Simple Summary
Allostatic load is a biomarker of chronic stress. It has been implicated in the etiology of multiple chronic diseases. However, the role of allostatic load in lung carcinogenesis is still largely unknown. Given cigarette smoking could modify levels of allostatic load, the investigation of the relationship between allostatic load and lung cancer risk is of particular interest. This work thus aims to carry out the first study to prospectively assess the association.
Abstract
Background: Allostatic load (AL) is a biomarker of chronic stress associated with various chronic diseases. No study has evaluated the relationship between AL and lung cancer risk. Methods: To address this gap, we analyzed the association between AL and the development of lung cancer in 344,380 participants from the UK Biobank. Results: During the follow-up period from 2006 to 2020, 2517 participants were diagnosed with incident lung cancer. Participants who developed lung cancer had significantly higher AL compared to cancer-free controls (mean: 3.49 vs. 2.87, p < 0.001). In the multivariate analysis, a marginally significant association was observed between higher AL and increased lung cancer risk (per one AL unit: Hazard Ratio [HR] = 1.02, 95% Confidence Interval [CI]: 0.99, 1.04). In the categorical analysis, individuals with high AL (AL > 2) had a 15% higher risk of lung cancer compared to those with low AL (AL ≤ 2) (HR = 1.15, 95% CI: 1.05, 1.25). Stratified analyses revealed that this increased risk was only observed in former (HR = 1.38, 95% CI: 1.06, 1.43) and current smokers (HR = 1.25, 95% CI: 1.10, 1.42) but not in never-smokers (HR = 0.93, 95% CI: 0.74, 1.17). Moreover, we found that demographics, socioeconomics, and other health behaviors could modify the risk association. Finally, among cigarette smoking-related variables, a significant trend of increasing AL was observed with higher pack-years, longer smoking duration, earlier age of smoking initiation, and later age of smoking cessation. Conclusions: These findings suggest that higher AL is associated with an increased risk of lung cancer. The results need to be further confirmed in additional studies.
1. Introduction
Allostatic load (AL), a biomarker of chronic stress, reflects the physiological “wear and tear” resulting from an individual’s exposure to stressors over their lifetime [1]. High AL scores have been significantly associated with worsened psychiatric symptoms, cognitive decline, physical deterioration, and increased risk of a variety of chronic diseases and mortalities [2]. Recent research has also explored AL’s role in cancer outcomes, mainly in breast cancer [3]. Elevated AL scores are linked to increased risk [4], poorer tumor characteristics [5], and mortality [6,7] in breast cancer. So far, only one study has ever been done on lung cancer. In non-small cell lung cancer patients, elevated AL was found to correlate with worse overall mortality [8].
In fact, lung cancer, the top cause of cancer-related death in the US and the second most prevalent cancer among both men and women, is of relevance to AL. Several lines of evidence support AL’s role in lung cancer etiology. First, past studies have demonstrated that cigarette smoking, a major risk factor for lung cancer, is associated with increased AL [9,10,11,12]. Our analysis of SWAN data revealed that both current and former smokers exhibit significantly higher AL levels compared to never-smokers [13]. Second, stressful life events have been linked to a higher risk of lung cancer [14,15,16,17]. Third, recent research indicates that stress contributes to lung carcinogenesis [18,19]. Chronic stress has been shown to facilitate lung tumorigenesis by promoting exocytosis of IGF2 in lung epithelial cells and increasing metastasis through neutrophil-mediated changes in the tumor microenvironment [19]. Fourth, individuals with higher levels of stress are more likely to engage in heavy smoking and are less likely to successfully quit in comparison to their counterparts [20,21]. Despite the compelling evidence above, no epidemiological studies have yet assessed the relationship between AL and lung cancer risk.
In this study, we aim to address this gap by using the valuable resources of the UK Biobank to assess whether AL is associated with an increased risk of lung cancer. We also analyzed smoking-related variables to clarify their relationship with AL.
2. Methods
Study Cohort: The UK Biobank project, conducted from 2006 to 2010, enrolled over 500,000 volunteers aged 46–69 across the UK [22]. Participants completed a detailed touch-screen questionnaire, provided biological samples, and underwent various physical measurements. For our study, we identified 502,241 participants as the initial cohort. We focused on individuals without a history of cancer at the time of enrollment, excluding those with benign tumors, carcinoma in situ, nonmelanoma skin cancer, or cancers of unknown prevalence. Additionally, participants lacking complete information on any of the 11 factors required to construct AL scores were excluded. This stringent selection process resulted in a final analysis cohort of 344,380 participants.
