Protein Structure Inspired Discovery of a Novel Inducer of Anoikis in Human Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Cell Lines
2.2. Development and Characterization of a Protein X-ray Crystallographic Library
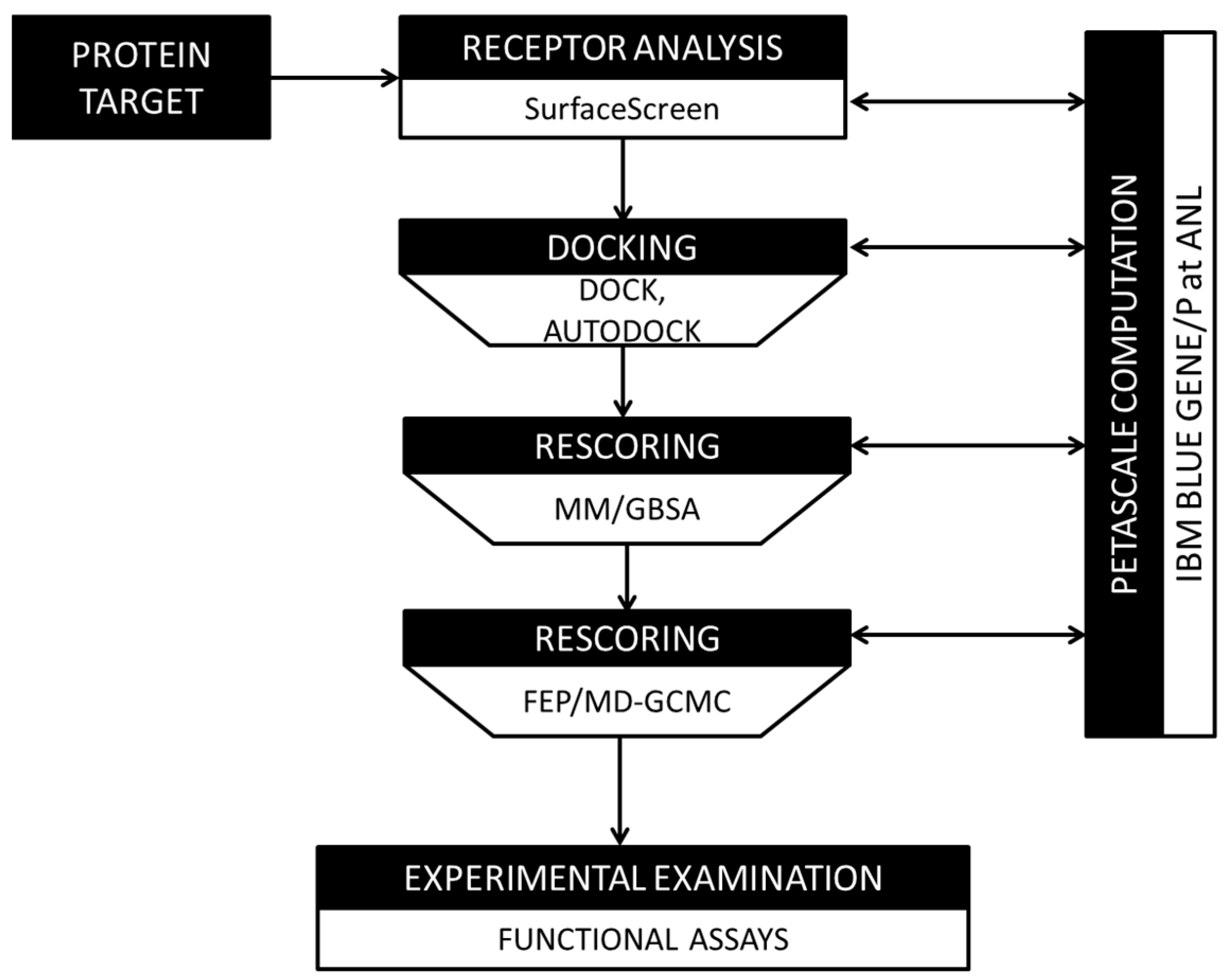
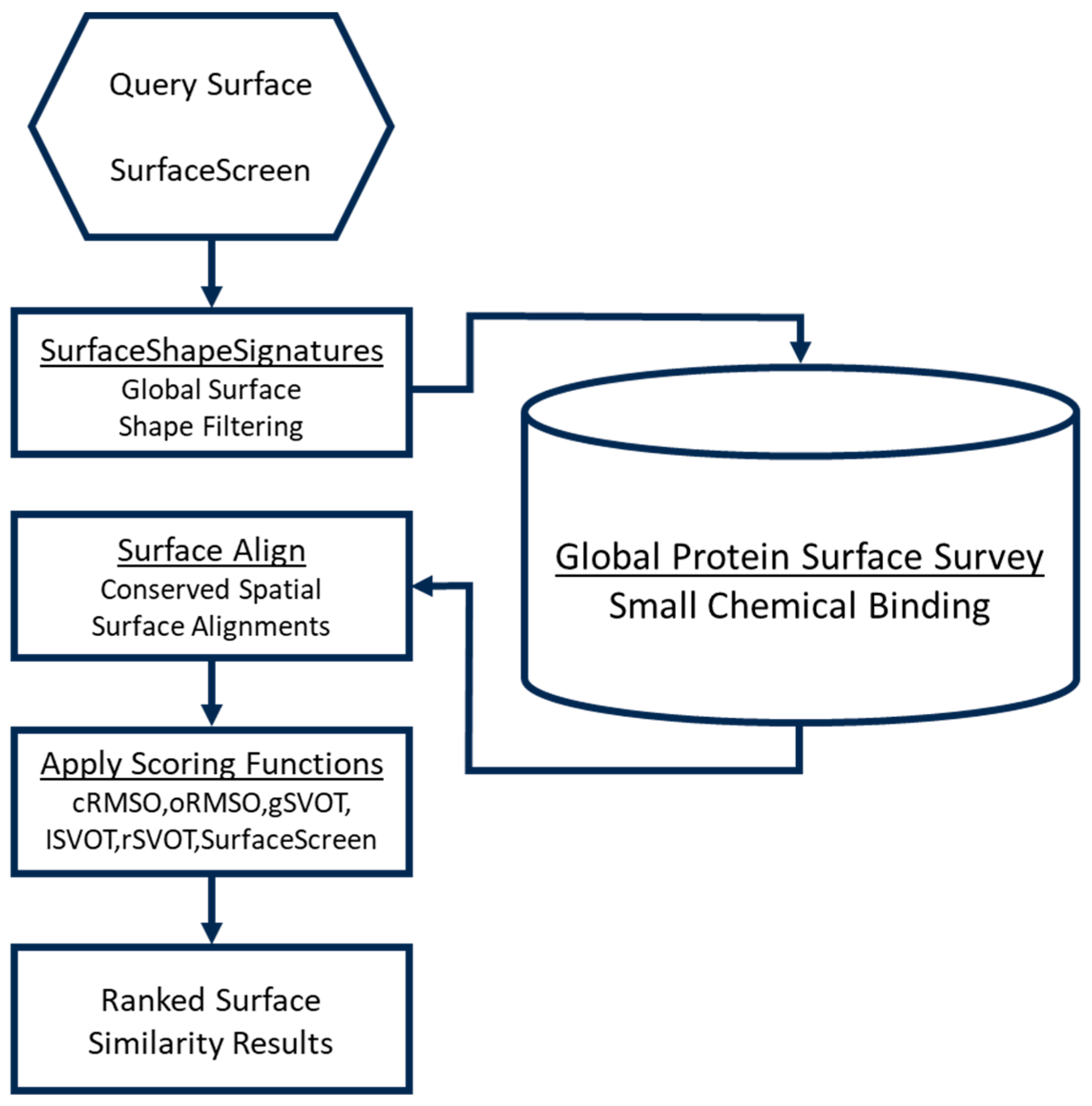
2.3. Development and Validation of a Physics-Based Protein Structure Analysis Platform
2.4. In Silico Compound Screening
2.5. Growth Assays
2.6. Bone Marrow Stem Cell Colony Formation Assays
2.7. Cell Cycle Analysis
2.8. Western Blot and Image Acquisition
3. Results
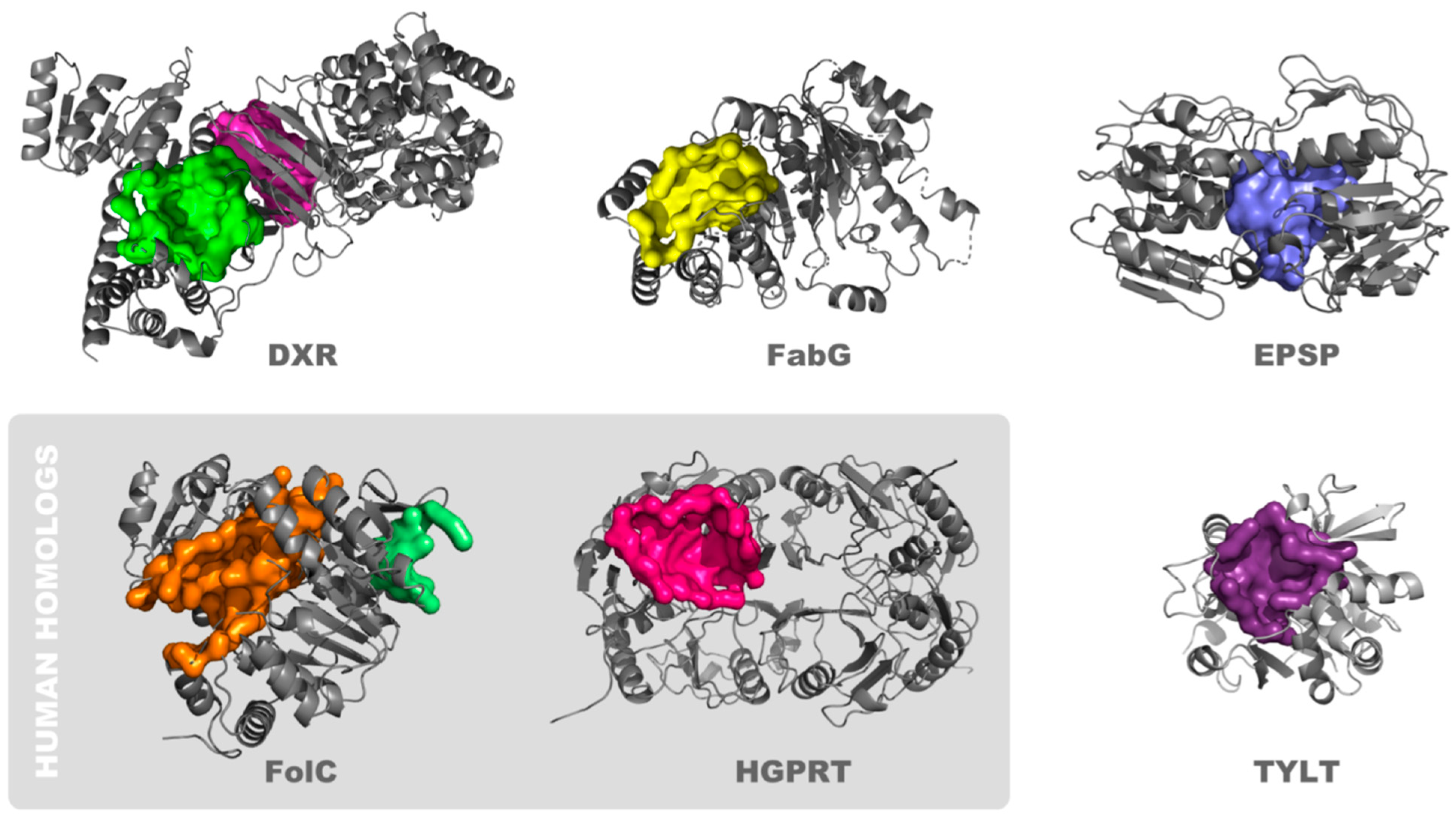
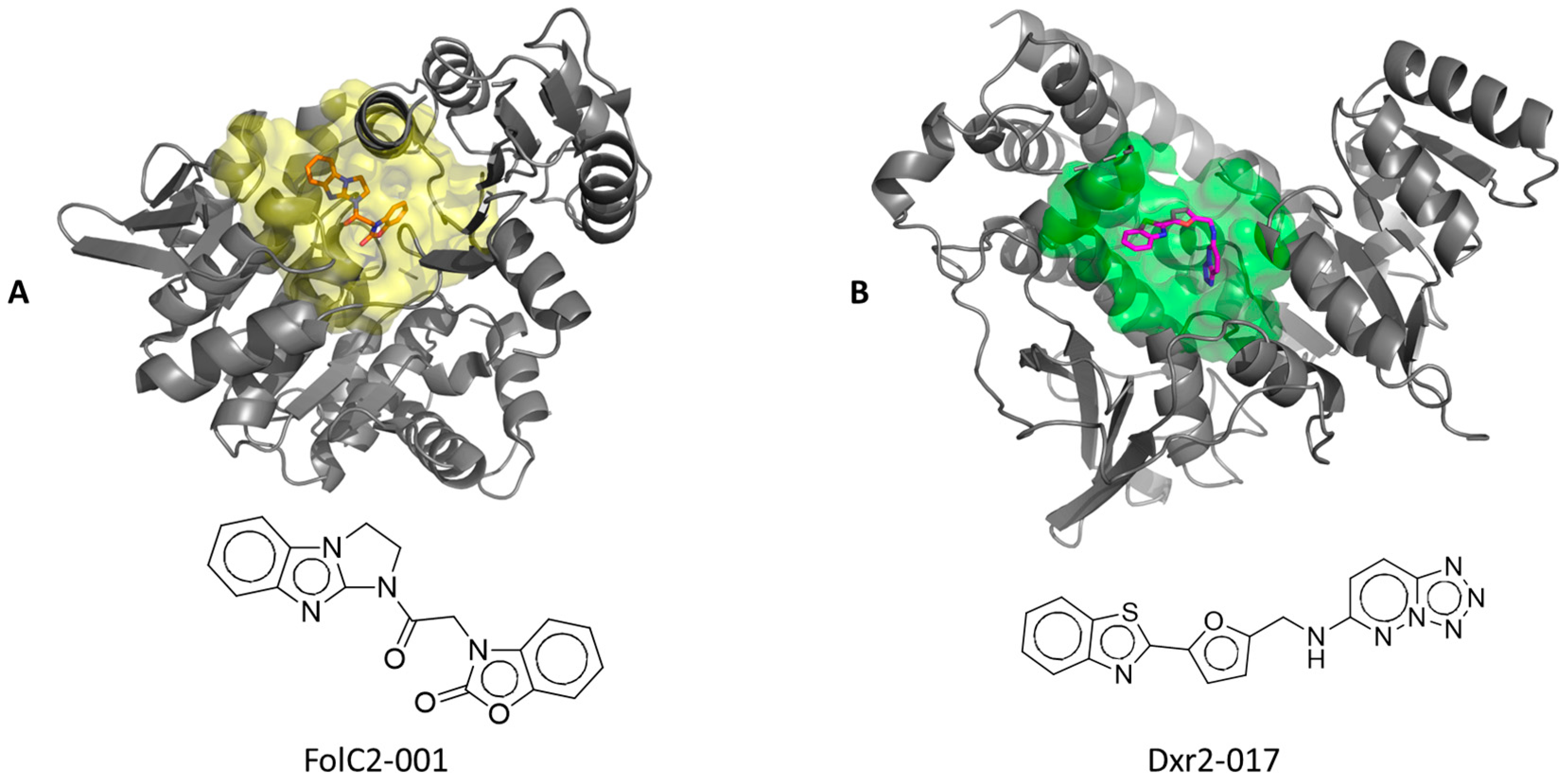
3.1. Identification of Unique Structural Motifs of High Potential Pharmacologic Value
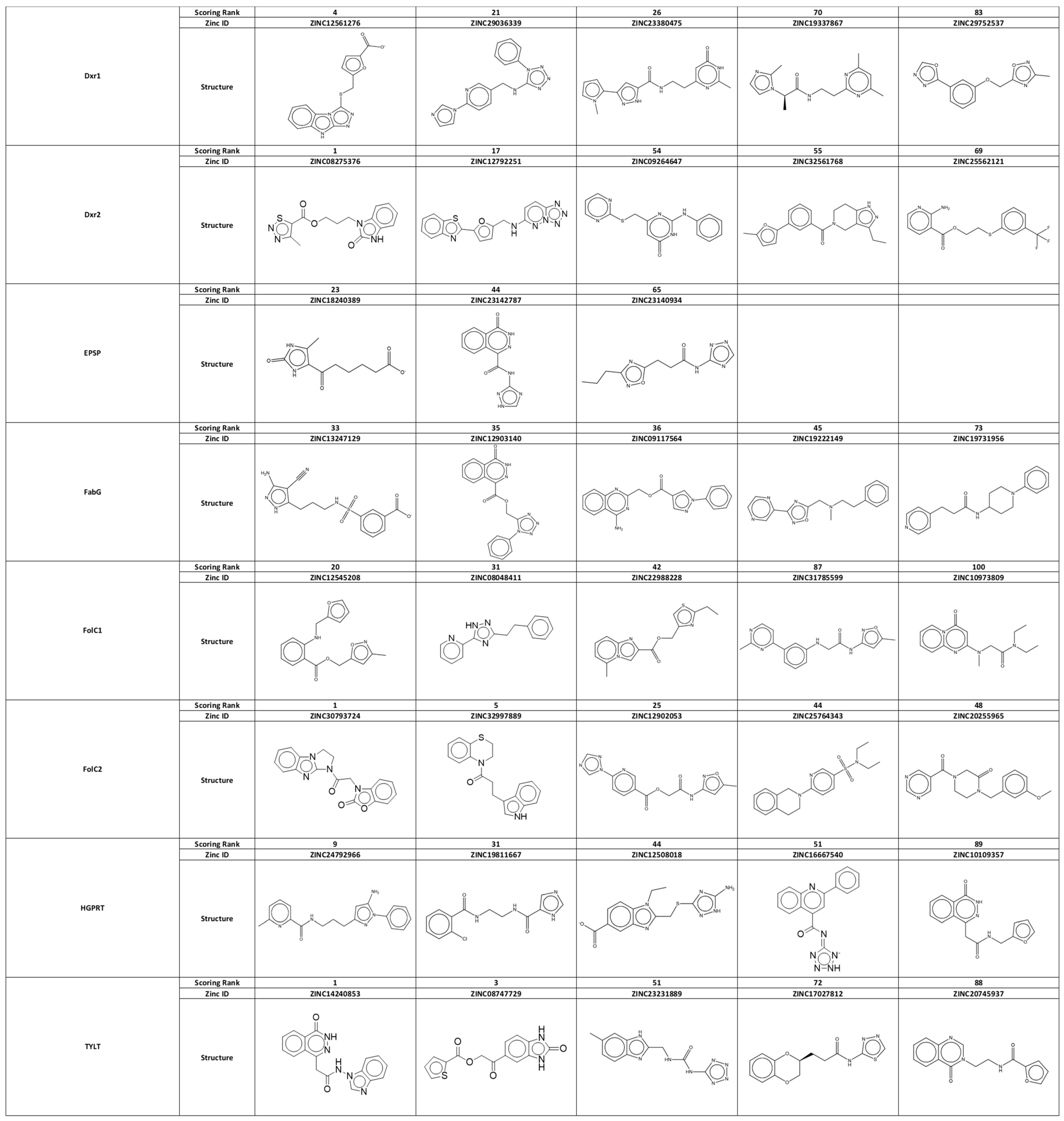
3.2. Identification of Small-Molecule Ligands with the Potential to Interact with Binding Pockets
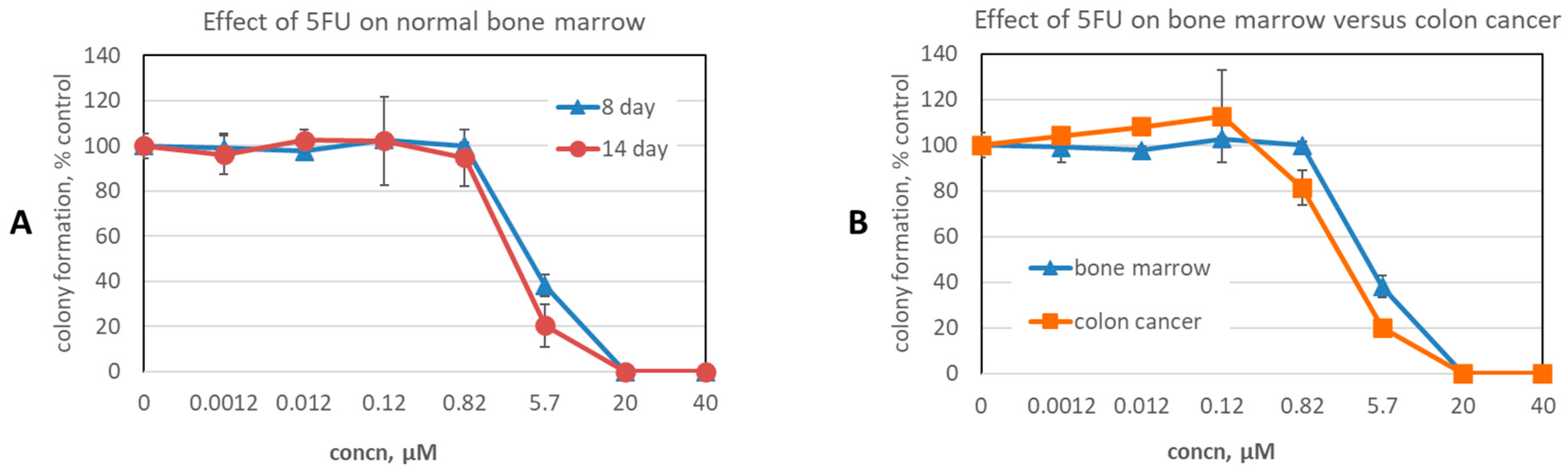
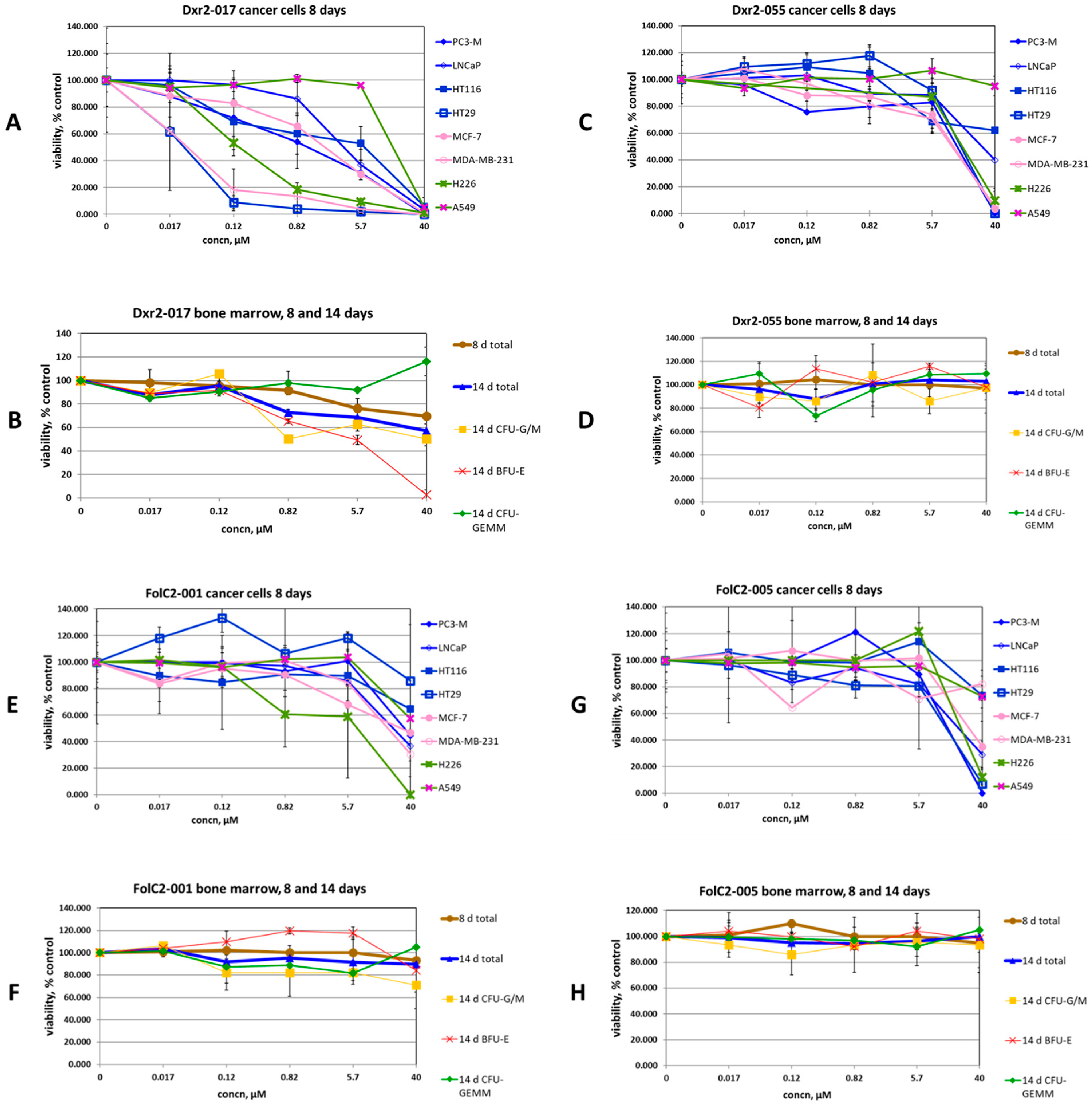
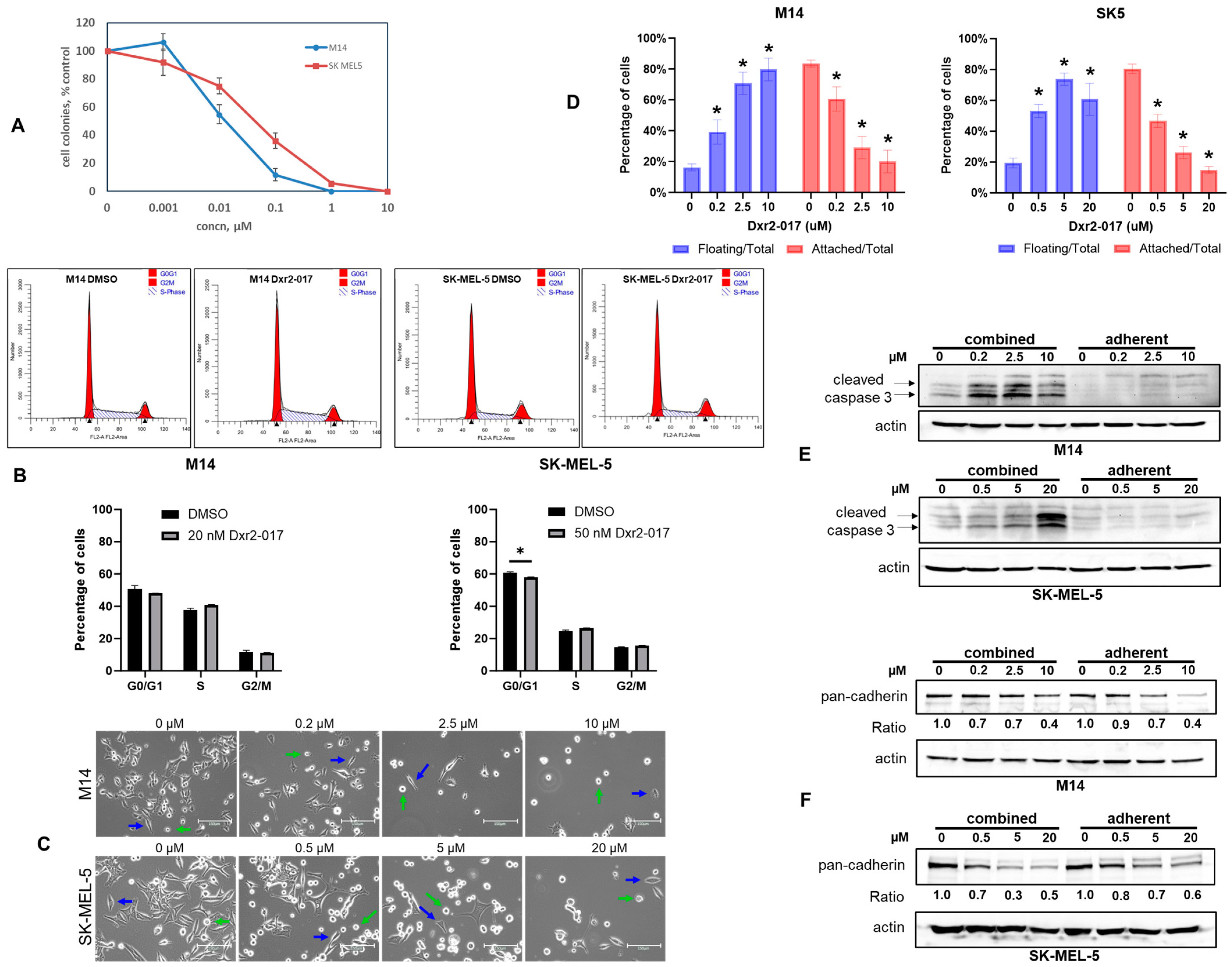
3.3. Screening for Therapeutic Efficacy
3.4. Expansion of Efficacy Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhung, W.; Kim, H.; Kim, W.Y. 3D molecular generative framework for interaction-guided drug design. Nat. Commun. 2024, 15, 2688. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Kobayashi, N.; Sugiki, T.; Nagashima, T.; Fujiwara, T.; Suzuki, K.; Kobayashi, N.; Murata, T.; Kosugi, T.; Tatsumi-Koga, R.; et al. Design of complicated all-alpha protein structures. Nat. Struct. Mol. Biol. 2024, 31, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Seoane, B.; Carbone, A. The complexity of protein interactions unravelled from structural disorder. PLoS Comput. Biol. 2021, 17, e1008546. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Li, Z.; Ding, M.; Chen, M. Three-dimensional protein model similarity analysis based on salient shape index. BMC Bioinform. 2016, 17, 131. [Google Scholar] [CrossRef]
- Batool, M.; Ahmad, B.; Choi, S. A Structure-Based Drug Discovery Paradigm. Int. J. Mol. Sci. 2019, 20, 2783. [Google Scholar] [CrossRef] [PubMed]
- Mohs, R.C.; Greig, N.H. Drug discovery and development: Role of basic biological research. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Barnash, K.D.; James, L.I.; Frye, S.V. Target class drug discovery. Nat. Chem. Biol. 2017, 13, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Childers, W.E.; Elokely, K.M.; Abou-Gharbia, M. The Resurrection of Phenotypic Drug Discovery. ACS Med. Chem. Lett. 2020, 11, 1820–1828. [Google Scholar] [CrossRef]
- Hingorani, A.D.; Kuan, V.; Finan, C.; Kruger, F.A.; Gaulton, A.; Chopade, S.; Sofat, R.; MacAllister, R.J.; Overington, J.P.; Hemingway, H.; et al. Improving the odds of drug development success through human genomics: Modelling study. Sci. Rep. 2019, 9, 18911. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Schlander, M.; Hernandez-Villafuerte, K.; Cheng, C.Y.; Mestre-Ferrandiz, J.; Baumann, M. How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment. Pharmacoeconomics 2021, 39, 1243–1269. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA 2020, 323, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994, 124, 619–626. [Google Scholar] [CrossRef]
