The Economic Impact of Treatment Sequencing in Chronic Lymphocytic Leukemia in Canada Using Venetoclax plus Obinutuzumab
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
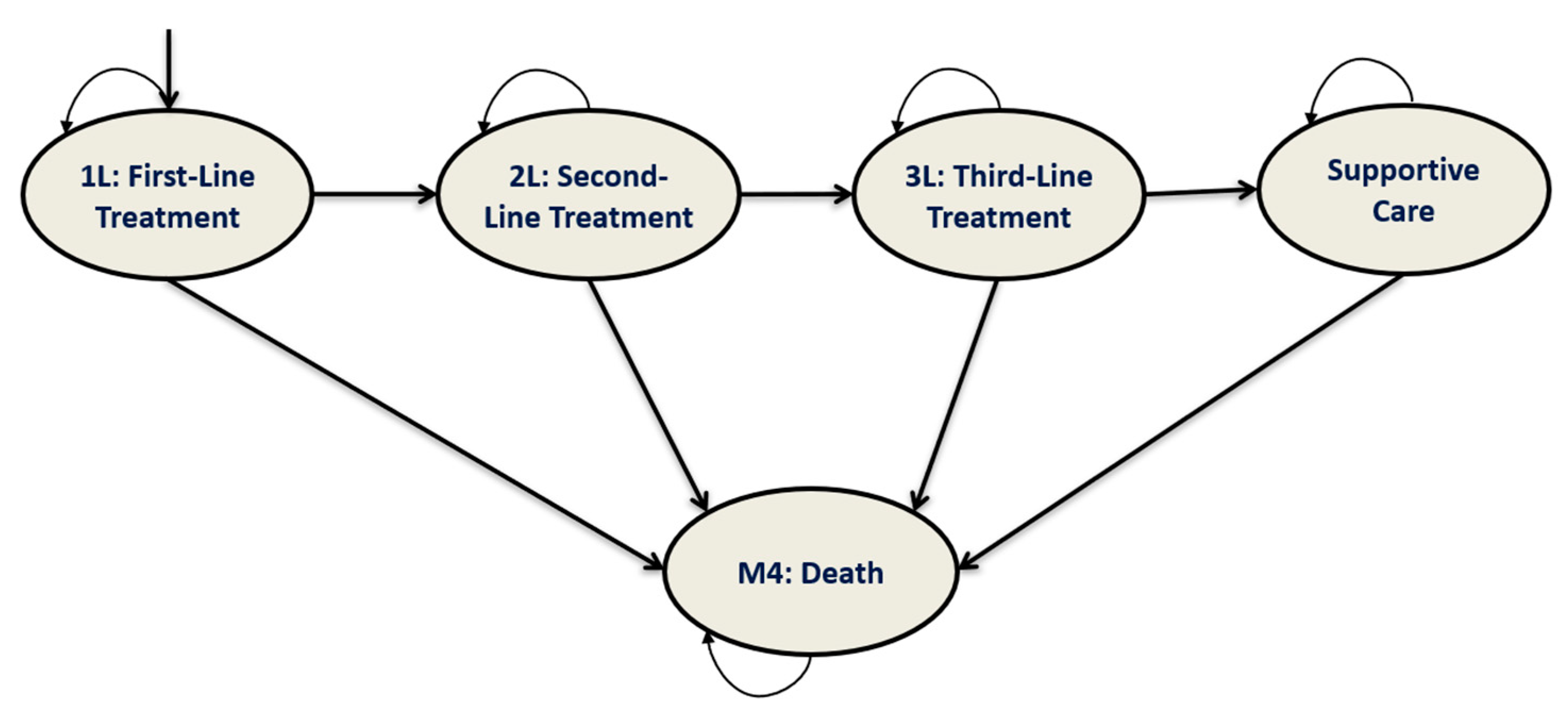
2.2. Model Structure
2.3. Simulated Clinical Pathway
2.4. Treatment Sequencing
2.5. Cost Data
2.5.1. Treatment Acquisition Costs
2.5.2. Administration Costs
2.5.3. Follow-Up and Monitoring Costs
2.5.4. Cost of Adverse Events
2.6. Model Outcomes
3. Results
3.1. 10-Year Cumulative Costs
3.1.1. First-Line Treatment
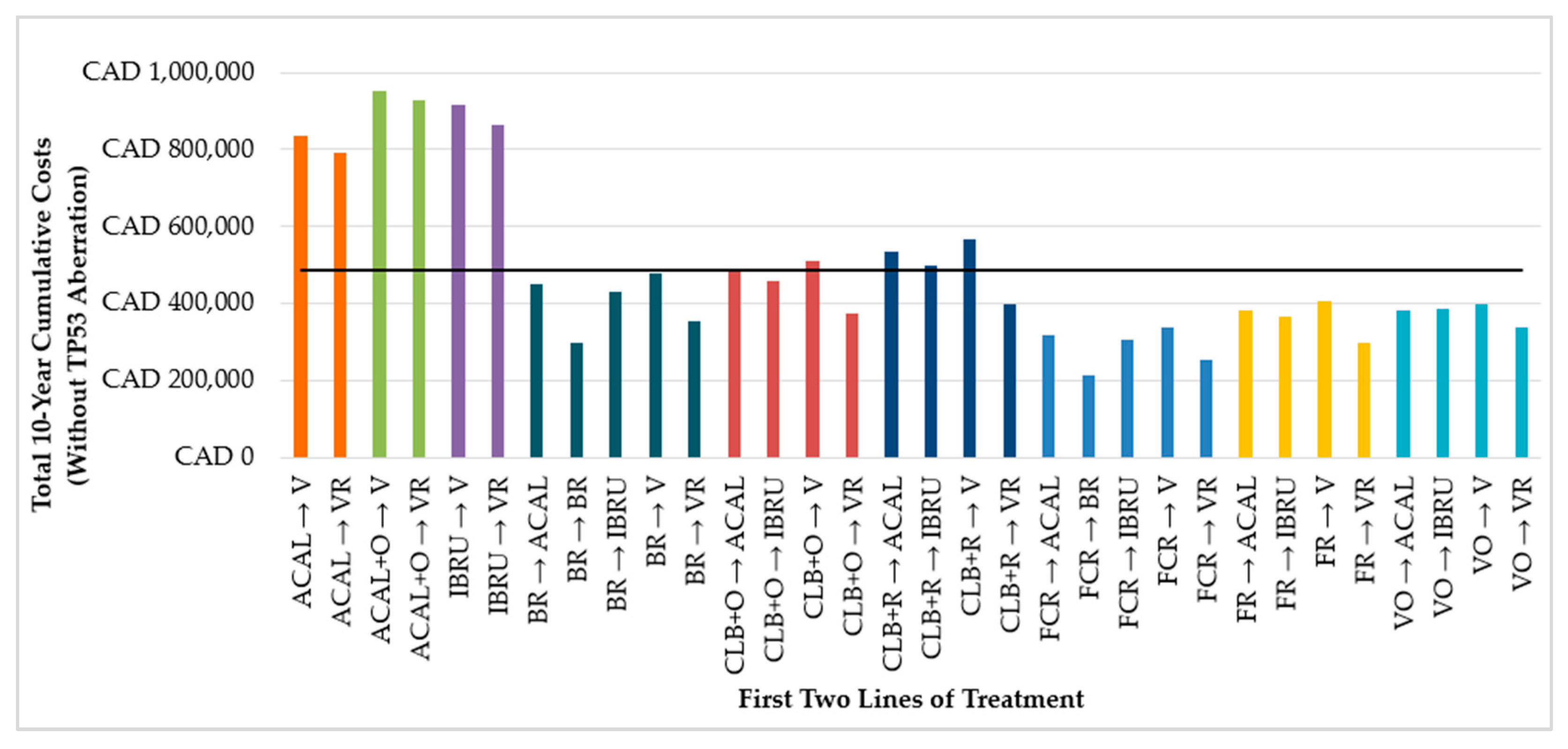
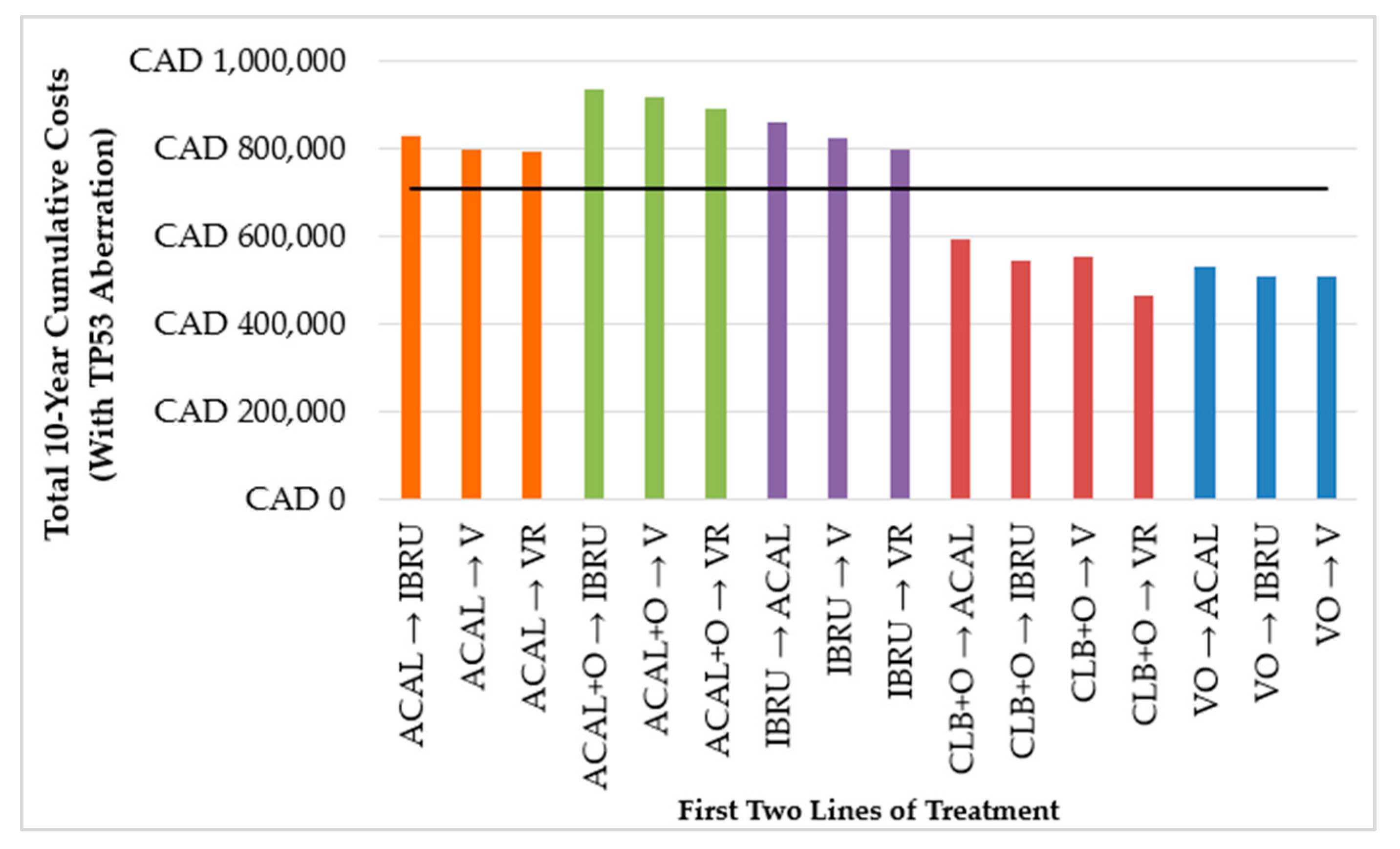
3.1.2. First Two Lines of Treatment
3.1.3. VO vs. Non-VO Treatment Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leukemia and Lymphoma Society of Canada. Blood Cancer in Canada—Facts and Stats. Available online: https://www.llscanada.org/disease-information/facts-and-statistics#Leukemia (accessed on 1 December 2021).
- Frey, S.; Blankart, C.R.; Stargardt, T. Economic burden and quality-of-life effects of chronic lymphocytic leukemia: A systematic review of the literature. Pharmacoeconomics 2016, 34, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Seftel, M.D.; Demers, A.A.; Banerji, V.; Gibson, S.B.; Morales, C.; Musto, G.; Pitz, M.W.; Johnston, J.B. High incidence of chronic lymphocytic leukemia (CLL) diagnosed by immunophenotyping: A population-based Canadian cohort. Leuk. Res. 2009, 33, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Iovino, L.; Shadman, M. Novel therapies in chronic lymphocytic leukemia: A rapidly changing landscape. Curr. Treat. Options Oncol. 2020, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.A. Chronic lymphocytic leukemia treatment algorithm 2018. Blood Cancer J. 2018, 8, 93. [Google Scholar] [CrossRef]
- Chen, Q.; Jain, N.; Ayer, T.; Wierda, W.G.; Flowers, C.R.; O’Brien, S.M.; Keating, M.J.; Kantarjian, H.M.; Chhatwal, J. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J. Clin. Oncol. 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Lachaine, J.; Beauchemin, C.; Guinan, K.; Thebault, P.; Aw, A.; Banerji, V.; Fleury, I.; Owen, C. Impact of oral targeted therapy on the economic burden of chronic lymphocytic leukemia in Canada. Curr. Oncol. 2021, 28, 332–345. [Google Scholar] [CrossRef]