Lung Cancer Cases Ascertainment: For this study, incident lung cancer cases were identified using ICD-10 codes reported in the UK Biobank database (Supplement Table S1). A stepwise selection of study participants was shown in Supplement Figure S1. Participants were followed up until 31 December 2020, with a median follow-up period of 11.6 years. Incident lung cancer cases were defined as those diagnosed with malignant neoplasms of the lung during the follow-up period. We excluded participants diagnosed with lung cancer within one year of their initial enrollment (N = 208) to ensure robust data. From the entire study population of 344,380 participants, we identified 2517 individuals with incident lung cancer.
AL Score Construction: In this study, we developed an Allostatic Load (AL) score utilizing eleven factors derived from baseline measurements (Supplement Table S2). Following the methodology outlined by Zhao and Chyu et al. [4,13,23], the AL score included biomarkers across three health domains: cardiovascular (Systolic Blood Pressure [SBP], Diastolic Blood Pressure [DBP], and Pulse Rate [PR]), inflammatory (C-reactive Protein [CRP]), and metabolic indicators (High-Density Lipoprotein [HDL], Waist-to-Hip Ratio, Abnormal Cholesterol, Triglycerides [TG], Hemoglobin A1c [HbA1c], and Creatinine). Each biomarker was assessed against established clinical thresholds, with scores of 0 or 1. Additionally, the history of medication use for metabolic diseases and hypertension was included as a factor, with a score of 1 given to those with such a history and 0 to those without. Consequently, the AL score ranged from 0 to 11, summing all factors. Based on the distribution, we further categorized the AL score into a dichotomized variable, either the low AL group (AL ≤ 2) or the high AL group (AL > 2).
Assessment of covariates: To adjust potential confounders, we included a list of covariates. They were age, gender, race, family history of lung cancer, education level, employment, family income, the Townsend deprivation index, cigarette smoking, alcohol consumption, sleep quality, and physical activity. For most categorical covariates (including gender, race/ethnicity, employment status, smoking status, alcohol consumption, and sleep quality), we used the UK Biobank codebook to define the category. Family history of lung cancer was determined by whether any first- or second-degree family member had been diagnosed with lung cancer. The income category was determined using £31,000 as the cutoff point. The Townsend deprivation index, derived from participants’ postcodes, was classified as low or high based on the median value. Education levels were categorized into “High school or less” and “College/professional”. Physical activity was evaluated using MET scores and classified into “Low”, “Moderate”, and “High”.
Statistical analysis: First, we compared differences in the mean (for continuous variables) and distribution (for categorical variables) of each covariate between lung cancer cases and cancer-free controls. Next, we compared the distribution of AL scores between these groups, treating AL as a continuous and categorical variable. The Student’s t-test was utilized to identify differences between continuous variables, while the Chi-square test was applied to detect differences between categorical variables. Then, the Cox Proportional Hazard regression model was employed to assess the association between the AL score and lung cancer risk. The event of interest was the first diagnosis of lung cancer. For lung cancer cases, follow-up time was defined from baseline to the date of lung cancer diagnosis, and for individuals who developed other cancers, the follow-up period extended from baseline to the date of diagnosis of these other cancers, with censoring at the time of diagnosis. For participants lost to follow-up, the period was from baseline to the date of the last follow-up. Additionally, Kaplan–Meier survival curves with the log-rank test were used to examine differences in lung cancer risk between different AL groups. To refine our analysis, we further conducted multivariate Cox Proportional Hazard regression models, adjusting for demographics (age, gender, and race), family history of lung cancer, socioeconomic status (education level, employment, and family income, and the Townsend deprivation index), and lifestyle factors (cigarette smoking, alcohol consumption, sleep quality, and physical activity). We included pack-years in the model to adjust the impact of cigarette smoking amount further. The proportional hazards assumption was tested, and a non-proportional hazards model was used if violated. Model fitting was assessed using the Likelihood Ratio Test. Subsequently, we conducted a stratified analysis to explore the association between AL and lung cancer risk factors that differed by covariates. Potential statistical interactions were noted. Finally, ANOVA was used to examine the relationship between smoking-related variables (pack-years, age of smoking initiation, age of smoking cessation, and years of smoking) and AL. Trend analyses assessed the dose–response relationship between smoking-related variables and AL scores. All statistical tests were two-sided, with p-values below 0.05 deemed statistically significant. The studies were performed using R version 4.3.0.