- Meredith, J.E., Jr.; Fazeli, B.; Schwartz, M.A. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 1993, 4, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta 2013, 1833, 3481–3498. [Google Scholar] [CrossRef] [PubMed]
- Neuendorf, H.M.; Simmons, J.L.; Boyle, G.M. Therapeutic targeting of anoikis resistance in cutaneous melanoma metastasis. Front. Cell Dev. Biol. 2023, 11, 1183328. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Kyle, E.; Patel, S.; Housseau, F.; Hakim, F.; Lieberman, R.; Pins, M.; Blagosklonny, M.V.; Bergan, R.C. Prostate cancer chemoprevention agents exhibit selective activity against early stage prostate cancer cells. Prostate Cancer Prostatic Dis. 2001, 4, 81–91. [Google Scholar] [CrossRef]
- Xu, L.; Ding, Y.; Catalona, W.J.; Yang, X.J.; Anderson, W.F.; Jovanovic, B.; Wellman, K.; Killmer, J.; Huang, X.; Scheidt, K.A.; et al. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J. Natl. Cancer Inst. 2009, 101, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pattanayak, A.; Li, W.; Ko, H.K.; Fowler, G.; Gordon, R.; Bergan, R. A Multifunctional Therapy Approach for Cancer: Targeting Raf1- Mediated Inhibition of Cell Motility, Growth, and Interaction with the Microenvironment. Mol. Cancer Ther. 2020, 19, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gordon, R.; Farmer, R.; Pattanayak, A.; Binkowski, A.; Huang, X.; Avram, M.; Krishna, S.; Voll, E.; Pavese, J.; et al. Precision therapeutic targeting of human cancer cell motility. Nat. Commun. 2018, 9, 2454. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F. Structural genomics and drug discovery for infectious diseases. Infect. Disord. Drug Targets 2009, 9, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Stacy, R.; Anderson, W.F.; Myler, P.J. Structural Genomics Support for Infectious Disease Drug Design. ACS Infect. Dis. 2015, 1, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F. (Ed.) Structural Genomics and Drug Discovery; Humana Press: New York, NY, USA, 2014; Volume 1140. [Google Scholar]
- Westbrook, J.; Feng, Z.; Chen, L.; Yang, H.; Berman, H.M. The Protein Data Bank and structural genomics. Nucleic Acids Res. 2003, 31, 489–491. [Google Scholar] [CrossRef]
- Binkowski, T.A.; Cuff, M.; Nocek, B.; Chang, C.; Joachimiak, A. Assisted assignment of ligands corresponding to unknown electron density. J. Struct. Funct. Genom. 2010, 11, 21–30. [Google Scholar] [CrossRef]
- Binkowski, T.A.; Joachimiak, A. Protein functional surfaces: Global shape matching and local spatial alignments of ligand binding sites. BMC Struct. Biol. 2008, 8, 45. [Google Scholar] [CrossRef]
- Binkowski, T.A.; Joachimiak, A.; Liang, J. Protein surface analysis for function annotation in high-throughput structural genomics pipeline. Protein Sci. A Publ. Protein Soc. 2005, 14, 2972–2981. [Google Scholar] [CrossRef]