- Waweru, C.; Kaur, S.; Sharma, S.; Mishra, N. Health-related quality of life and economic burden of chronic lymphocytic leukemia in the era of novel targeted agents. Curr. Med. Res. Opin. 2020, 36, 1481–1495. [Google Scholar] [CrossRef]
- Stephens, J.M.; Gramegna, P.; Laskin, B.; Botteman, M.F.; Pashos, C.L. Chronic lymphocytic leukemia: Economic burden and quality of life: Literature review. Am. J. Ther. 2005, 12, 460–466. [Google Scholar] [CrossRef]
- Wang, S.; Lafeuille, M.H.; Lefebvre, P.; Romdhani, H.; Emond, B.; Senbetta, M. Economic burden of treatment failure in chronic lymphocytic leukemia patients. Curr. Med. Res. Opin. 2018, 34, 1135–1142. [Google Scholar] [CrossRef]
- Brown, J.R.; Hallek, M.J.; Pagel, J.M. Chemoimmunotherapy versus targeted treatment in chronic lymphocytic leukemia: When, how long, how much, and in which combination? Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e387–e398. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Al-Sawaf, O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef]
- Harkins, R.A.; Patel, S.P.; Flowers, C.R. Cost-effectiveness of new targeted agents in the treatment of chronic lymphocytic leukemia. Cancer J. 2019, 25, 418–427. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib–rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, A.; Cavazzini, F.; Cavallari, M.; Foà, R.; Rigolin, G.M. Optimal management of chronic lymphocytic leukemia and economic constraints. Cancer J. 2021, 27, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Molica, S. The evolving role of time-limited targeted therapy in chronic lymphocytic leukemia. Expert Rev. Anticancer Ther. 2020, 20, 1015–1019. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Robrecht, S.; Kotak, A.; Chang, N.; Fink, A.M.; Tausch, E.; Schneider, C.; Ritgen, M.; Kreuzer, K.A.; et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemiaL: 5-year results of the randomized CLL 14 study. EHA 2022, 6, 49–50. [Google Scholar] [CrossRef]
