From 2D to 3D In Vitro World: Sonodynamically-Induced Prooxidant Proapoptotic Effects of C60-Berberine Nanocomplex on Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. C60-Berberine Complex Synthesis
2.3. Cell Culture and Treatment with Agents under Study
2.3.1. Preparation of Cell Monolayers
2.3.2. Preparation of Cell Spheroids
2.4. Ultrasound Exposure
2.5. Cell-Based Assays
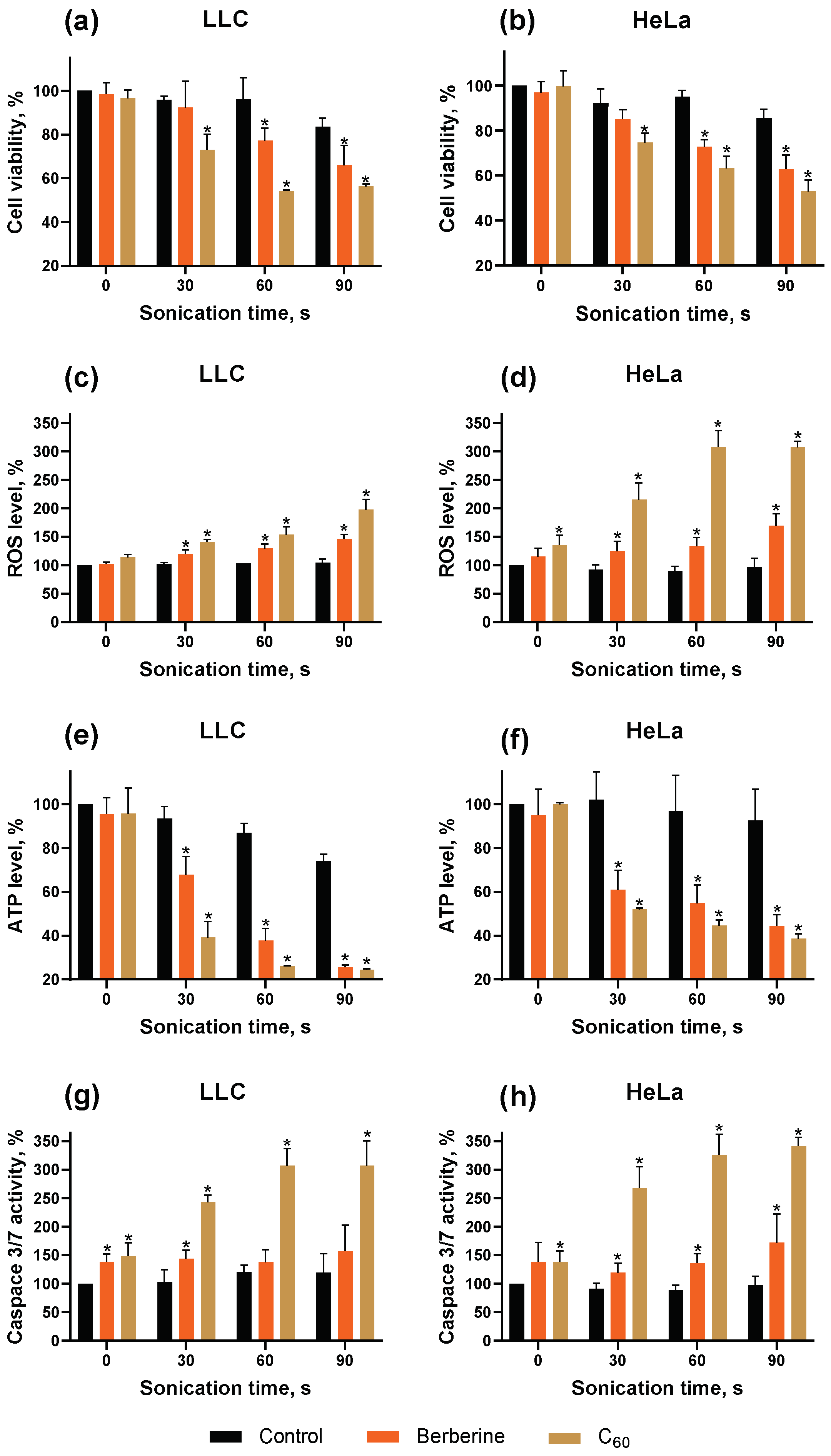
2.5.1. Cell Viability
2.5.2. Intracellular Reactive Oxygen Species Generation
2.5.3. Intercellular ATP Content
2.5.4. Caspase 3/7 Activity
2.6. Statistics
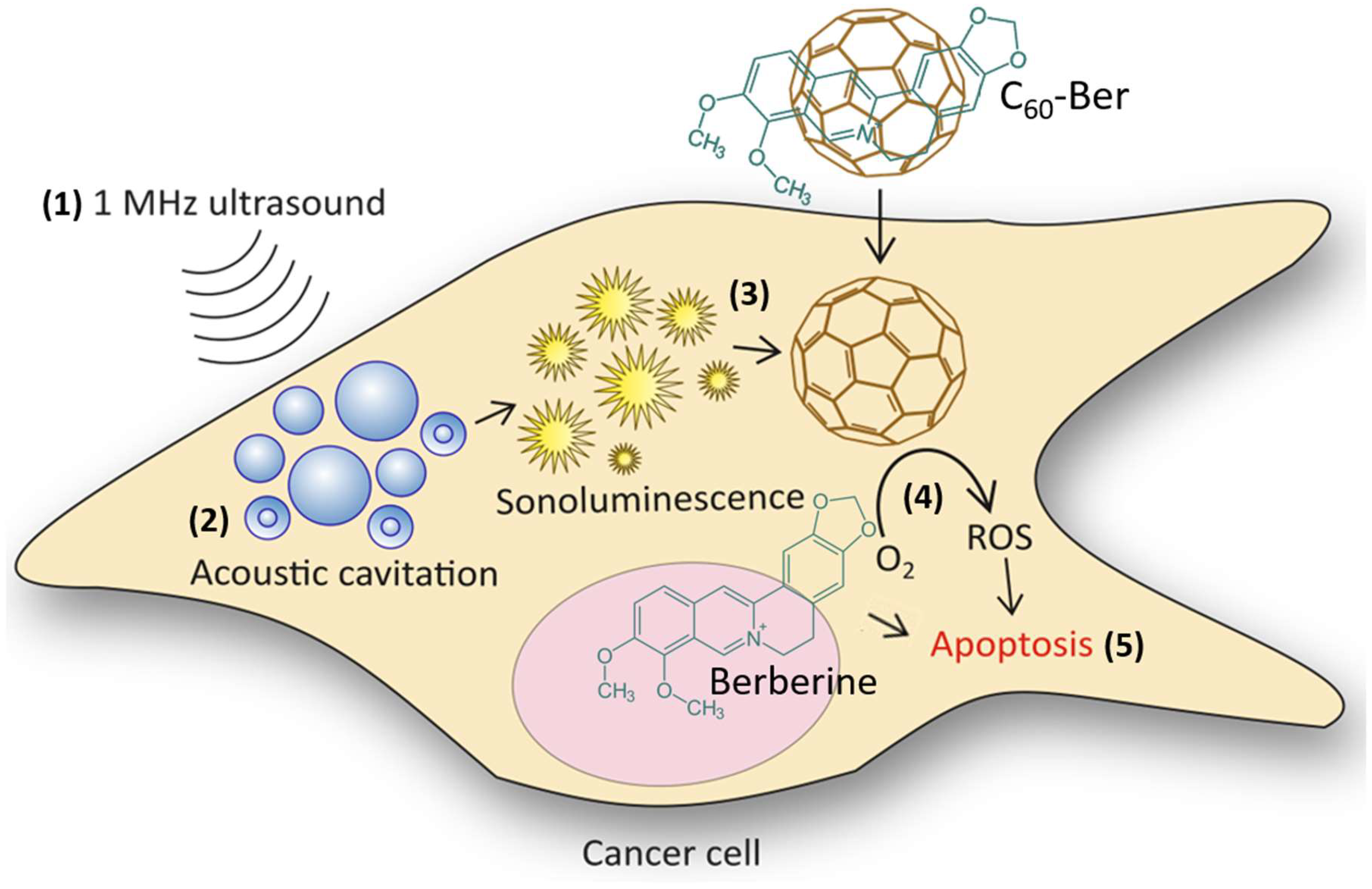
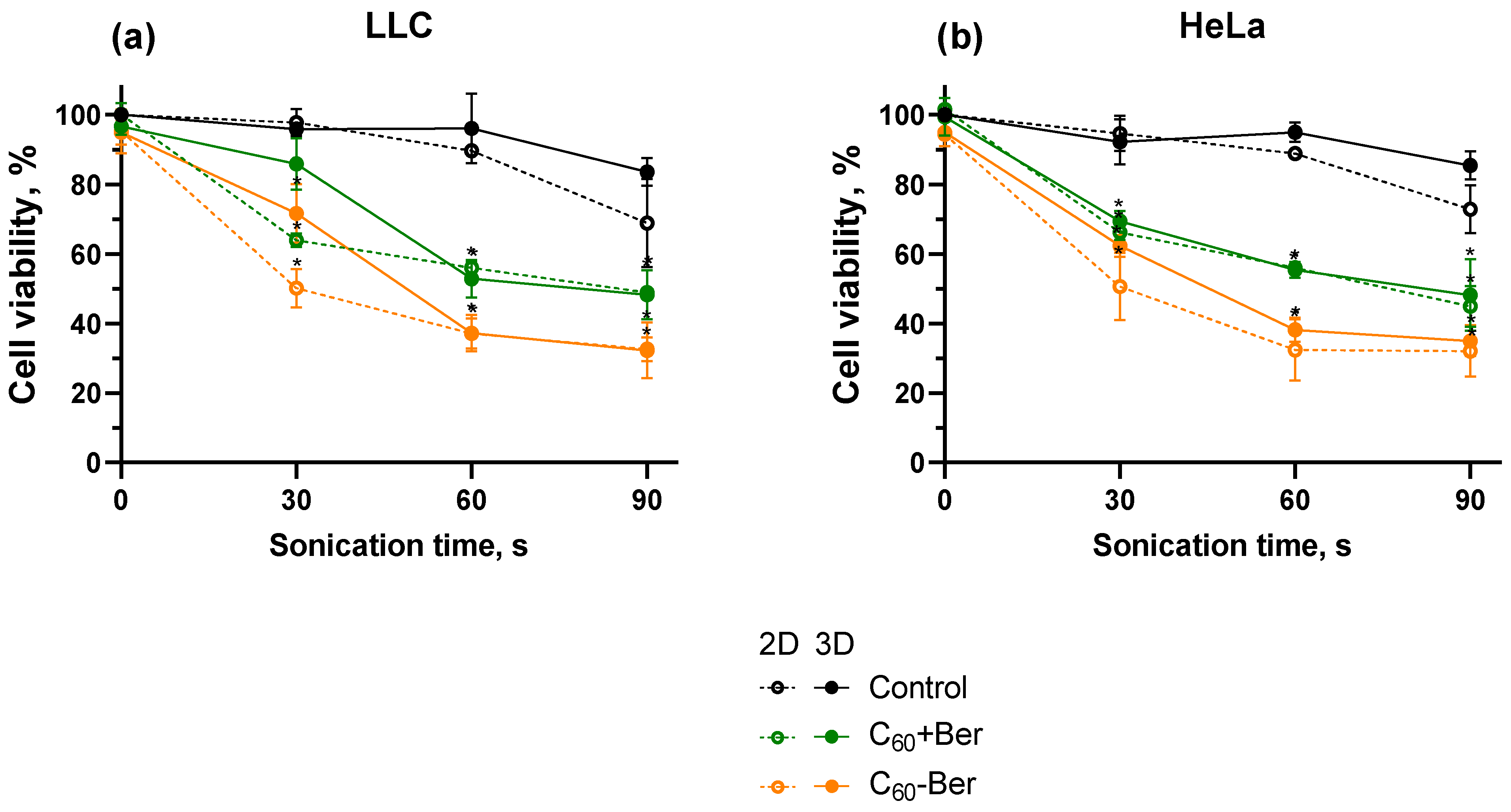