- Binkowski, T.A.; Adamian, L.; Liang, J. Inferring functional relationships of proteins from local sequence and spatial surface patterns. J. Mol. Biol. 2003, 332, 505–526. [Google Scholar] [CrossRef]
- Dundas, J.; Ouyang, Z.; Tseng, J.; Binkowski, A.; Turpaz, Y.; Liang, J. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006, 34, W116–W118. [Google Scholar] [CrossRef]
- Binkowski, T.A.; Freeman, P.; Liang, J. pvSOAR: Detecting similar surface patterns of pocket and void surfaces of amino acid residues on proteins. Nucleic Acids Res. 2004, 32, W555–W558. [Google Scholar] [CrossRef]
- Binkowski, T.A.; Naghibzadeh, S.; Liang, J. CASTp: Computed Atlas of Surface Topography of proteins. Nucleic Acids Res. 2003, 31, 3352–3355. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, D.T.; Lang, P.T.; Pegg, S.; Pettersen, E.; Kuntz, I.D.; Brooijmans, N.; Rizzo, R.C. Development and validation of a modular, extensible docking program: DOCK 5. J. Comput. Aided Mol. Des. 2006, 20, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, C.R.; Cardozo, M. MM-GB/SA rescoring of docking poses in structure-based lead optimization. J. Chem. Inf. Model. 2008, 48, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Roux, B. Computation of binding free energy with molecular dynamics and grand canonical Monte Carlo simulations. J. Chem. Phys. 2008, 128, 115103. [Google Scholar] [CrossRef] [PubMed]
- Dyda, F.; Klein, D.C.; Hickman, A.B. GCN5-related N-acetyltransferases: A structural overview. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, I.M.; Laha, R.G.; Roy, J. Handbook of Methods of Applied Statistics; John Wiley and Sons: Hoboken, NJ, USA, 1967; Volume I. [Google Scholar]
- Umeyama, S. Least-squares estimation of transformation paremeters between two point patterns. IEEE Trans. Pattern Anal. Mach. Intell. 1991, 13, 376–380. [Google Scholar] [CrossRef]
- Liang, J.; Edelsbrunner, H.; Fu, P.; Sudhakar, P.V.; Subramaniam, S. Analytical shape computation of macromolecules: II. Inaccessible cavities in proteins. Proteins 1998, 33, 18–29. [Google Scholar] [CrossRef]
- Liang, J.; Edelsbrunner, H.; Fu, P.; Sudhakar, P.V.; Subramaniam, S. Analytical shape computation of macromolecules: I. Molecular area and volume through alpha shape. Proteins 1998, 33, 1–17. [Google Scholar] [CrossRef]
- Liang, J.; Edelsbrunner, H.; Woodward, C. Anatomy of protein pockets and cavities: Measurement of binding site geometry and implications for ligand design. Protein Sci. 1998, 7, 1884–1897. [Google Scholar] [CrossRef]
- Rush, T.S., 3rd; Grant, J.A.; Mosyak, L.; Nicholls, A. A shape-based 3-D scaffold hopping method and its application to a bacterial protein-protein interaction. J. Med. Chem. 2005, 48, 1489–1495. [Google Scholar] [CrossRef]
- Huang, X.; Chen, S.; Xu, L.; Liu, Y.; Deb, D.K.; Platanias, L.C.; Bergan, R.C. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005, 65, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