- Owen, C.; Banerji, V.; Johnson, N.; Gerrie, A.; Aw, A.; Chen, C.; Robinson, S. Canadian evidence-based guideline for frontline treatment of chronic lymphocytic leukemia: 2022 update. Leuk. Res. 2023, 125, 107016. [Google Scholar] [CrossRef]
- Owen, C.; Eisinga, S.; Banerji, V.; Johnson, N.; Gerrie, A.S.; Aw, A.; Chen, C.; Robinson, S. Canadian evidence-based guideline for treatment of relapsed/refractory chronic lymphocytic leukemia. Leuk. Res. 2023, 133, 107372. [Google Scholar] [CrossRef]
- Woods, B.S.; Sideris, E.; Palmer, S.; Latimer, N.; Soares, M. Partitioned Survival and State Transition Models for Healthcare Decision Making in Oncology: Where Are We Now? Value Health 2020, 23, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.F.; Busch, R.; Stilgenbauer, S.; Stauch, M.; Bergmann, M.A.; Ritgen, M.; Kranzhofer, N.; Rohrberg, R.; Soling, U.; Burkhard, O.; et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 2009, 114, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Niederle, N.; Megdenberg, D.; Balleisen, L.; Heit, W.; Knauf, W.; Weiß, J.; Freier, W.; Hinke, A.; Ibach, S.; Eimermacher, H. Bendamustine compared to fludarabine as second-line treatment in chronic lymphocytic leukemia. Ann. Hematol. 2013, 92, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Dmoszynska, A.; Solal-Celigny, P.; Warzocha, K.; Loscertales, J.; Catalano, J.; Afanasiev, B.V.; Larratt, L.; Geisler, C.H.; Montillo, M.; et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J. Clin. Oncol. 2010, 28, 1756–1765. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Dyer, M.J.; Müller, L.; Smolej, L.; Di Bernardo, M.C.; Knapp, A.; Nielsen, T.; Hallek, M. Overall survival benefit of obinutuzumab over rituximab when combined with chlorambucil in patients with chronic lymphocytic leukemia and comorbidities: Final survival analaysis of CLL 11 study. EMA 2018, 215923, S151. [Google Scholar]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simlovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, S.; et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.S.