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Bailar, J.C.; Gornik, H.L. Cancer Undefeated. N. Engl. J. Med. 1997, 336, 1569–1574. [Google Scholar] [CrossRef]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A Concise Review on Cancer Treatment Methods and Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Loeb, K.R.; Loeb, L.A. Significance of Multiple Mutations in Cancer. Carcinogenesis 2000, 21, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; Chaturvedi, M.; Das, P.; Stephen, S.; Mathur, P. Cancer Incidence Estimates for 2022 & Projection for 2025: Result from National Cancer Registry Programme, India. Indian J. Med. Res. 2022, 156, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, J.; Feng, R.Q.; Fowlkes, J.B.; Brenner, D.E.; Cain, C.A. Sonodynamic Therapy: Activation of Anticancer Agents with Ultrasound. In Proceedings of the IEEE 1991 Ultrasonics Symposium, Orlando, FL, USA, 8–11 December 1991; Volume 2, pp. 1367–1370. [Google Scholar]
- Barbero, F.; Gul, S.; Perrone, G.; Fenoglio, I. Photoresponsive Inorganic Nanomaterials in Oncology. Technol. Cancer Res. Treat. 2023, 22, 15330338231192850. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Vasilyeva, A.; Hu, L.; Ruan, J.-L.; Gray, M.; Stride, E.P. Investigation of the Ultrasound-Mediated Toxicity Mechanisms of Various Sonosensitive Drugs. J. Acoust. Soc. Am. 2023, 153, A33. [Google Scholar] [CrossRef]
- Marsh, L. Pt(IV) Anticancer Prodrugs and Liposomal Encapsulation; University of Oxford: Oxford, UK, 2022; Available online: http://purl.org/dc/dcmitype/Text (accessed on 12 July 2024).