- Binkowski, T.A.; Jiang, W.; Roux, B.; Anderson, W.F.; Joachimiak, A. Virtual high-throughput ligand screening. Methods Mol. Biol. 2014, 1140, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, W.P.; Brylinski, M. Calculating an optimal box size for ligand docking and virtual screening against experimental and predicted binding pockets. J. Cheminform 2015, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I. Pharmacophore features of potential drugs. Chemistry 2002, 8, 1976–1981. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.G.; Sharp, K.A. Protein pockets: Inventory, shape, and comparison. J. Chem. Inf. Model. 2010, 50, 589–603. [Google Scholar] [CrossRef]
- Bastolla, U.; Porto, M.; Eduardo Roman, M.H.; Vendruscolo, M.H. Connectivity of neutral networks, overdispersion, and structural conservation in protein evolution. J. Mol. Evol. 2003, 56, 243–254. [Google Scholar] [CrossRef]
- Orengo, C.A.; Bray, J.E.; Buchan, D.W.; Harrison, A.; Lee, D.; Pearl, F.M.; Sillitoe, I.; Todd, A.E.; Thornton, J.M. The CATH protein family database: A resource for structural and functional annotation of genomes. Proteomics 2002, 2, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Erdin, S.; Ward, R.M.; Venner, E.; Lichtarge, O. Evolutionary trace annotation of protein function in the structural proteome. J. Mol. Biol. 2010, 396, 1451–1473. [Google Scholar] [CrossRef] [PubMed]
- Madabushi, S.; Yao, H.; Marsh, M.; Kristensen, D.M.; Philippi, A.; Sowa, M.E.; Lichtarge, O. Structural clusters of evolutionary trace residues are statistically significant and common in proteins. J. Mol. Biol. 2002, 316, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Lichtarge, O.; Bourne, H.R.; Cohen, F.E. An evolutionary trace method defines binding surfaces common to protein families. J. Mol. Biol. 1996, 257, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Landau, M.; Mayrose, I.; Rosenberg, Y.; Glaser, F.; Martz, E.; Pupko, T.; Ben-Tal, N. ConSurf 2005: The projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005, 33, W299–W302. [Google Scholar] [CrossRef] [PubMed]
- La, D.; Sutch, B.; Livesay, D.R. Predicting protein functional sites with phylogenetic motifs. Proteins 2005, 58, 309–320. [Google Scholar] [CrossRef] [PubMed]
- del Sol, A.; Pazos, F.; Valencia, A. Automatic methods for predicting functionally important residues. J. Mol. Biol. 2003, 326, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Molina, J.A.; Xu, Y.; Torres-Martinez, J.; Stevens, R.; Focia, P.J.; Eakin, A.E.; Sacchettini, J.C.; Craig, S.P., 3rd. Ternary complex structure of human HGPRTase, PRPP, Mg2+, and the inhibitor HPP reveals the involvement of the flexible loop in substrate binding. Protein Sci. 1999, 8, 1023–1031. [Google Scholar] [CrossRef]