3. Results
Three hundred forty-four thousand three hundred eighty participants were included in the final analysis, with a median follow-up of 11.6 years. During this period, 2517 incident lung cancer cases were recorded, with a median follow-up time of 7.0 years for these cases. The age-standardized incidence rate for males was 80.65 per 10,000 population, while for females, it was 78.03 per 10,000 population. Table 1 outlines the distribution of selected characteristics between lung cancer cases and the cancer-free controls. Compared to controls, lung cancer cases were older (61.6 vs. 56.2 years, p < 0.001) and more likely to be male (52.05% vs. 47.13%, p < 0.001), White (96.82% vs. 94.37%, p < 0.001), and have a family history of lung cancer (13.91% vs. 7.71%, p < 0.001). For socioeconomic status-related variables, lung cancer cases were more likely to be retired (52.01% vs. 31.91%, p < 0.001), have low income (59.00% vs. 40.32%, p < 0.001), and live in the area with high levels of deprivation, as indicated by the Townsend deprivation score (63.61% vs. 49.44%, p < 0.001). In terms of healthy behaviors, lung cancer cases were less likely to be never-smokers (13.35% vs. 55.11%, p < 0.01) and have quality sleep (19.67% vs. 24.51%, p < 0.001).

Table 1.
Comparison of characteristics between incident lung cancer cases and cancer-free controls.
The distribution of AL scores in lung cancer cases and cancer-free controls is presented in Table 2. The AL scores in this study ranged from 0 to 11. However, only 6 participants had an AL score of 10 or higher. The most frequent AL score was 3, representing approximately 22.61% of the cases and 20.40% of the controls. Overall, the AL distribution in the case group was left-skewed, with only about 11.36% of participants having an AL score of 6 or more. A significant difference was observed when comparing AL distribution between cases and controls (p < 0.001). Compared to cancer-free controls, lung cancer cases were more likely to be in the high AL categories (AL: 3 to 9) than the low AL categories (AL: 0 to 2). The mean AL score for lung cancer cases was 3.49, significantly higher than the mean score of 2.87 for the non-cases population (p < 0.001). Based on AL score distribution, we combined participants with AL scores of ≤2 into one category and AL scores of >2 into another category, creating a dichotomized variable, AL Category. A significant difference in the distribution of AL Category (high vs. low) was observed between lung cancer cases and cancer-free controls (p < 0.001), with lung cancer cases more likely to be in the high AL group than non-cases (71.75% vs. 55.51%, p < 0.01).

Table 2.
AL scores and score categories between lung cancer cases and controls.
Then, we investigated the association between AL and lung cancer risk, as detailed in Table 2. First, in the univariate Cox Proportional Hazard regression analysis, we found a 23% increase in lung cancer risk for each unit increase in the AL score (HR = 1.23, 95% CI: 1.20, 1.26) if AL was treated as a continuous variable. In the multivariate Cox regression analysis, demographic variables (age, gender, and race), family history of lung cancer, socioeconomic status (education, employment status, income, and Townsend deprivation index), and healthy behaviors (cigarette smoking, pack-years, alcohol consumption, sleep quality, and physical activity) were included in the model, and we observed a marginally significant association (HR = 1.02, 95% CI: 0.99, 1.04) between AL score and lung cancer risk. No significant violation of the Cox proportional hazards assumption for any model was confirmed using the Schoenfeld residuals test. Then, treated as a categorical variable, participants in the high AL group (AL > 2) had a 2.08-fold increased risk of lung cancer compared to those in the low AL group (AL ≤ 2) in the univariate analysis (HR = 2.08, 95%CI: 1.91, 2.27). Figure 1 illustrates the Kaplan–Meier survival curves, indicating that participants with high AL scores (AL > 2) had a significantly higher likelihood of developing lung cancer compared to those with low AL scores (AL ≤ 2) (p < 0.001). In further multivariate analysis, the risk associated with the high AL group remained (HR = 1.15, 95% CI: 1.05, 1.25).