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.; et al. Long-term results of alliance A041202 show continued advantage of ibrutinib-based regimens compared with bendamustine plus rituximab (BR) chemoimmunotherapy. Blood 2021, 138, 639. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Venclyxto—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/venclyxto#product-info (accessed on 1 May 2022).
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Mato, A.R.; Roeker, L.E.; Allan, J.N.; Pagel, J.M.; Brander, D.M.; Hill, B.T.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Tam, C.S.; et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am. J. Hematol. 2018, 93, 1394–1401. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. Am. Soc. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: Results from the full population of a phase II pivotal trial. J. Clin. Oncol. 2018, 36, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Zhang, C.; Lu, T.; Liao, M.; Panchal, A.; Robrecht, S. Minimal residual sisease dynamics after venetoclax-obinutuzumab treatment: Extended off-treatment follow-up from the randomized CLL14 study. J. Clin. Oncol. 2021, 39, 4049–4061. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.; Kamdar, M.; Flinn, I.W.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: Elevate-TN four-year follow up. J. Clin. Oncol. 2021, 39, 7509. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Peterson, B.L.; Gribben, J.G.; Morrison, V.A.; Rai, K.R.; Larson, R.A.; Byrd, J.C. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: Long-term follow-up of CALGB study 9712. J. Clin. Oncol. 2011, 29, 1349–1355. [Google Scholar] [CrossRef]
- Canadian Agency for Drug and Technologies in Health (CADTH). CADTH Reimbursement Review: Provisional Funding Algorithm—Chronic Lymphocytic Leukemia. Available online: https://www.cadth.ca/sites/default/files/attachments/2021-06/PH0004-CLL-Provisional-Algorithm-final-may18-rev.pdf (accessed on 1 December 2021).