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Francovich, A.; Serpe, L. The Bright Side of Sound: Perspectives on the Biomedical Application of Sonoluminescence. Photochem. Photobiol. Sci. 2020, 19, 1114–1121. [Google Scholar] [CrossRef]
- Luksiene, Z. Photodynamic Therapy: Mechanism of Action and Ways to Improve the Efficiency of Treatment. Med. Kaunas Lith. 2003, 39, 1137–1150. [Google Scholar]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic Therapy with Fullerenes in Vivo: Reality or a Dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef]
- Yamakoshi, Y.; Umezawa, N.; Ryu, A.; Arakane, K.; Miyata, N.; Goda, Y.; Masumizu, T.; Nagano, T. Active Oxygen Species Generated from Photoexcited Fullerene (C60) as Potential Medicines: O2−• versus 1O2. J. Am. Chem. Soc. 2003, 125, 12803–12809. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of Reactive Oxygen Species (ROS) in Apoptosis Induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, S. A Review of Mechanism of Actions of Ultrasound Waves for Treatment of Soft Tissue Injuries. Int. J. Green Pharm. IJGP 2017, 11, 13–20. [Google Scholar] [CrossRef]
- Aldalawi, A.; Suardi, N.; Ahmed, N.M.; A. S. Al-Farawn, M.; Dheyab, M.A.; I.jebur, W.; J.Kadhim, F. Comparison of Wavelength-Dependent Penetration Depth of 532 Nm and 660 Nm Lasers in Different Tissue Types: Comparison of Wavelength-Dependent Penetration Depth. J. Lasers Med. Sci. 2023, 14, e28. [Google Scholar] [CrossRef] [PubMed]
- Costley, D.; Mc Ewan, C.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating Cancer with Sonodynamic Therapy: A Review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef]
- Shibaguchi, H.; Tsuru, H.; Kuroki, M.; Kuroki, M. Sonodynamic Cancer Therapy: A Non-Invasive and Repeatable Approach Using Low-Intensity Ultrasound with a Sonosensitizer. Anticancer Res. 2011, 31, 2425–2429. [Google Scholar]
- Yamaguchi, T.; Kitahara, S.; Kusuda, K.; Okamoto, J.; Horise, Y.; Masamune, K.; Muragaki, Y. Current Landscape of Sonodynamic Therapy for Treating Cancer. Cancers 2021, 13, 6184. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Stride, E.P.; Borden, M.A.; Eisenbrey, J.R.; Dayton, P.A. Radiotherapy Sensitization With Ultrasound-Stimulated Intravenously Injected Oxygen Microbubbles Can Have Contrary Effects Depending on the Study Model. Ultrasound Med. Biol. 2023, 49, 2203–2204. [Google Scholar] [CrossRef]
- Gao, F.; He, G.; Yin, H.; Chen, J.; Liu, Y.; Lan, C.; Zhang, S.; Yang, B. Titania-Coated 2D Gold Nanoplates as Nanoagents for Synergistic Photothermal/Sonodynamic Therapy in the Second near-Infrared Window. Nanoscale 2019, 11, 2374–2384. [Google Scholar] [CrossRef]
- Wang, J.; Jiao, Y.; Shao, Y. Mesoporous Silica Nanoparticles for Dual-Mode Chemo-Sonodynamic Therapy by Low-Energy Ultrasound. Materials 2018, 11, 2041. [Google Scholar] [CrossRef]
- Radivoievych, A.; Prylutska, S.; Zolk, O.; Ritter, U.; Frohme, M.; Grebinyk, A. Comparison of Sonodynamic Treatment Set-Ups for Cancer Cells with Organic Sonosensitizers and Nanosonosensitizers. Pharmaceutics 2023, 15, 2616. [Google Scholar] [CrossRef]