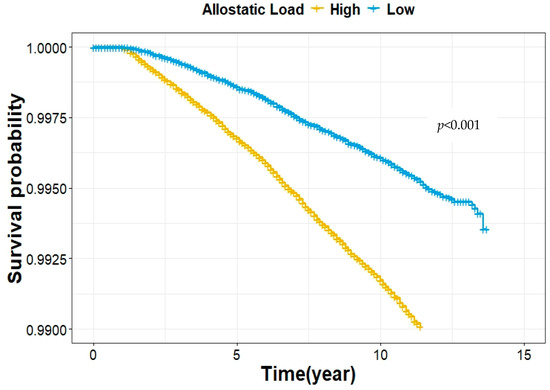
Figure 1.
Kaplan–Meier survival estimates for the association between the AL score category and lung cancer risk.
Subsequently, we investigated whether the association between AL and lung cancer risk differed by covariates (Table 3). The risk associated with high AL was slightly higher among those who were younger (<57 years old), women, Whites, and had higher levels of socioeconomic status (>high school education, higher income, employed, retired, and low Townsend deprivation index) than their counterparts. No statistical interaction was observed. For cigarette smoking, the risk was only observed among former (HR = 1.38, 95% CI: 1.20, 1.59) and current smokers (HR = 1.17, 95% CI: 1.02, 1.35), and not among never-smokers (HR = 0.93, 95% CI: 0.74, 1.17). Similarly, the risk was only observed among those with moderate (HR = 1.24, 95% CI: 1.06, 1.46) and heavy alcohol consumption (HR = 1.25, 95% CI: 1.09, 1.44), and not among never/occasional drinkers (HR = 1.11, 95% CI: 0.93, 1.34). In addition, significant interactions were observed between cigarette smoking and alcohol drinking with the AL category (former and current vs. never-smokers: p = 0.001 and 0.031, respectively; heavy vs. never/occasional drinkers: p = 0.030). The risk also differed by physical activity and sleep quality. A significant association was only observed among those with moderate and high levels of physical activity, and never/rarely and sometimes insomnia, but not among those with low levels of physical activity and frequent insomnia.

Table 3.
AL and lung cancer risk stratified by sociodemographic factors and healthy behaviors.
Our final analysis further investigated the association between various smoking-related factors and AL (Table 4). As expected, all analyzed smoking-related variables, including smoking status, pack-years of smoking among all study participants, age of smoking initiation, and years of smoking among former and current smokers, respectively, and age of smoking cessation among former smokers, were significantly associated with levels of AL (p < 0.001, respectively). Additionally, a significant trend of increasing AL was observed for changing smoking status from never or former to current (p < 0.001) and increasing pack-years from never to the highest quartile (p < 0.001) (Figure 2). A similar trend was also observed for increasing years of smoking among both former and current smokers and increasing age of smoking cessation among former smokers (p < 0.001, respectively). On the other hand, a significant trend of increasing AL was observed for increasing age of smoking initiation (p < 0.001).

Table 4.
Comparison of AL score by selected smoking-related variables.
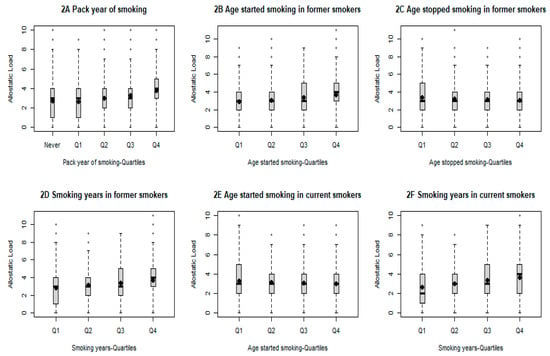
Figure 2.
Comparison of AL score by selected smoking-related variables. All p for trend less than 0.001.
4. Discussion
This is the first study to assess the relationship between AL and lung cancer risk in a cohort. In summary, we found that higher levels of AL are associated with a 15% increased risk of lung cancer after adjusting for demographic, socioeconomic, and health behavioral factors, as well as a family history of cancer. In stratified analysis, the significant risk association with AL was only observed among former and current smokers but not among non-smokers. We also found that demographics (age, gender, and race), socioeconomics (education, income, employment, and Townsend deprivation index), and other health behaviors (alcohol drinking, physical activity, and sleep quality) could modify the risk association. In a further analysis of the relationship between smoking-related variables and AL, significant trends of increasing AL were observed with the increase in pack-years, smoking years, and age of smoking cessation (former smokers only) and the decrease in age of smoking initiation.