- Fleurence, R.L.; Hollenbeak, C.S. Rates and probabilities in economic modelling: Transformation, translation and appropriate application. Pharmacoeconomics 2007, 25, 3–6. [Google Scholar] [CrossRef]
- Byrd, J.C.; Petersonm, B.L.; Morrison, V.A.; Park, K.; Jacobson, R.; Hoke, E.; Vardiman, J.W.; Rai, K.; Schiffer, C.A.; Larson, R.A. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-Cellchronic lymphocytic leukemia: Results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 2003, 101, 6–14. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.S.; Illmer, T.; et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.W.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Tandon, M.; Sinha, A.; Fink, A.M.; Robrecht, S.; Samoylova, O.; Liberati, A.M.; Pinilla-Ibarz, J.; Opat, S.; et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): Follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Burger, J.A.; Blum, K.A.; Coleman, M.; Wierda, W.G.; Jones, J.A.; Zhao, W.; Heerema, N.A.; et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015, 125, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Mato, A.R.; Wierda, W.G.; Davids, M.S.; Choi, M.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Barr, P.M.; Zhou, L.; et al. Venetoclax for Chronic Lymphocytic Leukaemia Progressing after Ibrutinib: A Multicentre, Open-Label Phase 2 Trial. Lancet Oncol. 2018, 19, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Cancer Care Alberta. Chronic Lymphocytic Leukemia: Clinical Practice Guideline LYHE-007—Version 7 (Effective Date: March, 2022). Available online: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-lyhe007-cll.pdf (accessed on 1 December 2021).
- Cancer Care Ontario. Available online: https://www.cancercareontario.ca/en/drugformulary/regimens (accessed on 1 December 2021).
- Ministry of Health and Long Term Care Ontario Health Insurance Plan. Schedule of Benefits—Physician Services under the Health Insurance Act; Ministry of Health and Long Term Care Ontario Health Insurance Plan: Toronto, ON, USA, 2021. [Google Scholar]
- Government of Canada. Job Bank—Wage Report. Available online: https://www.jobbank.gc.ca/wagereport/location/geo9219 (accessed on 1 December 2021).
- Statistics Canada. Average Usual Hours and Wages by Selected Characteristics, Monthly, Unadjusted for Seasonality (Table: 14-10-0320-02). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410032002 (accessed on 1 December 2021).
- Ministry of Health and Long Term Care Ontario Health Insurance Plan. Schedule of Benefits for Laboratory Services; Ministry of Health and Long Term Care Ontario Health Insurance Plan: Toronto, ON, USA, 2020. [Google Scholar]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Canadian Institute for Health Information (CIHI). Patient Cost Estimator; Canadian Institute for Health Information (CIHI): Ottawa, ON, USA, 2021. [Google Scholar]
- Ontario Ministry of Health and Long Term Care. Ontario Care Costing Analysis Tool. Available online: https://data.ontario.ca/en/dataset/ontario-case-costing-initiative-occi (accessed on 1 December 2021).
- de Oliveira, C.; Pataky, R.; Bremner, K.E.; Rangrej, J.; Chan, K.K.; Cheung, W.Y.; Hoch, J.S.; Peacock, S.; Krahn, M.D. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer 2016, 16, 809. [Google Scholar] [CrossRef]
- Statistics Canada. Consumer Price Index by Product Group, Monthly, Percentage Change, Not Seasonally Adjusted, Canada, Provinces, Whitehorse, Yellowknife and Iqaluit (Table: 18-10-0004-13). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000413 (accessed on 1 December 2021).
