 | 0.087 |
| Unadjusted Model | Multivariable-Adjusted Model ^ | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | p (Int *) | HR (95% CI) | p | p (Int *) | |
| TTP (78 events) | ||||||
| Overall: RT-treated vs. RT-omitted | 0.81 (0.45, 1.48) | 0.492 | - | 0.80 (0.44, 1.47) | 0.466 | - |
| DV4-5: RT-treated vs. RT-omitted | 0.33 (0.13, 0.88) | 0.027 | 0.133 | 0.29 (0.10, 0.80) | 0.017 | 0.087 |
| DV1-3: RT-treated vs. RT-omitted | 0.85 (0.40, 1.81) | 0.671 | 0.86 (0.40, 1.86) | 0.707 | ||
| Unadjusted Model | Multivariable-Adjusted Model ^ | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | p (Int *) | HR (95% CI) | p | p (Int *) | |
| TTP Censored at 24 months # (55 events) | ||||||
| Overall: RT-treated vs. RT-omitted | 0.83 (0.43, 1.60) | 0.564 | - | 0.77 (0.40, 1.51) | 0.437 | - |
| DV4-5: RT-treated vs. RT-omitted | 0.35 (0.13, 0.94) | 0.037 | 0.166 | 0.28 (0.10, 0.79) | 0.017 | 0.114 |
| DV1-3: RT-treated vs. RT-omitted | 0.90 (0.37, 2.20) | 0.820 | 0.84 (0.34, 2.08) | 0.711 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yau, C.E.; Low, C.E.; Ong, W.S.; Khoo, L.P.; Hoe, J.T.M.; Tan, Y.H.; Chang, E.W.Y.; Yang, V.S.; Poon, E.Y.L.; Chan, J.Y.; et al. Using Deauville Scoring to Guide Consolidative Radiotherapy in Diffuse Large B-Cell Lymphoma. Cancers 2024, 16, 3311. https://doi.org/10.3390/cancers16193311
Yau CE, Low CE, Ong WS, Khoo LP, Hoe JTM, Tan YH, Chang EWY, Yang VS, Poon EYL, Chan JY, et al. Using Deauville Scoring to Guide Consolidative Radiotherapy in Diffuse Large B-Cell Lymphoma. Cancers. 2024; 16(19):3311. https://doi.org/10.3390/cancers16193311
Chicago/Turabian StyleYau, Chun En, Chen Ee Low, Whee Sze Ong, Lay Poh Khoo, Joshua Tian Ming Hoe, Ya Hwee Tan, Esther Wei Yin Chang, Valerie Shiwen Yang, Eileen Yi Ling Poon, Jason Yongsheng Chan, and et al. 2024. "Using Deauville Scoring to Guide Consolidative Radiotherapy in Diffuse Large B-Cell Lymphoma" Cancers 16, no. 19: 3311. https://doi.org/10.3390/cancers16193311
APA StyleYau, C. E., Low, C. E., Ong, W. S., Khoo, L. P., Hoe, J. T. M., Tan, Y. H., Chang, E. W. Y., Yang, V. S., Poon, E. Y. L., Chan, J. Y., Sin, I. H., Yeoh, K. W., Somasundaram, N., Harunal Rashid, M. F. B., Tao, M., Lim, S. T., & Chiang, J. (2024). Using Deauville Scoring to Guide Consolidative Radiotherapy in Diffuse Large B-Cell Lymphoma. Cancers, 16(19), 3311. https://doi.org/10.3390/cancers16193311