A significant association between AL and lung cancer is expected—evidence from epidemiologic and laboratory studies supports the role of stress in the etiology of lung cancer. In a case–control study, Jafri et al. reported that lung cancer patients are significantly more likely to have had a major stressful life event within the preceding 5 years [14]. The use of β-blockers may be protective against lung cancer. In an earlier study, Levav et al. found that bereaved parents who had lost an adult son in an accident had a significantly higher chance of developing lung cancer later in life [24]. Furthermore, in a recent study in Chicago, exposure to increased neighborhood violence was found to be associated with an increased risk of lung cancer, possibly through activating physiological stress responses [25]. In laboratory mice, Jang et al. reported that chronic stress could accelerate lung tumorigenesis by promoting the exocytosis of IGF2 in lung epithelial cells [18]. Also, glucocorticoids released during chronic stress cause neutrophil extracellular trap (NET) formation, establish a metastasis-promoting microenvironment, and ultimately accelerate lung tumor metastasis [19].
Furthermore, Liu et al. reported that the combination of chronic stress and smoke could exacerbate the development of lung cancer in A/J mice [26]. Such a synergistic effect was also reported in a human study to show the combination of smoking and depression can contribute to decreases in NK cell activity, which serves as the first line of defense against tumors [27]. Our findings are consistent with those literature reports. In this study, we found significant statistical interactions between AL and smoking status. Specifically, the risk association with AL only exists among former and current smokers but not among non-smokers. In further analysis, we investigated whether the association between AL and lung cancer risk differed by the amount and duration of cigarette smoking. Among former smokers, there was a significant trend of increasing lung cancer risk with the increase in pack-years and years of smoking from lowest to highest quartile (both p for trend < 0.001). This further strengthens the notion that there is a synergistic effect between stress, represented by AL here, and cigarette smoking on lung cancer risk. In addition, we found a significant statistical interaction between alcohol consumption and AL in lung cancer risk. This is likely because heavy drinkers are more likely to be ever smokers in this study (p < 0.001).
One exciting but puzzling finding in this study is that the risk association is more evident among those with higher levels of socioeconomic status (SES) at both individual (education, employment, and income) and neighborhood levels (Townsend deprivation index). On the one hand, in our study population, individuals with lower levels of education and income, unemployment, and living in areas with high levels of Townsend deprivation index have statistically significantly higher AL levels than their counterparts. On the other hand, lower SES has been linked to worse lung cancer outcomes [28]. For example, in a pooled case–control study with 17,021 cases and 20,885 controls, an elevated risk between lung cancer and low SES was observed, even after the adjustment for cigarette smoking [29]. To eliminate the potential impact of cigarette smoking, we compared smoking status and pack-years by SES status. We found that those with higher SES were more likely to be never-smokers and consume fewer cigarettes. Thus, the difference cannot be explained by the smoking difference. More research is needed to clarify the association further.
Previous studies have shown that AL is higher among smokers than never-smokers [2,13,30,31]. Still, there is no study to assess the relationship between smoking-related variables beyond smoking status and AL. In this study, we assessed the impact of smoking status, pack-years of smoking among all study participants, age of smoking initiation, and years of smoking among former and current smokers, respectively, and age of smoking cessation among former smokers. Our results have demonstrated cigarette smoking—not only the amount and duration but also the timing—had a significant influence on AL. As mentioned above, individuals with higher levels of stress are more likely to engage in heavy smoking and are less likely to quit successfully than their counterparts [20,21]. Thus, those who have elevated levels of stress are more susceptible and addicted to smoking behavior, which, consequently, leads to further increased levels of stress. Such action creates a vicious cycle of exacerbating smoking and stress. This is also consistent with our finding that the risk association was only observed among former and never-smokers.
The main limitation of this study is the lack of information on tumor subtypes. So, we don’t know whether the association between adenocarcinoma and squamous carcinoma differs. Also, we only have a one-time measurement of AL, which does not allow us to assess the dynamic changes in AL during the follow-up. Nevertheless, this is the first study to demonstrate the risk association between AL as a biomarker of chronic stress and lung cancer risk in a large epidemiologic study. Further research is warranted to confirm the results of this study.