- Keough, D.T.; Hockova, D.; Holy, A.; Naesens, L.M.; Skinner-Adams, T.S.; Jersey, J.; Guddat, L.W. Inhibition of hypoxanthine-guanine phosphoribosyltransferase by acyclic nucleoside phosphonates: A new class of antimalarial therapeutics. J. Med. Chem. 2009, 52, 4391–4399. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Dikhit, M.R.; Sahoo, G.C.; Das, P. Comparative modeling of HGPRT enzyme of L. donovani and binding affinities of different analogs of GMP. Int. J. Biol. Macromol. 2012, 50, 637–649. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Equbal, A.; Dikhit, M.R.; Mansuri, R.; Rana, S.; Ali, V.; Sahoo, G.C.; Das, P. Establishment of correlation between in-silico and in-vitro test analysis against Leishmania HGPRT to inhibitors. Int. J. Biol. Macromol. 2016, 83, 78–96. [Google Scholar] [CrossRef]
- Bognar, A.L.; Osborne, C.; Shane, B.; Singer, S.C.; Ferone, R. Folylpoly-gamma-glutamate synthetase-dihydrofolate synthetase. Cloning and high expression of the Escherichia coli folC gene and purification and properties of the gene product. J. Biol. Chem. 1985, 260, 5625–5630. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Q.; Yang, Y.; Coward, J.K.; Nzila, A.; Sims, P.F.; Hyde, J.E. Characterisation of the bifunctional dihydrofolate synthase-folylpolyglutamate synthase from Plasmodium falciparum; a potential novel target for antimalarial antifolate inhibition. Mol. Biochem. Parasitol. 2010, 172, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kuzuyama, T.; Watanabe, H.; Seto, H. A 1-deoxy-D-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-D-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 9879–9884. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Diao, J.; Chen, P.; Pujari, V.; Yao, Y.; Cheng, G.; Crick, D.C.; Prasad, B.V.; Song, Y. Inhibition of 1-deoxy-D-xylulose-5-phosphate reductoisomerase by lipophilic phosphonates: SAR, QSAR, and crystallographic studies. J. Med. Chem. 2011, 54, 4721–4734. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, Y.; Cao, X.; Li, X.; Xue, S. Structure-directed construction of a high-performance version of the enzyme FabG from the photosynthetic microorganism Synechocystis sp. PCC 6803. FEBS Lett. 2015, 589, 3052–3057. [Google Scholar] [CrossRef]
- Hoang, T.T.; Sullivan, S.A.; Cusick, J.K.; Schweizer, H.P. Beta-ketoacyl acyl carrier protein reductase (FabG) activity of the fatty acid biosynthetic pathway is a determining factor of 3-oxo-homoserine lactone acyl chain lengths. Microbiology 2002, 148, 3849–3856. [Google Scholar] [CrossRef] [PubMed]
- Gurvitz, A. The essential mycobacterial genes, fabG1 and fabG4, encode 3-oxoacyl-thioester reductases that are functional in yeast mitochondrial fatty acid synthase type 2. Mol. Genet. Genom. 2009, 282, 407–416. [Google Scholar] [CrossRef]
- Ducasse-Cabanot, S.; Cohen-Gonsaud, M.; Marrakchi, H.; Nguyen, M.; Zerbib, D.; Bernadou, J.; Daffe, M.; Labesse, G.; Quemard, A. In vitro inhibition of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein reductase MabA by isoniazid. Antimicrob. Agents Chemother. 2004, 48, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Samuel, G.; Reeves, P. Biosynthesis of O-antigens: Genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr. Res. 2003, 338, 2503–2519. [Google Scholar] [CrossRef] [PubMed]