- Guamán Ortiz, L.M.; Lombardi, P.; Tillhon, M.; Scovassi, A.I. Berberine, an Epiphany Against Cancer. Molecules 2014, 19, 12349–12367. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, T.; Zhou, Z.; Hu, A.; Li, M.; Zhang, Z.; Yu, G.; Feng, H.; An, Y.; Peng, J.; et al. Berberine Nanoparticles for Promising Sonodynamic Therapy of a HeLa Xenograft Tumour. RSC Adv. 2019, 9, 10528–10535. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian Haftcheshmeh, S.; Momtazi-Borojeni, A.A. Berberine as a Promising Natural Compound for the Treatment of Periodontal Disease: A Focus on Anti-Inflammatory Properties. J. Cell. Mol. Med. 2021, 25, 11333–11337. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Kamble, S.; Deshkar, S.; Kothapalli, L.; Chitlange, S. Bioavailability of Berberine: Challenges and Solutions. İstanbul J. Pharm. 2021, 51, 141–153. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, D.W.; Jones, V.O.; Choi, Y.; Ferry, V.E.; Geller, M.A.; Azarin, S.M. Sonosensitizer-Functionalized Graphene Nanoribbons for Adhesion Blocking and Sonodynamic Ablation of Ovarian Cancer Spheroids. Adv. Healthc. Mater. 2021, 10, e2001368. [Google Scholar] [CrossRef]
- Radivoievych, A.; Kolp, B.; Grebinyk, S.; Prylutska, S.; Ritter, U.; Zolk, O.; Glökler, J.; Frohme, M.; Grebinyk, A. Silent Death by Sound: C60 Fullerene Sonodynamic Treatment of Cancer Cells. Int. J. Mol. Sci. 2023, 24, 1020. [Google Scholar] [CrossRef]
- Geng, B.; Xu, S.; Li, P.; Li, X.; Fang, F.; Pan, D.; Shen, L. Platinum Crosslinked Carbon Dot@TiO2− x p-n Junctions for Relapse-Free Sonodynamic Tumor Eradication via High-Yield ROS and GSH Depletion. Small Weinh. Bergstr. Ger. 2022, 18, e2103528. [Google Scholar] [CrossRef]
- Behzadpour, N.; Ranjbar, A.; Azarpira, N.; Sattarahmady, N. Development of a Composite of Polypyrrole-Coated Carbon Nanotubes as a Sonosensitizer for Treatment of Melanoma Cancer under Multi-Step Ultrasound Irradiation. Ultrasound Med. Biol. 2020, 46, 2322–2334. [Google Scholar] [CrossRef]
- Yang, C.-C.; Wang, C.-X.; Kuan, C.-Y.; Chi, C.-Y.; Chen, C.-Y.; Lin, Y.-Y.; Chen, G.-S.; Hou, C.-H.; Lin, F.-H. Using C-Doped TiO2 Nanoparticles as a Novel Sonosensitizer for Cancer Treatment. Antioxidants 2020, 9, E880. [Google Scholar] [CrossRef]
- Gorgizadeh, M.; Azarpira, N.; Lotfi, M.; Daneshvar, F.; Salehi, F.; Sattarahmady, N. Sonodynamic Cancer Therapy by a Nickel Ferrite/Carbon Nanocomposite on Melanoma Tumor: In Vitro and in Vivo Studies. Photodiagnosis Photodyn. Ther. 2019, 27, 27–33. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Fan, F.; Zhu, X.; Jia, L.; Chen, M.; Du, P.; Yang, L.; Yang, S. Boosting Antitumor Sonodynamic Therapy Efficacy of Black Phosphorus via Covalent Functionalization. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2021, 8, e2102422. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Katayama, R.; Kojima, C.; Matsumoto, A.; Ishihara, K.; Yusa, S. Singlet Oxygen Generation by Sonication Using a Water-Soluble Fullerene (C60) Complex: A Potential Application for Sonodynamic Therapy. Polym. J. 2020, 52, 1387–1394. [Google Scholar] [CrossRef]
- Haddon, R.C. Electronic Structure, Conductivity and Superconductivity of Alkali Metal Doped C60. Pure Appl. Chem. 1993, 65, 11–15. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical Engineers Get to Revisit an Old Friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Petrenko, V.I.; Ivankov, O.I.; Kyzyma, O.A.; Bulavin, L.A.; Litsis, O.O.; Evstigneev, M.P.; Cherepanov, V.V.; Naumovets, A.G.; Ritter, U. On the Origin of C60 Fullerene Solubility in Aqueous Solution. Langmuir 2014, 30, 3967–3970. [Google Scholar] [CrossRef]
