Isotopic Composition of C, N, and S as an Indicator of Endometrial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
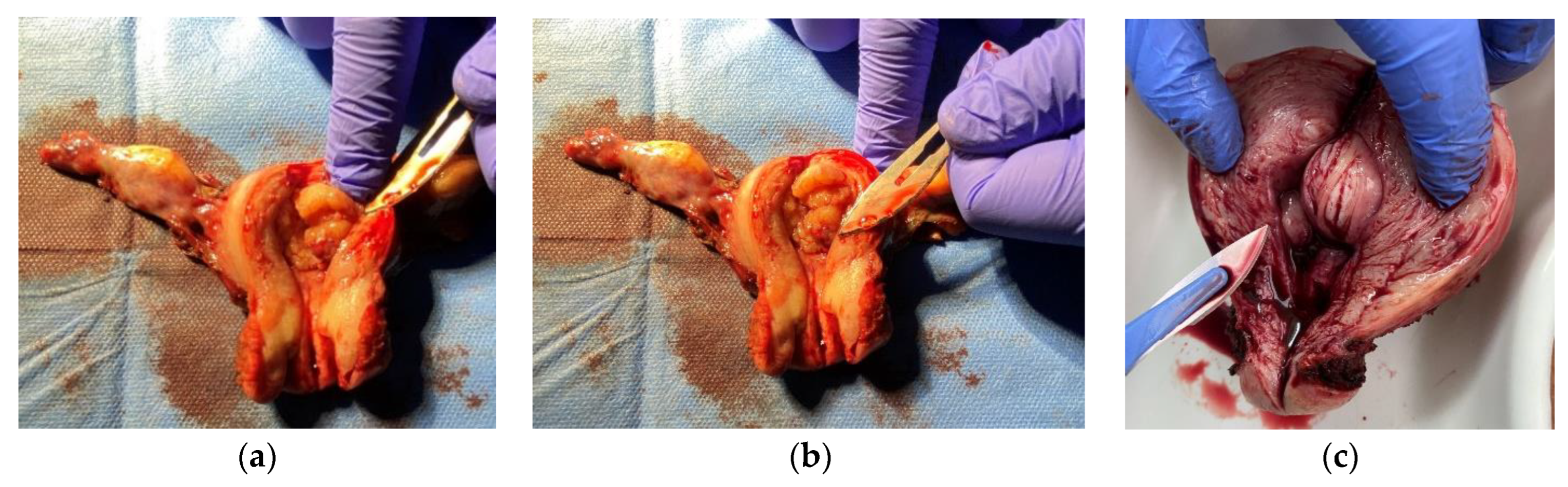
2. Materials and Methods
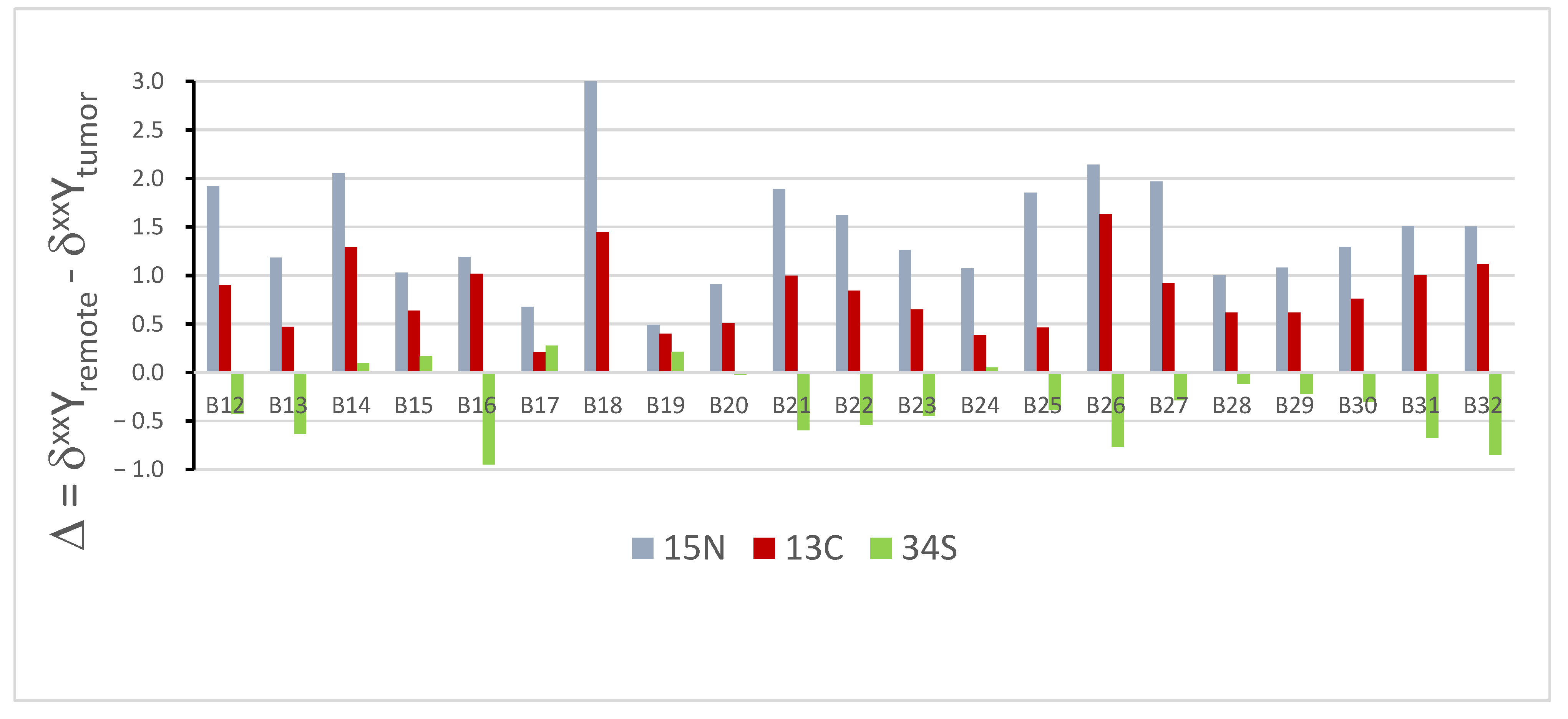
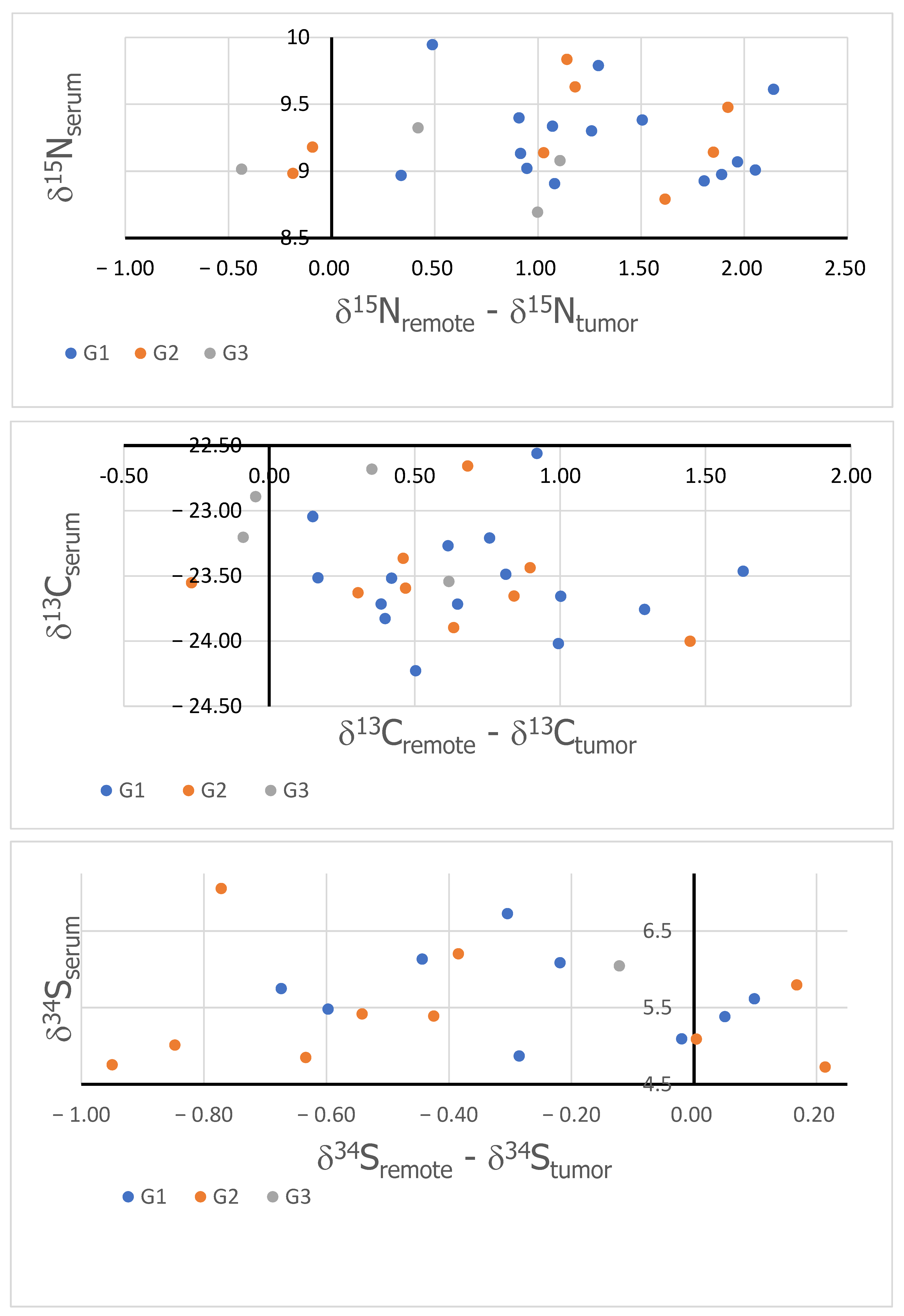
3. Results
4. Discussion
- -
- They are a substrate for the synthesis of many compounds that play a role in the metabolism of malignant tumor cells;
- -
- They modify mitochondrial tRNA [54];
- -
- They regulate osmotic processes and play an important antioxidant function for the homeostasis of the tumor microenvironment [55];
- -
- They regulate the glucose and lipid metabolism;
- -
- They affect the processes of oxidative phosphorylation and protein sulfurization;
- -
- -
- They play a role in the metabolism of malignant tumor cells. In healthy cells, they show strong antioxidant properties and maintain cellular homeostasis [58]. However, it has been shown that, under conditions of severe oxidative stress, elevated glutathione levels contribute to the accelerated progression of malignant tumors and resistance to chemotherapeutic treatment [59,60].