- Cho, S.K.; Manzoor, B.S.; Sail, K.R.; Parise, H.; Ravelo, A.; Shapouri, S.; Kapustyan, T.; Sharmokh, S.; Virabhak, S.; Davids, M.S.; et al. Budget impact of 12-month fixed Tteatment duration venetoclax in combination with obinutuzumab in previously untreated chronic lymphocytic leukemia patients in the United States. Pharmacoeconomics 2020, 38, 941–951. [Google Scholar] [CrossRef]
- Fang, H.; Ravonimbola, H.; Hazra, N.C.; Zhou, Z.; Manzoor, B.S.; Levy, V.; Ysebaert, L.; Kabore, N.; Sail, K. Economic impact of treatment sequences for chronic lymphocytic leukemia (CLL) and budget impact analysis of venetoclax plus obinutuzumab sequences for CLL patients not eligible for full dose fludarabine in France. Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]
- Davids, M.S.; Manzoor, B.S.; Hazra, N.C.; Fang, H.; Ravelo, A.; Han, F.; Guerin, A.; Sail, K.; Shapouri, S.; Shadman, M. The economic impact of treatment sequences for chronic lymphocytic leukemia in the United States: A cost of care and budget impact model of venetoclax plus obinutuzumab sequences. J. Clin. Pathw. 2022, 8, 36–46. [Google Scholar] [CrossRef]
- Alrawashdh, N.; McBride, A.; Erstad, B.; Sweasy, J.; Persky, D.O.; Abraham, I. Cost-effectiveness and economic burden analyses on all first-line treatments of chronic lymphocytic leukemia. Value Health 2022, 25, 1685–1695. [Google Scholar] [CrossRef]
- Chatterjee, A.; van de Wetering, G.; Goeree, R.; Owen, C.; Desbois, A.M.; Barakat, S.; Manzoor, B.S.; Sail, K. A probabilistic cost-effectiveness analysis of venetoclax and obinutuzumab as a first-line therapy in chronic lymphocytic leukemia in Canada. PharmacoEconomics-Open 2023, 7, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Gribben, J.G. How and when I do allogeneic transplant in CLL. Blood 2018, 132, 31–39. [Google Scholar] [CrossRef]
- Roeker, L.E.; Dreger, P.; Brown, J.R.; Lahoud, O.B.; Eyre, T.A.; Brander, D.M.; Skarbnik, A.; Coombs, C.C.; Kim, H.T.; Davids, M.; et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020, 4, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.; Wiedmeier-Nutor, J.E.; Parikh, S.A.; McCabe, C.E.; O’Brien, D.R.; Boddicker, N.J.; Kleinstern, G.; Rabe, K.G.; Bruins, L.; Brown, S.; et al. Differential prognosis of single and multiple TP53 abnormalities in high-count MBL and untreated CLL. Blood Adv. 2023, 7, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Cymbalista, F.; Ghia, P.; Jager, U.; Pospisilova, S.; Rosenquist, R.; Schuh, A.; Stilgenbauer, S. TP53 aberrations in chronic lymphocytic leukemia: An overview of the clinical implications of improved diagnostics. Haematologica 2018, 103, 1956–1968. [Google Scholar] [CrossRef]
- Wierda, W.G.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Opat, S.; Tedeschi, A.; Badoux, X.C.; Kuss, B.J.; Jackson, S.; Moreno, C.; et al. Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results from the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study. J. Clin. Oncol. 2021, 39, 3853–3865. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.D.; Benjamini, O.; for the GLOW Investigators. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Evid 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Janssen Inc. Product Monograph: IMBRUVICA® (Ibrutinib Tablets); Janssen Inc.: Toronto, ON, USA, 2023. [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health (CADTH). CADTH Reimbursement Recommendation: Ibrutinib (Imbruvica) in Combination with Venetoclax. Available online: https://www.cda-amc.ca/sites/default/files/DRR/2023/PC0317REC-Imbruvica-Final-Recommendation-meta.pdf (accessed on 6 September 2024).
| Treatments | PFS and OS (Transition Probability by 28-Day Cycle) * | Grade 3/4 AEs (%) | Drug Cost a (CAD/Cycle) | References |
|---|---|---|---|---|
| 1L | ||||