- Torres, V.M.; Posa, M.; Srdjenovic, B.; Simplício, A.L. Solubilization of Fullerene C60 in Micellar Solutions of Different Solubilizers. Colloids Surf. B Biointerfaces 2011, 82, 46–53. [Google Scholar] [CrossRef]
- Ritter, U.; Prylutskyy, Y.I.; Evstigneev, M.P.; Davidenko, N.A.; Cherepanov, V.V.; Senenko, A.I.; Marchenko, O.A.; Naumovets, A.G. Structural Features of Highly Stable Reproducible C60 Fullerene Aqueous Colloid Solution Probed by Various Techniques. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 530–534. [Google Scholar] [CrossRef]
- Prylutska, S.V.; Grebinyk, A.G.; Lynchak, O.V.; Byelinska, I.V.; Cherepanov, V.V.; Tauscher, E.; Matyshevska, O.P.; Prylutskyy, Y.I.; Rybalchenko, V.K.; Ritter, U.; et al. In Vitro and in Vivo Toxicity of Pristine C60 Fullerene Aqueous Colloid Solution. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 715–728. [Google Scholar] [CrossRef]
- Zolfagharpour, F.; Khalilabad, M.H.R.; Nikkhoo, N.S.; Mousavi, M.H.; Hatampanah, S. Spectrum of Emitted Light from Sonoluminescence Bubbles. Adv. Appl. Phys. 2013, 1, 93–103. [Google Scholar] [CrossRef][Green Version]
- Franskevych, D.; Palyvoda, K.; Petukhov, D.; Prylutska, S.; Grynyuk, I.; Schuetze, C.; Drobot, L.; Matyshevska, O.; Ritter, U. Fullerene C60 Penetration into Leukemic Cells and Its Photoinduced Cytotoxic Effects. Nanoscale Res. Lett. 2017, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Grebinyk, A.; Grebinyk, S.; Prylutska, S.; Ritter, U.; Matyshevska, O.; Dandekar, T.; Frohme, M. C60 Fullerene Accumulation in Human Leukemic Cells and Perspectives of LED-Mediated Photodynamic Therapy. Free Radic. Biol. Med. 2018, 124, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Prylutskyy, Y.; Bychko, A.; Sokolova, V.; Prylutska, S.; Evstigneev, M.; Rybalchenko, V.; Epple, M.; Scharff, P. Interaction of C60 Fullerene Complexed to Doxorubicin with Model Bilipid Membranes and Its Uptake by HeLa Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Grebinyk, A.; Prylutska, S.; Grebinyk, S.; Prylutskyy, Y.; Ritter, U.; Matyshevska, O.; Dandekar, T.; Frohme, M. Complexation with C60 Fullerene Increases Doxorubicin Efficiency against Leukemic Cells In Vitro. Nanoscale Res. Lett. 2019, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Panchuk, R.; Gołuński, G.; Skivka, L.; Prylutskyy, Y.; Hurmach, V.; Skorohyd, N.; Borowik, A.; Woziwodzka, A.; Piosik, J.; et al. C60 Fullerene Enhances Cisplatin Anticancer Activity and Overcomes Tumor Cell Drug Resistance. Nano Res. 2017, 10, 652–671. [Google Scholar] [CrossRef]
- Prylutska, S.; Grynyuk, I.; Skaterna, T.; Horak, I.; Grebinyk, A.; Drobot, L.; Matyshevska, O.; Senenko, A.; Prylutskyy, Y.; Naumovets, A.; et al. Toxicity of C60 Fullerene-Cisplatin Nanocomplex against Lewis Lung Carcinoma Cells. Arch. Toxicol. 2019, 93, 1213–1226. [Google Scholar] [CrossRef]
- Grebinyk, A.; Prylutska, S.; Buchelnikov, A.; Tverdokhleb, N.; Grebinyk, S.; Evstigneev, M.; Matyshevska, O.; Cherepanov, V.; Prylutskyy, Y.; Yashchuk, V.; et al. C60 Fullerene as an Effective Nanoplatform of Alkaloid Berberine Delivery into Leukemic Cells. Pharmaceutics 2019, 11, 586. [Google Scholar] [CrossRef]