5. Conclusions
In this epidemiologic study, we have reported a significant relationship between higher AL and lung cancer risk. The risk association could be modified by cigarette smoking, demographics, socioeconomics, and other health behaviors. Future studies are needed to further assess the role of AL in lung carcinogenesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16183235/s1, Table S1: Codes used to identify lung cancer cases (Study censoring date: 31 December 2020). Table S2: Distribution and high-risk cutoff points for individual biomarkers of AL scores (N = 456,263). Figure S1: Flow chart of the study population.
Author Contributions
Y.G., J.S., K.Z., B.F.F. and H.Z. helped in conceptualization; Y.G. and J.S. helped in methodology; Y.G. and H.Z. worked in software; K.Z. helped in formal analysis; Y.G. and J.S. helped in data curation; Y.G., J.S. and H.Z. contributed to writing—original draft preparation; all contributed to writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by U01 CA179655, U01-260731, and R21CA267975 from NCI/NIH.
Institutional Review Board Statement
The study was approved by the relevant ethical committees for the UK Biobank (#94449, approved date: 09/272022) and Virginia Commonwealth University HM20214587, approved date: 11/052022).
Informed Consent Statement
Written informed consent was obtained from all participants.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McEwen, B.S. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Doorenbos, A.Z.; Li, H.; Jang, M.K.; Park, C.G.; Bronas, U.G. Allostatic Load in Cancer: A Systematic Review and Mini Meta-Analysis. Biol. Res. Nurs. 2021, 23, 341–361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guan, Y.; Shen, J.; Lu, J.; Fuemmeler, B.F.; Shock, L.S.; Zhao, H. Association between allostatic load and breast cancer risk: A cohort study. Breast Cancer Res. 2023, 25, 155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, H.; Song, R.; Ye, Y.; Chow, W.H.; Shen, J. Allostatic score and its associations with demographics, healthy behaviors, tumor characteristics, and mitochondrial DNA among breast cancer patients. Breast Cancer Res. Treat. 2021, 187, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Elsaid, M.I.; Handley, D.; Plascak, J.J.; Andersen, B.L.; Carson, W.E.; Pawlik, T.M.; Fareed, N.; Obeng-Gyasi, S. Association between Neighborhood Opportunity, Allostatic Load, and All-Cause Mortality in Patients with Breast Cancer. J. Clin. Oncol. 2024, 42, 1788–1798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obeng-Gyasi, S.; Elsaid, M.I.; Lu, Y.; Chen, J.C.; Carson, W.E.; Ballinger, T.J.; Andersen, B.L. Association of Allostatic Load with All-Cause Mortality in Patients with Breast Cancer. JAMA Netw. Open. 2023, 6, e2313989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obeng-Gyasi, S.; Li, Y.; Carson, W.E.; Reisenger, S.; Presley, C.J.; Shields, P.G.; Carbone, D.P.; Ceppa, D.P.; Carlos, R.C.; Andersen, B.L. Association of Allostatic Load with Overall Mortality among Patients with Metastatic Non-Small Cell Lung Cancer. JAMA Netw. Open. 2022, 5, e2221626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suvarna, B.; Suvarna, A.; Phillips, R.; Juster, R.P.; McDermott, B.; Sarnyai, Z. Health risk behaviours and allostatic load: A systematic review. Neurosci. Biobehav. Rev. 2020, 108, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Doan, S.N.; Dich, N.; Evans, G.W. Childhood cumulative risk and later allostatic load: Mediating role of substance use. Health Psychol. 2014, 33, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Falcon, L.M.; Gao, X.; Tucker, K.L.; Mattei, J. A Healthy Lifestyle Score Is Associated with Cardiometabolic and Neuroendocrine Risk Factors among Puerto Rican Adults. J. Nutr. 2015, 145, 1531–1540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriquez, E.J.; Livaudais-Toman, J.; Gregorich, S.E.; Jackson, J.S.; Napoles, A.M.; Perez-Stable, E.J. Relationships between allostatic load, unhealthy behaviors, and depressive disorder in U.S. adults, 2005–2012 NHANES. Prev. Med. 2018, 110, 9–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, J.; Fuemmeler, B.F.; Guan, Y.; Zhao, H. Association of Allostatic Load and All Cancer Risk in the SWAN Cohort. Cancers 2022, 14, 3044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jafri, S.H.; Ali, F.; Mollaeian, A.; Mojiz Hasan, S.; Hussain, R.; Akkanti, B.; Williams, J.; Shoukier, M.; El-Osta, H. Major Stressful Life Events and Risk of Developing Lung Cancer: A Case-Control Study. Clin. Med. Insights Oncol. 