- Alphey, M.S.; Pirrie, L.; Torrie, L.S.; Boulkeroua, W.A.; Gardiner, M.; Sarkar, A.; Maringer, M.; Oehlmann, W.; Brenk, R.; Scherman, M.S.; et al. Allosteric competitive inhibitors of the glucose-1-phosphate thymidylyltransferase (RmlA) from Pseudomonas aeruginosa. ACS Chem. Biol. 2013, 8, 387–396. [Google Scholar] [CrossRef]
- Xiao, G.; Alphey, M.S.; Tran, F.; Pirrie, L.; Milbeo, P.; Zhou, Y.; Bickel, J.K.; Kempf, O.; Kempf, K.; Naismith, J.H.; et al. Next generation Glucose-1-phosphate thymidylyltransferase (RmlA) inhibitors: An extended SAR study to direct future design. Bioorganic Med. Chem. 2021, 50, 116477. [Google Scholar] [CrossRef] [PubMed]
- Lewendon, A.; Coggins, J.R. 3-Phosphoshikimate 1-carboxyvinyltransferase from Escherichia coli. Methods Enzymol. 1987, 142, 342–348. [Google Scholar]
- Herrmann, K.M.; Weaver, L.M. The Shikimate Pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Stallings, W.C.; Abdel-Meguid, S.S.; Lim, L.W.; Shieh, H.S.; Dayringer, H.E.; Leimgruber, N.K.; Stegeman, R.A.; Anderson, K.S.; Sikorski, J.A.; Padgette, S.R.; et al. Structure and topological symmetry of the glyphosate target 5-enolpyruvylshikimate-3-phosphate synthase: A distinctive protein fold. Proc. Natl. Acad. Sci. USA 1991, 88, 5046–5050. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.; Irwin, J.J. ZINC 15–Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.D.; Wagers, A.J. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 643–655. [Google Scholar] [CrossRef]
- Hart, L.; Ogbonnaya, A.; Boykin, K.; Deyoung, K.; Bailey, R.; Heritage, T.; Lopez-Gonzalez, L.; Huang, H.; Gordan, L. Burden of chemotherapy-induced myelosuppression among patients with extensive-stage small cell lung cancer: A retrospective study from community oncology practices. Cancer Med. 2023, 12, 10020–10030. [Google Scholar] [CrossRef] [PubMed]
- Epstein, R.S.; Aapro, M.S.; Basu Roy, U.K.; Salimi, T.; Krenitsky, J.; Leone-Perkins, M.L.; Girman, C.; Schlusser, C.; Crawford, J. Patient Burden and Real-World Management of Chemotherapy-Induced Myelosuppression: Results from an Online Survey of Patients with Solid Tumors. Adv. Ther. 2020, 37, 3606–3618. [Google Scholar] [CrossRef] [PubMed]
- Kurtin, S. Myeloid toxicity of cancer treatment. J. Adv. Pr. Oncol. 2012, 3, 209–224. [Google Scholar]
- Purgato, M.; Barbui, C. What is the WHO essential medicines list? Epidemiol. Psychiatr. Sci. 2012, 21, 343–345. [Google Scholar] [CrossRef]
- Grem, J.L. 5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Investig. New Drugs 2000, 18, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, M.; Xu, L.; Ananthanarayanan, V.; Cooper, J.; Takimoto, C.H.; Helenowski, I.; Pelling, J.C.; Bergan, R.C. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008, 68, 2024–2032. [Google Scholar] [CrossRef]
- Tedja, R.; Fox, A.; Alvero, A.B. Detection of Anoikis Using Cell Viability Dye and Quantitation of Caspase Activity. Methods Mol. Biol. 2021, 2255, 69–76. [Google Scholar] [CrossRef]
