The Role of Circulating Tumor DNA in Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
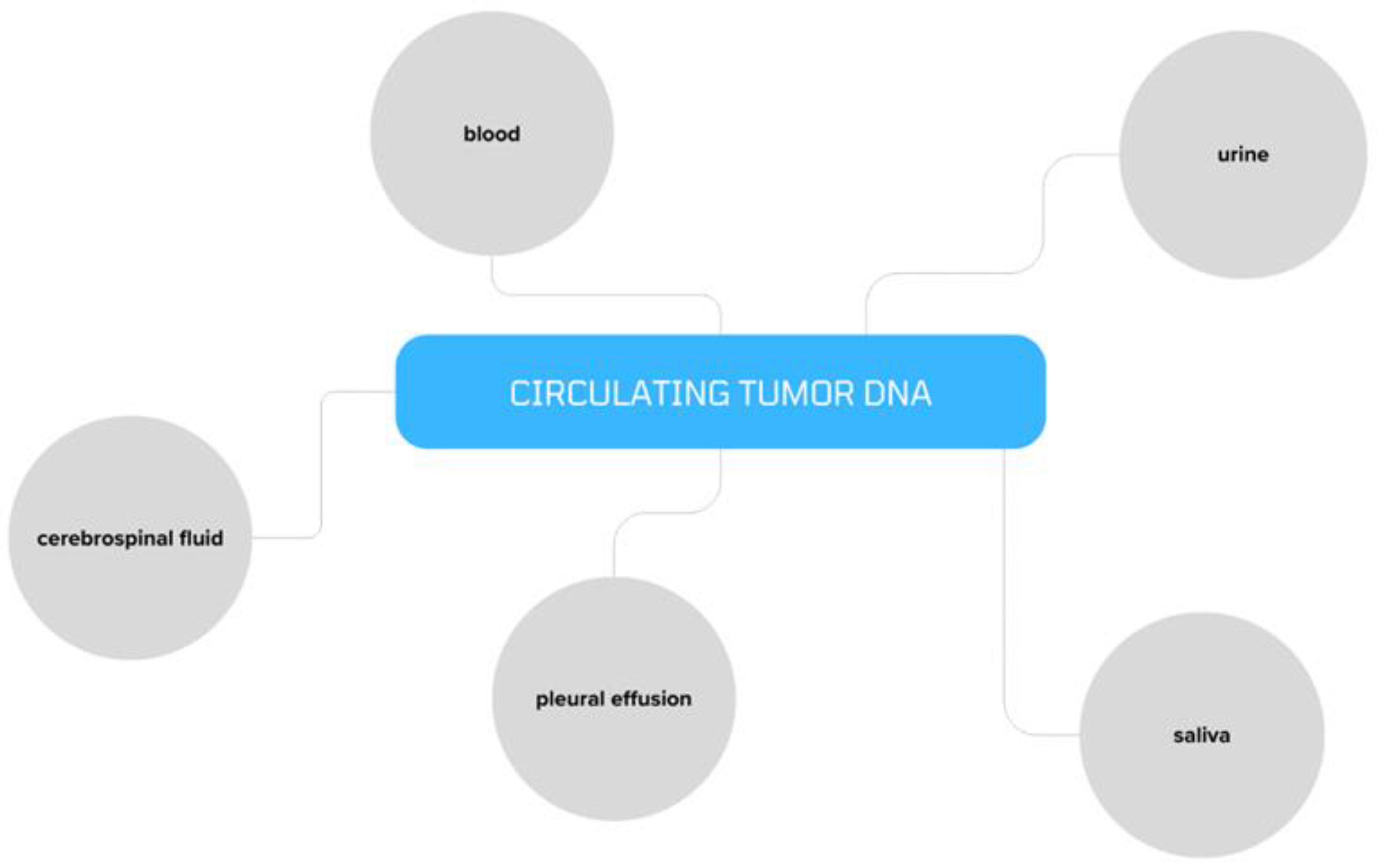
2. ctDNA in Ovarian Cancer
2.1. Chromosomal Instability
2.2. Somatic Mutations
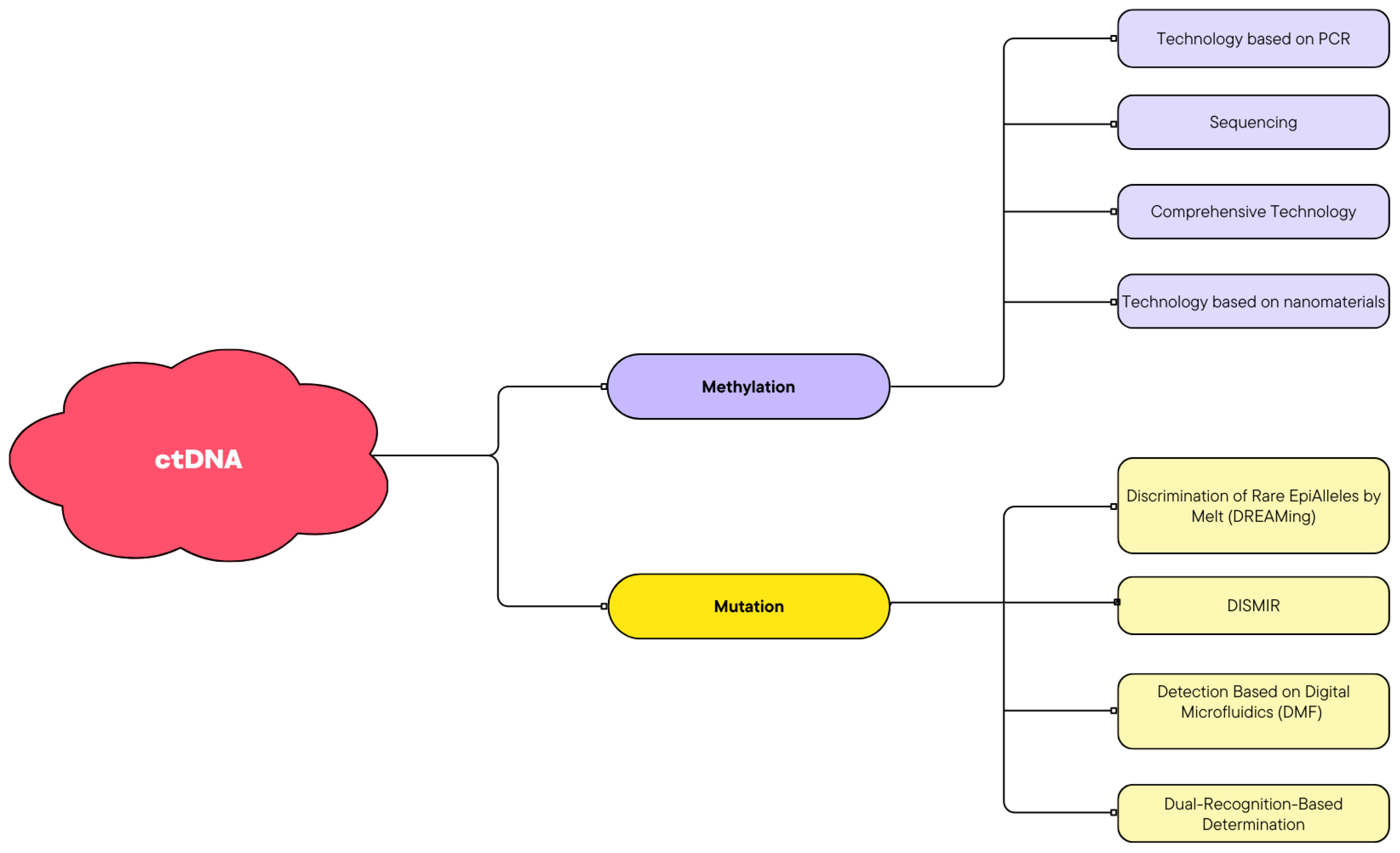
2.3. Methylation
3. Diagnostics
4. Prognosis and Prediction of Response to Treatment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bois, A.D.; Pfisterer, J. Future options for first-line therapy of advanced ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2005, 15, 42–50. [Google Scholar] [CrossRef]
- Gasparri, A.P.M.L.; Savone, D.; Besharat, R.A.; Farooqi, A.A.; Bellati, F.; Ruscito, I.; Panici, P.B. Circulating tumor cells as trigger to hematogenous spreads and potential biomarkers to predict the prognosis in ovarian cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Niepel, M.; Mitchison, T.K.; Sorger, P.K. Dissecting variability in responses to cancer chemotherapy through systems pharmacology. Clin. Pharmacol. Ther. 2010, 88, 34–38. [Google Scholar] [CrossRef]
- Yeung, T.-L.; Leung, C.S.; Yip, K.-P.; Yeung, C.L.A.; Wong, S.T.C.; Mok, S.C. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol. Cell Physiol. 2015, 309, C444–C456. [Google Scholar] [CrossRef]
- Tan, D.S.P.; Agarwal, R.; Kaye, S.B. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet. Oncol. 2006, 7, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Marzese, D.M.; Hirose, H.; Hoon, D.S.B. Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients. Expert Rev. Mol. Diagn. 2013, 13, 827–844. [Google Scholar] [CrossRef]
- Kuhlmann, J.D.; Schwarzenbach, H.; Wimberger, P.; Poetsch, M.; Kimmig, R.; Kasimir-Bauer, S. LOH at 6q and 10q in fractionated circulating DNA of ovarian cancer patients is predictive for tumor cell spread and overall survival. BMC Cancer 2012, 12, 325. [Google Scholar] [CrossRef]