- Grebinyk, A.; Prylutska, S.; Chepurna, O.; Grebinyk, S.; Prylutskyy, Y.; Ritter, U.; Ohulchanskyy, T.Y.; Matyshevska, O.; Dandekar, T.; Frohme, M. Synergy of Chemo- and Photodynamic Therapies with C60 Fullerene-Doxorubicin Nanocomplex. Nanomaterials 2019, 9, 1540. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-Based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Grebinyk, A.; Grebinyk, S.; Prylutska, S.; Ritter, U.; Matyshevska, O.; Dandekar, T.; Frohme, M. HPLC-ESI-MS Method for C60 Fullerene Mitochondrial Content Quantification. Data Brief 2018, 19, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Grebinyk, A.; Prylutska, S.; Grebinyk, S.; Evstigneev, M.; Krysiuk, I.; Skaterna, T.; Horak, I.; Sun, Y.; Drobot, L.; Matyshevska, O.; et al. Antitumor Efficiency of the Natural Alkaloid Berberine Complexed with C60 Fullerene in Lewis Lung Carcinoma In Vitro and In Vivo. Cancer Nanotechnol. Basic Transl. Clin. Res. 2021, 12, 24. [Google Scholar] [CrossRef]
- Kudo, N.; Miyaoka, T.; Okada, K.; Yamamoto, K.; Niwa, K. Study on Mechanism of Cell Damage Caused by Microbubbles Exposed to Ultrasound. In Proceedings of the 2002 IEEE Ultrasonics Symposium, Munich, Germany, 8–11 October 2002; Volume 2, pp. 1383–1386. [Google Scholar]
- Ellwart, J.W.; Brettel, H.; Kober, L.O. Cell Membrane Damage by Ultrasound at Different Cell Concentrations. Ultrasound Med. Biol. 1988, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hensel, K.; Mienkina, M.P.; Schmitz, G. Analysis of Ultrasound Fields in Cell Culture Wells for In Vitro Ultrasound Therapy Experiments. Ultrasound Med. Biol. 2011, 37, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Yumita, N.; Watanabe, T.; Chen, F.-S.; Momose, Y.; Umemura, S.-I. Induction of Apoptosis by Functionalized Fullerene-Based Sonodynamic Therapy in HL-60 Cells. Anticancer Res. 2016, 36, 2665–2674. [Google Scholar]
- Gardiner, B.; Dougherty, J.A.; Ponnalagu, D.; Singh, H.; Angelos, M.; Chen, C.-A.; Khan, M. Measurement of Oxidative Stress Markers In Vitro Using Commercially Available Kits. In Measuring Oxidants and Oxidative Stress in Biological Systems; Berliner, L.J., Parinandi, N.L., Eds.; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-47317-4. [Google Scholar]
- Cho, H.-Y.; Reddy, S.P.; Kleeberger, S.R. Nrf2 Defends the Lung from Oxidative Stress. Antioxid. Redox Signal. 2006, 8, 76–87. [Google Scholar] [CrossRef]
- Liang, H.L.; Sedlic, F.; Bosnjak, Z.; Nilakantan, V. SOD1 and MitoTEMPO Partially Prevent Mitochondrial Permeability Transition Pore Opening, Necrosis, and Mitochondrial Apoptosis after ATP Depletion Recovery. Free Radic. Biol. Med. 2010, 49, 1550–1560. [Google Scholar] [CrossRef]
- Richter, C.; Schweizer, M.; Cossarizza, A.; Franceschi, C. Control of Apoptosis by the Cellular ATP Level. FEBS Lett. 1996, 378, 107–110. [Google Scholar] [CrossRef]
- Arnoult, D. Mitochondrial Fragmentation in Apoptosis. Trends Cell Biol. 2007, 17, 6–12. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis—Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, Pyroptosis and Apoptosis: An Intricate Game of Cell Death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, N.; Zhao, Y.; Kiyomi, A.; Yuan, B.; Onda, K.; Tanaka, S.; Sugiyama, K.; Sugiura, M.; Takagi, N.; Hayakawa, A.; et al. Chemo-Sensitivity of Two-Dimensional Monolayer and Three-Dimensional Spheroid of Breast Cancer MCF-7 Cells to Daunorubicin, Docetaxel, and Arsenic Disulfide. Anticancer Res. 2018, 38, 2101–2108. [Google Scholar] [PubMed]