| FR | Median PFS, 45.0 months (1.4%) Median OS, 105.0 months (0.6%) | Anemia, 4 Neutropenia, 76 Thrombocytopenia, 20 Infection, 20 | IV: C1: 3194 C2–6: 3896 Oral: C1: 3620 C2–6: 4322 | Woyach, 2011 [38], Byrd, 2003 [41] |
| FCR | 3-year PFS, 72.9% (0.8%) 3-year OS, 91.5% (0.2%) | Anemia, 14.6 Neutropenia, 45 Febrile neutropenia, 15.8 Thrombocytopenia, 15.2 Infection, 9.5 Atrial fibrillation, 1.2 | IV: C1: 3071 C2–6: 3773 Oral: C1: 3069 C2–6: 3770 | Shanafelt, 2019 [15] |
| CLB + O | Median PFS, 29.8 months (2.2%) TP53+, median PFS, 11.3 months (5.5%) 5-year OS, 66.0% (0.6%) TP53+, 30-month OS, 85.0% (0.5%) | Anemia, 5 Neutropenia, 35 Thrombocytopenia, 11 Infection, 11 | C1: 16,498 C2–6: 5542 | Goede, 2018 [26], Moreno, 2019 [27], Goede, 2014 [42] |
| CLB + R | Median PFS, 15.7 months (4.0%) Median OS, 73.1 months (0.9%) | Anemia, 4 Neutropenia, 27 Thrombocytopenia, 4 Infection, 13 | C1: 2169 C2–6: 2871 | Goede, 2018 [26], Goede, 2014 [42] |
| BR | Median PFS, 15.7 months (1.4%) 2-year OS, 95.0% (0.2%) | Anemia, 12 Febrile neutropenia, 7 Infection, 15 Atrial fibrillation, 3 | C1: 5720 C2–6: 6421 | Woyach, 2018 [43] |
| IBRU | 5-year PFS, 73.0% (0.5%) TP53+, 1-year PFS, 87.0% (1.1%) 5-year OS, 83.0% (0.3%) TP53+, 1-year OS, 89.0% (0.9%) | Anemia, 7 Neutropenia, 13 Infection, 12 Atrial fibrillation, 5 | 8386 | Burger, 2020 [30], Mato, 2018 [32] |
| VO | 5-year PFS, 62.6% (0.7%) TP53+, 5-year PFS, 40.6% (1.4%) 4-year OS, 85.4% (0.3%) | Anemia, 9 Neutropenia, 53 Febrile neutropenia, 5 Thrombocytopenia, 13 Infection, 7 Atrial fibrillation, 2 | C1: 16,532 C2: 9153 C3–6: 13,318 C7–13: 7840 | Al-Sawaf, 2022 [19], Al-Sawaf, 2020 [44] |
| ACAL | 4-year PFS, 78.0% (0.5%) TP53+, 39-month PFS, 74.0% (0.7%) 4-year OS, 88.0% (0.2%) TP53+, 2-year OS, 95.0% (0.2%) | Neutropenia, 11.2 Bleeding, 2.8 Atrial fibrillation, 1.1 | 7615 | Sharman, 2021 [37], Sharman, 2020 [36] |
| ACAL + O | 4-year PFS, 87.0% (0.3%) TP53+, 33-month PFS, 70.2% (1.0%) 4-year OS, 93.0% (0.1%) TP53+, 2-year OS, 95.0% (0.2%) | Neutropenia, 30.9 Bleeding, 2.9 Atrial fibrillation, 0.6 | C1: 7615 C2: 24,048 C3: 13,092 C8+: 7615 | Sharman, 2021 [37], Sharman, 2020 [36] |
| 2L/3L ** | ||||
| FCR | Median PFS, 30.6 months (2.1%) 55-month OS, 60.0% (0.9%) | Anemia, 12 Neutropenia, 42 Febrile neutropenia, 12 Thrombocytopenia, 11 Infection, 5 | IV: C1: 3071 C2–6: 3773 Oral: C1: 3069 C2–6: 3770 | Robak, 2010 [25] |
| BR | Median PFS, 21.6 months (2.9%) TP53+, median PFS, 14.6 months (4.3%) 5-year OS, 62.2% (0.7%) | Anemia, 13.8 Neutropenia, 38.8 Febrile neutropenia, 9.6 Thrombocytopenia, 10.1 Infection, 8 | C1: 5720 C2–6: 6421 | EMA, 2020 [29], Seymour, 2018 [45] |
| IBRU | Median PFS, 42.5 months (1.5%) TP53+, median PFS, 40.6 months (1.6%) Median OS, 67.7 months (0.9%) TP53+, median OS, 61.8 months (1.0%) | Anemia, 9 Neutropenia, 25 Thrombocytopenia, 10 Bleeding, 10 Infection, 21 Atrial fibrillation, 6 | 8386 | Munir, 2019 [31] Byrd, 2003 [46], Munir, 2019 [31] |
| V | 2-year PFS, 24.0 months (2.3%) TP53+, 1-year PFS, 72.0% (2.5%) 12-month OS, 92.0% (0.6%) TP53+, 2-year OS, 73.0% (1.2%) | Anemia, 29 Neutropenia, 51 Febrile neutropenia, 13 Thrombocytopenia, 29 Infection, 12 | C1: 1813 C2+: 7840 | EMA, 2020 [29], Jones, 2018 [47] |
| VR | Median PFS, 55.1 months (1.2%) TP53+, median PFS, 47.9 months (1.3%) 5-year OS, 82.1% (0.3%) | Anemia, 10.8 Neutropenia, 57.7 Febrile neutropenia, 3.6 Thrombocytopenia, 5.7 Infection, 17.5 | Ramp-up: 3773 C1: 9945 C2–6: 10,647 C7–26: 7840 | EMA, 2020 [29], Seymour, 2018 [45] |
| ACAL | 22-month PFS, 74.7% (1.2%) TP53+, 19-month PFS, 80.2% (1.1%) 12-month OS, 94.0% (0.5%) | Anemia, 11 Neutropenia, 15 Febrile neutropenia, 0 Thrombocytopenia, 4 Infection, 5 Atrial fibrillation, 2 | 7615 | Ghia, 2020 [33] |
| Parameters | Model | Reference |