- Warton, K.; Samimi, G. Methylation of cell-free circulating DNA in the diagnosis of cancer. Front. Mol. Biosci. 2015, 2, 13. [Google Scholar] [CrossRef]
- Patel, K.M.; Tsui, D.W.Y. The translational potential of circulating tumour DNA in oncology. Clin. Biochem. 2015, 48, 957–961. [Google Scholar] [CrossRef]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a cancer biomarker: A broad overview. Crit. Rev. Oncol. 2020, 155, 103109. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011, 1, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Gormally, E.; Caboux, E.; Vineis, P.; Hainaut, P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: Practical aspects and biological significance. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007, 635, 105–117. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L. The Prognostic Value of Circulating Tumor DNA in Ovarian Cancer: A Meta-Analysis. Technol. Cancer Res. Treat. 2021, 20, 15330338211043784. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, L.; Chen, Y.; Qing, C. Circulating cell-free DNA and circulating tumor cells, the ‘liquid biopsies’ in ovarian cancer. J. Ovarian Res. 2017, 10, 75. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [PubMed]
- Hufnagl, C.; Leisch, M.; Weiss, L.; Melchardt, T.; Moik, M.; Asslaber, D.; Roland, G.; Steininger, P.; Meissnitzer, T.; Neureiter, D.; et al. Evaluation of circulating cell-free DNA as a molecular monitoring tool in patients with metastatic cancer. Oncol. Lett. 2020, 19, 1551–1558. [Google Scholar] [CrossRef]