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global cancer burden growing, amidst mounting need for services. Saudi Med. J. 2024, 45, 326–327. [Google Scholar]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Conley, B.A.; Taube, S.E. Prognostic and predictive markers in cancer. Dis. Markers 2004, 20, 35–43. [Google Scholar] [CrossRef]
- Thenrajan, T.; Alwarappan, S.; Wilson, J. Molecular Diagnosis and Cancer Prognosis—A Concise Review. Diagnostics 2023, 13, 766. [Google Scholar] [CrossRef]
- Rabin, B.A.; Gaglio, B.; Sanders, T.; Nekhlyudov, L.; Dearing, J.W.; Bull, S.; Glasgow, R.E.; Marcus, A. Predicting cancer prognosis using interactive online tools: A systematic review and implications for cancer care providers. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1645–1656. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Feng, Y.; Zhang, J.; Swinnen, J.; Li, Y.; Ni, Y. A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed. Cancers 2019, 11, 1782. [Google Scholar] [CrossRef]
- Albarede, F.; Télouk, P.; Balter, V.; Bondanese, V.P.; Albalat, E.; Oger, P.; Bonaventura, P.; Miossec, P.; Fujii, T. Medical applications of Cu, Zn, and S isotope effects. Metallomics 2016, 8, 1056–1070. [Google Scholar] [CrossRef]
- Hastuti, A.A.M.B.; Costas-Rodríguez, M.; Matsunaga, A.; Ichinose, T.; Hagiwara, S.; Shimura, M.; Vanhaecke, F. Cu and Zn isotope ratio variations in plasma for survival prediction in hematological malignancy cases. Sci. Rep. 2020, 10, 16389. [Google Scholar] [CrossRef]
- Balter, V.; da Costa, A.N.; Bondanese, V.P.; Jaouen, K.; Lamboux, A.; Sangrajrang, S.; Vincent, N.; Fourel, F.; Télouk, P.; Gigou, M.; et al. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, 982–985. [Google Scholar] [CrossRef]
- Télouk, P.; Puisieux, A.; Fujii, T.; Balter, V.; Bondanese, V.P.; Morel, A.-P.; Clapisson, G.; Lamboux, A.; Albarede, F. Copper isotope effect in serum of cancer patients. A pilot study. Metallomics 2015, 7, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Costas-Rodríguez, M.; Anoshkina, Y.; Lauwens, S.; Van Vlierberghe, H.; Delanghe, J.; Vanhaecke, F. Isotopic analysis of Cu in blood serum by multi-collector ICP-mass spectrometry: A new approach for the diagnosis and prognosis of liver cirrhosis? Metallomics 2015, 7, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Toubhans, B.; Gourlan, A.; Telouk, P.; Lutchman-Singh, K.; Francis, L.; Conlan, R.; Margarit, L.; Gonzalez, D.; Charlet, L. Cu isotope ratios are meaningful in ovarian cancer diagnosis. J. Trace Elem. Med. Biol. 2020, 62, 126611. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Zhang, C.; Sheng, F.; Song, S.; Li, P.; Dai, S.; Wang, B.; Lu, D.; Zhang, L.; et al. Identification of two-dimensional copper signatures in human blood for bladder cancer with machine learning. Chem. Sci. 2022, 13, 1648–1656. [Google Scholar] [CrossRef]
- Miaou, E.; Tissot, F.L.H. Copper isotope ratios in serum do not track cancerous tumor evolution, but organ failure. Metallomics 2023, 15, mfad060. [Google Scholar] [CrossRef]
- Tea, I.; De Luca, A.; Schiphorst, A.-M.; Grand, M.; Barillé-Nion, S.; Mirallié, E.; Drui, D.; Krempf, M.; Hankard, R.; Tcherkez, G. Stable Isotope Abundance and Fractionation in Human Diseases. Metabolites 2021, 11, 370. [Google Scholar] [CrossRef]