- Breslin, S.; O’Driscoll, L. The Relevance of Using 3D Cell Cultures, in Addition to 2D Monolayer Cultures, When Evaluating Breast Cancer Drug Sensitivity and Resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lou, X.; Zhang, Z.; Ingram, P.; Yoon, E. High-Throughput Cancer Cell Sphere Formation for Characterizing the Efficacy of Photo Dynamic Therapy in 3D Cell Cultures. Sci. Rep. 2015, 5, 12175. [Google Scholar] [CrossRef]
- Klameth, L.; Rath, B.; Hochmaier, M.; Moser, D.; Redl, M.; Mungenast, F.; Gelles, K.; Ulsperger, E.; Zeillinger, R.; Hamilton, G. Small Cell Lung Cancer: Model of Circulating Tumor Cell Tumorospheres in Chemoresistance. Sci. Rep. 2017, 7, 5337. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Deng, C.-X. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int. J. Biol. Sci. 2018, 14, 2083–2093. [Google Scholar] [CrossRef]
- Henriquez, M.; Armisen, R.; Stutzin, A.; Quest, A.F.G. Cell Death by Necrosis, a Regulated Way to Go. Curr. Mol. Med. 2008, 8, 187–206. [Google Scholar] [CrossRef]
- Jiang, Z.; Xiao, W.; Fu, Q. Stimuli Responsive Nanosonosensitizers for Sonodynamic Therapy. J. Control. Release 2023, 361, 547–567. [Google Scholar] [CrossRef]
- Moosavi Nejad, S.; Takahashi, H.; Hosseini, H.; Watanabe, A.; Endo, H.; Narihira, K.; Kikuta, T.; Tachibana, K. Acute Effects of Sono-Activated Photocatalytic Titanium Dioxide Nanoparticles on Oral Squamous Cell Carcinoma. Ultrason. Sonochem. 2016, 32, 95–101. [Google Scholar] [CrossRef]
- Tserkovsky, D.A.; Alexandrova, E.N.; Chalau, V.N.; Istomin, Y.P. Effects of Combined Sonodynamic and Photodynamic Therapies with Photolon on a Glioma C6 Tumor Model. Exp. Oncol. 2012, 34, 332–335. [Google Scholar]
- Yang, Y.; Tu, J.; Yang, D.; Raymond, J.L.; Roy, R.A.; Zhang, D. Photo- and Sono-Dynamic Therapy: A Review of Mechanisms and Considerations for Pharmacological Agents Used in Therapy Incorporating Light and Sound. Curr. Pharm. Des. 2019, 25, 401–412. [Google Scholar] [CrossRef] [PubMed]





| CI | LLC | HeLa |
|---|---|---|
| 2D | 0.25 (strong synergism) | 0.39 (synergism) |
| 3D | 0.44 (synergism) | 0.40 (synergism) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radivoievych, A.; Schnepel, S.; Prylutska, S.; Ritter, U.; Zolk, O.; Frohme, M.; Grebinyk, A. From 2D to 3D In Vitro World: Sonodynamically-Induced Prooxidant Proapoptotic Effects of C60-Berberine Nanocomplex on Cancer Cells. Cancers 2024, 16, 3184. https://doi.org/10.3390/cancers16183184
Radivoievych A, Schnepel S, Prylutska S, Ritter U, Zolk O, Frohme M, Grebinyk A. From 2D to 3D In Vitro World: Sonodynamically-Induced Prooxidant Proapoptotic Effects of C60-Berberine Nanocomplex on Cancer Cells. Cancers. 2024; 16(18):3184. https://doi.org/10.3390/cancers16183184
Chicago/Turabian StyleRadivoievych, Aleksandar, Sophia Schnepel, Svitlana Prylutska, Uwe Ritter, Oliver Zolk, Marcus Frohme, and Anna Grebinyk. 2024. "From 2D to 3D In Vitro World: Sonodynamically-Induced Prooxidant Proapoptotic Effects of C60-Berberine Nanocomplex on Cancer Cells" Cancers 16, no. 18: 3184. https://doi.org/10.3390/cancers16183184
APA StyleRadivoievych, A., Schnepel, S., Prylutska, S., Ritter, U., Zolk, O., Frohme, M., & Grebinyk, A. (2024). From 2D to 3D In Vitro World: Sonodynamically-Induced Prooxidant Proapoptotic Effects of C60-Berberine Nanocomplex on Cancer Cells. Cancers, 16(18), 3184. https://doi.org/10.3390/cancers16183184