- Ko, H.; Kim, S.; Jin, C.H.; Lee, E.; Ham, S.; Yook, J.I.; Kim, K. Protein kinase casein kinase 2-mediated upregulation of N-cadherin confers anoikis resistance on esophageal carcinoma cells. Mol. Cancer Res. 2012, 10, 1032–1038. [Google Scholar] [CrossRef]
- Sousa, B.; Pereira, J.; Marques, R.; Grilo, L.F.; Pereira, S.P.; Sardao, V.A.; Schmitt, F.; Oliveira, P.J.; Paredes, J. P-cadherin induces anoikis-resistance of matrix-detached breast cancer cells by promoting pentose phosphate pathway and decreasing oxidative stress. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165964. [Google Scholar] [CrossRef]
- Hu, W.; Ohue, M. SpatialPPI: Three-dimensional space protein-protein interaction prediction with AlphaFold Multimer. Comput. Struct. Biotechnol. J. 2024, 23, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Gorostiola Gonzalez, M.; Janssen, A.P.A.; AP, I.J.; Heitman, L.H.; van Westen, G.J.P. Oncological drug discovery: AI meets structure-based computational research. Drug Discov. Today 2022, 27, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Arul Murugan, N.; Ruba Priya, G.; Narahari Sastry, G.; Markidis, S. Artificial intelligence in virtual screening: Models versus experiments. Drug Discov. Today 2022, 27, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Gedgaudas, M.; Baronas, D.; Kazlauskas, E.; Petrauskas, V.; Matulis, D. Thermott: A comprehensive online tool for protein-ligand binding constant determination. Drug Discov. Today 2022, 27, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, R.; Bajorath, J.; Aoki, K. From traditional to data-driven medicinal chemistry: A case study. Drug Discov. Today 2022, 27, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, I.; Isin, E.M.; Barton, P.; Cillero-Pastor, B.; Heeren, R.M.A. Multimodal molecular imaging in drug discovery and development. Drug Discov. Today 2022, 27, 2086–2099. [Google Scholar] [CrossRef]
- AI’s potential to accelerate drug discovery needs a reality check. Nature 2023, 622, 217. [CrossRef]
| Mean Number of Colonies, % of Control * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Dxr2-001 | Dxr2-017 | Dxr2-054 | Dxr2-055 | Dxr2-069 | FolC2-001 | FolC2-005 | FolC2-025 | FolC2-044 | FolC2-048 |
| 8 days | 109 | 70 | 79 | 99 | 108 | 108 | 105 | 93 | 96 | 87 |
| 14 days | 93 | 57 | 112 | 115 | 75 | 87 | 101 | 109 | 73 | 120 |
| Compound | FolC1-020 | FolC1-031 | FolC1-042 | FolC1-087 | FolC1-100 | Dxr1-005 | Dxr1-021 | Dxr1-026 | Dxr1-070 | Dxr1-083 |
| 8 days | 107 | 80 | 105 | 107 | 98 | 106 | 101 | 100 | 96 | 97 |
| 14 days | 97 | 85 | 102 | 94 | 96 | 90 | 80 | 128 | 97 | 93 |
| Compound | TYLT-001 | TYLT-003 | TYLT-051 | TYLT-072 | TYLT-088 | FabG-033 | FabG-035 | FabG-036 | FabG-045 | FabG-073 |
| 8 days | 117 | 97 | 84 | 88 | 101 | 102 | 113 | 106 | 97 | 97 |
| 14 days | 103 | 94 | 103 | 104 | 112 | 94 | 105 | 100 | 103 | 92 |
| Compound | PRT-009 | PRT-031 | PRT-044 | PRT-051 | PRT-P89 | EPSP-023 | EPSP-044 | EPSP-065 | ||
| 8 days | 111 | 106 | 101 | 93 | 111 | 94 | 98 | 99 | ||
| 14 days | 101 | 97 | 93 | 119 | 93 | 114 | 98 | 98 | ||