- Straub, M.; Sigman, D.M.; Auderset, A.; Ollivier, J.; Petit, B.; Hinnenberg, B.; Rubach, F.; Oleynik, S.; Vozenin, M.-C.; Martínez-García, A. Distinct nitrogen isotopic compositions of healthy and cancerous tissue in mice brain and head&neck micro-biopsies. BMC Cancer 2021, 21, 805. [Google Scholar]
- Straub, M.; Auderset, A.; de Leval, L.; Piazzon, N.; Maison, D.; Vozenin, M.-C.; Ollivier, J.; Petit, B.; Sigman, D.M.; Martínez-García, A. Nitrogen isotopic composition as a gauge of tumor cell anabolism-to-catabolism ratio. Sci. Rep. 2023, 13, 19796. [Google Scholar] [CrossRef]
- Madej, A.; Forma, E.; Golberg, M.; Kamiński, R.; Paneth, P.; Kobos, J.; Różański, W.; Lipiński, M. 13C Natural Isotope Abundance in Urothelium as a New Marker in the Follow-Up of Patients with Bladder Cancer. Cancers 2022, 14, 2423. [Google Scholar] [CrossRef]
- Wolfsberg, M.; Van Hook, W.A.; Paneth, P.; Rebelo, L.P. Isotope Effects: In the Chemical, Geological, and BioSciences; Springer: Berlin/Heidelberg, Germany, 2009; 466p. [Google Scholar]
- Tcherkez, G.; Tea, I. 32S/34S isotope fractionation in plant sulphur metabolism. New Phytol. 2013, 200, 44–53. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Epstein, T.; Xu, L.; Gillies, R.J.; AGatenby, R. Separation of metabolic supply and demand: Aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab. 2014, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Reinfeld, B.I.; Madden, M.Z.; Wolf, M.M.; Chytil, A.; Bader, J.E.; Patterson, A.R.; Sugiura, A.; Cohen, A.S.; Ali, A.; Do, B.T.; et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 2021, 593, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Wang, Z.; Schey, K.L.; Truscott, R.J.W. Spontaneous Cleavage at Glu and Gln Residues in Long-Lived Proteins. ACS Chem. Biol. 2021, 16, 2244–2254. [Google Scholar] [CrossRef]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New aspects of amino acid metabolism in cancer. Br. J. Cancer 2019, 122, 150–156. [Google Scholar] [CrossRef]
- Anderson, N.M.; Mucka, P.; Kern, J.G.; Feng, H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 2018, 9, 216–237. [Google Scholar] [CrossRef]
- Li, T.; Copeland, C.; Le, A. Glutamine Metabolism in Cancer. Adv. Exp. Med. Biol. 2021, 1311, 17–38. [Google Scholar]
- Shim, H.; Dolde, C.; Lewis, B.C.; Wu, C.-S.; Dang, G.; Jungmann, R.A.; Dalla-Favera, R.; Dang, C.V. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA 1997, 94, 6658–6663. [Google Scholar] [CrossRef]
- Matés, J.M.; Campos-Sandoval, J.A.; Santos-Jiménez, J.d.L.; Márquez, J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett. 2019, 467, 29–39. [Google Scholar] [CrossRef]
- Bott, A.J.; Shen, J.; Tonelli, C.; Zhan, L.; Sivaram, N.; Jiang, Y.P.; Yu, X.; Bhatt, V.; Chiles, E.; Zhong, H.; et al. Glutamine Anabolism Plays a Critical Role in Pancreatic Cancer by Coupling Carbon and Nitrogen Metabolism. Cell Rep. 2019, 29, 1287–1298.e6. [Google Scholar] [CrossRef] [PubMed]
- Peyton, K.J.; Liu, X.-M.; Yu, Y.; Yates, B.; Behnammanesh, G.; Durante, W. Glutaminase-1 stimulates the proliferation, migration, and survival of human endothelial cells. Biochem. Pharmacol. 2018, 156, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Adler, L.; Karathia, H.; Carmel, N.; Rabinovich, S.; Auslander, N.; Keshet, R.; Stettner, N.; Silberman, A.; Agemy, L.; et al. Urea Cycle Dysregulation Generates Clinically Relevant Genomic and Biochemical Signatures. Cell 2018, 174, 1559–1570.e22. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H.; Sun, W.; Yang, X.; Nie, Q.; Fang, X. Role of glutamine and its metabolite ammonia in crosstalk of cancer-associated fibroblasts and cancer cells. Cancer Cell Int. 2021, 21, 479. [Google Scholar] [CrossRef]