2019, 13, 1179554919835798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Qiao, Y.; Xiang, S.; Li, W.; Gan, Y.; Chen, Y. Work stress and the risk of cancer: A meta-analysis of observational studies. Int. J. Cancer 2019, 144, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Heikkila, K.; Nyberg, S.T.; Theorell, T.; Fransson, E.I.; Alfredsson, L.; Bjorner, J.B.; Williams, J.; Shoukier, M.; El-Osta, H. Work stress and risk of cancer: Meta-analysis of 5700 incident cancer events in 116,000 European men and women. BMJ 2013, 346, f165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jang, H.J.; Boo, H.J.; Lee, H.J.; Min, H.Y.; Lee, H.Y. Chronic Stress Facilitates Lung Tumorigenesis by Promoting Exocytosis of IGF2 in Lung Epithelial Cells. Cancer Res. 2016, 76, 6607–6619. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Gao, Y.; Ng, D.; Michalopoulou, E.; George, S.; Adrover, J.M.; Sun, L.; Albrengues, J.; Daßler-Plenker, J.; Han, X.; et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell. 2024, 42, 474–486.e12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stubbs, B.; Veronese, N.; Vancampfort, D.; Prina, A.M.; Lin, P.Y.; Tseng, P.T.; Evangelou, E.; Solmi, M.; Kohler, C.; Carvalho, A.F.; et al. Perceived stress and smoking across 41 countries: A global perspective across Europe, Africa, Asia and the Americas. Sci. Rep. 2017, 7, 7597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawless, M.H.; Harrison, K.A.; Grandits, G.A.; Eberly, L.E.; Allen, S.S. Perceived stress and smoking-related behaviors and symptomatology in male and female smokers. Addict. Behav. 2015, 51, 80–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chyu, L.; Upchurch, D.M. A Longitudinal Analysis of Allostatic Load among a Multi-Ethnic Sample of Midlife Women: Findings from the Study of Women’s Health Across the Nation. Womens Health Issues 2018, 28, 258–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levav, I.; Kohn, R.; Iscovich, J.; Abramson, J.H.; Tsai, W.Y.; Vigdorovich, D. Cancer incidence and survival following bereavement. Am. J. Public. Health. 2000, 90, 1601–1607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.J.; Kery, C.; An, J.; Rineer, J.; Bobashev, G.; Matthews, A.K. Racial/Ethnic disparities in exposure to neighborhood violence and lung cancer risk in Chicago. Soc. Sci. Med. 2024, 340, 116448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.P.; Zhang, C.; Zhang, Y.P.; Li, K.W.; Song, C. The combination of chronic stress and smoke exacerbated depression-like changes and lung cancer factor expression in A/J mice: Involve inflammation and BDNF dysfunction. PLoS ONE 2022, 17, e0277945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, W.; Irwin, M. Reduction of natural killer cytotoxic activity in major depression: Interaction between depression and cigarette smoking. Psychosom. Med. 1999, 61, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Sanchez, D.; Petrova, D.; Rodriguez-Barranco, M.; Fernandez-Navarro, P.; Jimenez-Moleon, J.J.; Sanchez, M.J. Socio-Economic Inequalities in Lung Cancer Outcomes: An Overview of Systematic Reviews. Cancers 2022, 14, 398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hovanec, J.; Siemiatycki, J.; Conway, D.I.; Olsson, A.; Stucker, I.; Guida, F.; Jöckel, K.-H.; Pohlabeln, H.; Ahrens, W.; Brüske, I.; et al. Lung cancer and socioeconomic status in a pooled analysis of case-control studies. PLoS ONE 2018, 13, e0192999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hicks, B.; Veronesi, G.; Ferrario, M.M.; Forrest, H.; Whitehead, M.; Diderichsen, F.; Tunstall-Pedoe, H.; Kuulasmaa, K.; Sans, S.; Salomaa, V.; et al. Roles of allostatic load, lifestyle and clinical risk factors in mediating the association between education and coronary heart disease risk in Europe. J. Epidemiol. Community Health 2021, 75, 1147–1154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forrester, S.N.; Leoutsakos, J.M.; Gallo, J.J.; Thorpe, R.J.; Jr Seeman, T.E. Association between allostatic load and health behaviours: A latent class approach. J. Epidemiol. Community Health 2019, 73, 340–345. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).