|---|---|---|
| Probabilities, % | ||
| Patients on IV therapy | 100.00 | When both formulas are available |
| Costs, CAD | ||
| Follow-up and laboratory monitoring costs | ||
| Electrolyte panel | 18.08 | L226, L204, L053, L165, L194, L061, L700 |
| Renal panel | 25.48 | L251, L065, L700 |
| Liver function test | 21.15 | L223, L222, L191, L029, L030, L031, L005, L208, L700 |
| CBC panel | 14.74 | L393, L700 |
| Coagulation parameters | 13.42 | L445, L700 |
| Serology | 21.01 | L319, L700 |
| Professional fees | ||
| Consultation, Hematology | 168.75 | Schedule of Benefits, code A615 [50] |
| Partial assessment, Hematology | 38.05 | Schedule of Benefits, code A618 [50] |
| Administration costs | ||
| Physician fee for administration | 105.15 | Schedule of Benefits, code G359 [50] |
| Nurse average wage (CAD/min) | 0.67 * | Statistic Canada [52]; Job Bank Canada, NOC 3012 [51] |
| Pharmacist average wage (CAD/min) | 0.87 * | Statistic Canada [52]; Job Bank Canada, NOC 3131 [51] |
| Adverse events b | ||
| Anemia | 793.02 * | OCC, code D649 [56] Assuming 2% managed inpatient |
| Neutropenia | 553.32 * | OCC, code D700 [56] Assuming 100% managed outpatient |
| Febrile neutropenia | 10,918.00 * | OCC, code R508 [56] Assuming 100% managed inpatient |
| Thrombocytopenia | 467.27 * | OCC, code D696 [56] Assuming 100% managed outpatient |
| Bleeding | 943.96 * | OCC, code D473 [56] Assuming 4% managed inpatient |
| Infection | 1840.27 * | OCC, code A499/B349 [56] Assuming 25% managed inpatient |
| Atrial fibrillation | 1443.66 * | OCC, code I4890 [56] Assuming 10% managed inpatient |
| TLS Prophylaxis | ||
| VO regimens | 1290.44 | See Supplementary Material, Tables S7–S9 |
| V and VR regimens | 1805.91 | See Supplementary Material, Tables S7, S8 and S10 |
| Palliative care c | 6103.46/cycle * | De Oliveira (2016) [57] |
| 1L Treatment | 10-Year Cumulative Costs (2022 CAD) | ||
|---|---|---|---|
| Without TP53 Aberration | With TP53 Aberration | ||
| BTKis | ACAL | 772,127–854,077 | 770,737–832,182 |
| ACAL + O | 914,811–966,247 | 890,042–942,126 | |
| IBRU | 844,607–930,700 | 789,732–862,554 | |
| Chemoimmunotherapy | BR | 264,460–486,313 | * |
| CLB + O | 346,879–519,606 | 448,574–603,645 | |
| CLB + R | 365,926–577,797 | * | |
| FCR | 190,282–343,317 | * | |
| FR | 277,979–413,126 | * | |
| BCL-2i | VO | 327,574–418,213 | 500,639–536,507 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guinan, K.; Mathurin, K.; Lachaine, J.; Roc, N.P.; Bull, S.-J.; Tankala, D.; Barakat, S.; Manzoor, B.S.; Hillis, C.; Banerji, V. The Economic Impact of Treatment Sequencing in Chronic Lymphocytic Leukemia in Canada Using Venetoclax plus Obinutuzumab. Cancers 2024, 16, 3182. https://doi.org/10.3390/cancers16183182
Guinan K, Mathurin K, Lachaine J, Roc NP, Bull S-J, Tankala D, Barakat S, Manzoor BS, Hillis C, Banerji V. The Economic Impact of Treatment Sequencing in Chronic Lymphocytic Leukemia in Canada Using Venetoclax plus Obinutuzumab. Cancers. 2024; 16(18):3182. https://doi.org/10.3390/cancers16183182
Chicago/Turabian StyleGuinan, Kimberly, Karine Mathurin, Jean Lachaine, Nancy Paul Roc, Sarah-Jane Bull, Dipti Tankala, Stephane Barakat, Beenish S. Manzoor, Christopher Hillis, and Versha Banerji. 2024. "The Economic Impact of Treatment Sequencing in Chronic Lymphocytic Leukemia in Canada Using Venetoclax plus Obinutuzumab" Cancers 16, no. 18: 3182. https://doi.org/10.3390/cancers16183182
APA StyleGuinan, K., Mathurin, K., Lachaine, J., Roc, N. P., Bull, S.-J., Tankala, D., Barakat, S., Manzoor, B. S., Hillis, C., & Banerji, V. (2024). The Economic Impact of Treatment Sequencing in Chronic Lymphocytic Leukemia in Canada Using Venetoclax plus Obinutuzumab. Cancers, 16(18), 3182. https://doi.org/10.3390/cancers16183182