- Kurmi, K.; Haigis, M.C. Nitrogen Metabolism in Cancer and Immunity. Trends Cell Biol. 2020, 30, 408–424. [Google Scholar] [CrossRef]
- Bogusiak, K.; Kozakiewicz, M.; Puch, A.; Mostowski, R.; Paneth, P.; Kobos, J. Oral Cavity Cancer Tissues Differ in Isotopic Composition Depending on Location and Staging. Cancers 2023, 15, 4610. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Locasale, J.W.; Cantley, L.C. Metabolic Flux and the Regulation of Mammalian Cell Growth. Cell Metab. 2011, 14, 443–451. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Zhang, L.; Zhang, S.; Dai, Y. Metabolic reprogramming and interventions in endometrial carcinoma. Biomed. Pharmacother. 2023, 161, 114526. [Google Scholar] [CrossRef]
- Šimaga, S.; Abramić, M.; Osmak, M.; Babić, D.; Ilić-Forko, J. Total tissue lactate dehydrogenase activity in endometrial carcinoma. Int. J. Gynecol. Cancer 2008, 18, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J. The Role of Metabolic Syndrome in Endometrial Cancer: A Review. Front. Oncol. 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Raglan, O.; Kalliala, I.; Markozannes, G.; Cividini, S.; Gunter, M.J.; Nautiyal, J.; Gabra, H.; Paraskevaidis, E.; Martin-Hirsch, P.; Tsilidis, K.K.; et al. Risk factors for endometrial cancer: An umbrella review of the literature. Int. J. Cancer 2019, 145, 1719–1730. [Google Scholar] [CrossRef]
- Lambe, M.; Wigertz, A.; Garmo, H.; Walldius, G.; Jungner, I.; Hammar, N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control 2011, 22, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Cormio, A.; Cormio, G.; Musicco, C.; Sardanelli, A.M.; Gasparre, G.; Gadaleta, M.N. Mitochondrial changes in endometrial carcinoma: Possible role in tumor diagnosis and prognosis (Review). Oncol. Rep. 2015, 33, 1011–1018. [Google Scholar] [CrossRef]
- Song, J.; Lee, K.; Park, S.W.; Chung, H.; Jung, D.; Na, Y.R.; Quan, H.; Cho, C.S.; Che, J.-H.; Kim, J.H.; et al. Lactic Acid Upregulates VEGF Expression in Macrophages and Facilitates Choroidal Neovascularization. Investig. Opthalmology Vis. Sci. 2018, 59, 3747–3754. [Google Scholar] [CrossRef]
- Oh, S.; Seo, S.B.; Kim, G.; Batsukh, S.; Son, K.H.; Byun, K. Poly-D,L-Lactic Acid Stimulates Angiogenesis and Collagen Synthesis in Aged Animal Skin. Int. J. Mol. Sci. 2023, 24, 7986. [Google Scholar] [CrossRef]
- Ward, N.P.; DeNicola, G.M. Sulfur metabolism and its contribution to malignancy. Int. Rev. Cell Mol. Biol. 2019, 347, 39–103. [Google Scholar]
- Sanderson, S.M.; Gao, X.; Dai, Z.; Locasale, J.W. Methionine metabolism in health and cancer: A nexus of diet and precision medicine. Nat. Rev. Cancer 2019, 19, 625–637. [Google Scholar] [CrossRef]
- El-Khairy, L.; Ueland, P.M.; Nygård, O.; Refsum, H.; EVollset, S. Lifestyle and cardiovascular disease risk factors as determinants of total cysteine in plasma: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 1999, 70, 1016–1024. [Google Scholar] [CrossRef]