| Ratio of Cancer Cell Colonies to Bone Marrow Colonies at 8 Days * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell Line | Dxr2-017 | Dxr2-055 | Dxr2-069 | FolC2-001 | FolC2-005 | FolC2-044 | FolC1-042 | FolC1-020 | Dxr1-021 | TYLT-072 |
| HT116 | 0.08 | 0.63 | 0.93 | 0.60 | 0.70 | 0.03 | 0.51 | 0.23 | 0.95 | 1.18 |
| HT29 | 0.00 | 0.00 | 0.01 | 0.79 | 0.06 | 0.00 | 0.10 | 0.12 | 0.59 | 2.05 |
| MCF-7 | 0.02 | 0.04 | 0.03 | 0.43 | 0.33 | 0.01 | 0.64 | 0.01 | 0.81 | 0.98 |
| MDAMB231 | 0.00 | 0.05 | 0.59 | 0.28 | 0.78 | 0.16 | 0.65 | 0.65 | 0.62 | 0.97 |
| H226 | 0.02 | 0.10 | 0.03 | 0.00 | 0.11 | 0.00 | 0.01 | 0.26 | 1.26 | 0.14 |
| A549 | 0.07 | 0.96 | 0.15 | 0.53 | 0.69 | 0.15 | 0.96 | 0.89 | 0.72 | 1.17 |
| PC3-M | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 | 0.18 | 0.13 | 0.38 | 0.99 |
| LNCaP ** | 0.05 | 0.40 | - | 0.34 | 0.28 | - | - | - | - | - |
| Relative activity for a given compound, % ¥ | 100 | 75 | 71 | 63 | 63 | 100 | 43 | 73 | 14 | 14 |
| Activity ** | ||||
|---|---|---|---|---|
| Site | Active Compounds, % | Mean | SEM | Range, % ¥ |
| Dxr2 | 60 | 0.06 | 0.02 | 71–100 |
| FolC2 | 60 | 0.15 | 0.04 | 63–100 |
| FolC1 | 40 | 0.13 | 0.03 | 43–73 |
| Dxr1 | 20 | 0.38 | 0 | 14 |
| TYLT | 20 | 0.14 | 0 | 14 |
| PRT | 0 | NA | NA | NA |
| EPSP | 0 | NA | NA | NA |
| FabG | 0 | NA | NA | NA |
| Ratio of Cancer Cell Colonies to Bone Marrow Colonies at 8 Days * | ||||
|---|---|---|---|---|
| Cell Line | Dxr2-017 | FolC2-001 | FolC1-020 | TYLT-072 |
| HT116 | 0.69 | 0.85 | 0.88 | 0.83 |
| HT29 | 0.03 | 1.13 | 0.69 | 1.22 |
| MCF-7 | 0.39 | 0.65 | 0.08 | 0.94 |
| MDAMB231 | 0.05 | 0.81 | 0.96 | 0.95 |
| H226 | 0.12 | 0.56 | 0.96 | 0.26 |
| A549 | 1.26 | 0.99 | 0.84 | 0.99 |
| PC3-M | 0.41 | 0.96 | 0.76 | 1.02 |
| LNCaP ** | 0.49 | 0.82 | - | - |
| Relative activity for a given compound, % ¥ | 75 | 13 | 14 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, F.; Binkowski, T.A.; Broughan, I.; Chen, W.; Natarajan, A.; Schiltz, G.E.; Scheidt, K.A.; Anderson, W.F.; Bergan, R. Protein Structure Inspired Discovery of a Novel Inducer of Anoikis in Human Melanoma. Cancers 2024, 16, 3177. https://doi.org/10.3390/cancers16183177
Qiao F, Binkowski TA, Broughan I, Chen W, Natarajan A, Schiltz GE, Scheidt KA, Anderson WF, Bergan R. Protein Structure Inspired Discovery of a Novel Inducer of Anoikis in Human Melanoma. Cancers. 2024; 16(18):3177. https://doi.org/10.3390/cancers16183177
Chicago/Turabian StyleQiao, Fangfang, Thomas Andrew Binkowski, Irene Broughan, Weining Chen, Amarnath Natarajan, Gary E. Schiltz, Karl A. Scheidt, Wayne F. Anderson, and Raymond Bergan. 2024. "Protein Structure Inspired Discovery of a Novel Inducer of Anoikis in Human Melanoma" Cancers 16, no. 18: 3177. https://doi.org/10.3390/cancers16183177
APA StyleQiao, F., Binkowski, T. A., Broughan, I., Chen, W., Natarajan, A., Schiltz, G. E., Scheidt, K. A., Anderson, W. F., & Bergan, R. (2024). Protein Structure Inspired Discovery of a Novel Inducer of Anoikis in Human Melanoma. Cancers, 16(18), 3177. https://doi.org/10.3390/cancers16183177