- Minton, D.R.; Nam, M.; McLaughlin, D.J.; Shin, J.; Bayraktar, E.C.; Alvarez, S.W.; Sviderskiy, V.O.; Papagiannakopoulos, T.; Sabatini, D.M.; Birsoy, K.; et al. Serine Catabolism by SHMT2 Is Required for Proper Mitochondrial Translation Initiation and Maintenance of Formylmethionyl-tRNAs. Mol. Cell 2018, 69, 610–621.e5. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.L.; Sufrin, J.R.; Porter, C.W. Relative effects of S-adenosylmethionine depletion on nucleic acid methylation and polyamine biosynthesis. Biochem. J. 1987, 247, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002, 21, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Kyriakopoulos, A.M.; Goulielmaki, M.; Panayiotidis, M.I.; Spandidos, D.A.; Zoumpourlis, V. Significance of taurine transporter (TauT) in homeostasis and its layers of regulation (Review). Mol. Med. Rep. 2020, 22, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.-Q.; Chen, H.-J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.-Y.; et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules 2022, 27, 3389. [Google Scholar] [CrossRef]
- Shackelford, R.E.; Mohammad, I.Z.; Meram, A.T.; Kim, D.; Alotaibi, F.; Patel, S.; Ghali, G.E.; Kevil, C.G. Molecular Functions of Hydrogen Sulfide in Cancer. Pathophysiology 2021, 28, 437–456. [Google Scholar] [CrossRef]
- Rai, S.R.; Bhattacharyya, C.; Sarkar, A.; Chakraborty, S.; Sircar, E.; Dutta, S.; Sengupta, R. Glutathione: Role in Oxidative/Nitrosative Stress, Antioxidant Defense, and Treatments. ChemistrySelect 2021, 6, 4566–4590. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Bansal, A.; Celeste Simon, M. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Pasqualini, J.R. Estrogen sulfotransferases in breast and endometrial cancers. Ann. N. Y. Acad. Sci. 2009, 1155, 88–98. [Google Scholar] [CrossRef]
- Huber, W.W.; Parzefall, W. Thiols and the chemoprevention of cancer. Curr. Opin. Pharmacol. 2007, 7, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Eiler, J.; Cesar, J.; Chimiak, L.; Dallas, B.; Grice, K.; Griep-Raming, J.; Juchelka, D.; Kitchen, N.; Lloyd, M.; Makarov, A.; et al. Analysis of molecular isotopic structures at high precision and accuracy by Orbitrap mass spectrometry. Int. J. Mass Spectrom. 2017, 422, 126–142. [Google Scholar] [CrossRef]
- Hofmann, A.E.; Chimiak, L.; Dallas, B.; Griep-Raming, J.; Juchelka, D.; Makarov, A.; Schwieters, J.; Eiler, J.M. Using Orbitrap mass spectrometry to assess the isotopic compositions of individual compounds in mixtures. Int. J. Mass Spectrom. 2020, 457, 116410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuzak, T.; Bogaczyk, A.; Krata, A.A.; Kamiński, R.; Paneth, P.; Kluz, T. Isotopic Composition of C, N, and S as an Indicator of Endometrial Cancer. Cancers 2024, 16, 3169. https://doi.org/10.3390/cancers16183169
Zuzak T, Bogaczyk A, Krata AA, Kamiński R, Paneth P, Kluz T. Isotopic Composition of C, N, and S as an Indicator of Endometrial Cancer. Cancers. 2024; 16(18):3169. https://doi.org/10.3390/cancers16183169
Chicago/Turabian StyleZuzak, Tomasz, Anna Bogaczyk, Agnieszka Anna Krata, Rafał Kamiński, Piotr Paneth, and Tomasz Kluz. 2024. "Isotopic Composition of C, N, and S as an Indicator of Endometrial Cancer" Cancers 16, no. 18: 3169. https://doi.org/10.3390/cancers16183169
APA StyleZuzak, T., Bogaczyk, A., Krata, A. A., Kamiński, R., Paneth, P., & Kluz, T. (2024). Isotopic Composition of C, N, and S as an Indicator of Endometrial Cancer. Cancers, 16(18), 3169. https://doi.org/10.3390/cancers16183169