- Kamat, A.A.; Bischoff, F.Z.; Dang, D.; Baldwin, M.F.; Han, L.Y.; Lin, Y.G.; Merritt, W.M.; Landen, C.N., Jr.; Lu, C.; Gershenson, D.M.; et al. Circulating cell-free DNA: A novel biomarker for response to therapy in ovarian carcinoma. Cancer Biol. Ther. 2006, 5, 1369–1374. [Google Scholar] [CrossRef]
- Cheng, C.; Omura-Minamisawa, M.; Kang, Y.; Hara, T.; Koike, I.; Inoue, T. Quantification of circulating cell-free DNA in the plasma of cancer patients during radiation therapy. Cancer Sci. 2009, 100, 303–309. [Google Scholar] [CrossRef]
- Blitzer, G.C.; Zhao, S.G.; Bradley, K.A.; Hartenbach, E.M. The role of ctDNA in endometrial cancer: A tool for risk stratification and disease monitoring. Gynecol. Oncol. 2023, 178, 170–171. [Google Scholar] [CrossRef]
- Li, L.; Tong, Y.; Wu, J.; Xu, X. Clinical applications and utility of ctDNA in cervical cancer and its precursor lesions: From screening to predictive biomarker. Cancer Cell Int. 2023, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, E.; Latouche, A.; Bonneau, C.; Calméjane, M.-A.; Beaufort, C.; Ruigrok-Ritstier, K.; Bataillon, G.; Chérif, L.L.; Dupain, C.; Lecerf, C.; et al. Circulating HPV DNA as a Marker for Early Detection of Relapse in Patients with Cervical Cancer. Clin. cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Panet, F.; Papakonstantinou, A.; Borrell, M.; Vivancos, J.; Vivancos, A.; Oliveira, M. Use of ctDNA in early breast cancer: Analytical validity and clinical potential. Nat. Partn. J. Breast Cancer 2024, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Schøler, L.V.; Reinert, T.; Ørntoft, M.-B.W.; Kassentoft, C.G.; Árnadóttir, S.S.; Vang, S.; Nordentoft, I.; Knudsen, M.; Lamy, P.; Andreasen, D.; et al. Clinical Implications of Monitoring Circulating Tumor DNA in Patients with Colorectal Cancer. Clin. cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5437–5445. [Google Scholar] [CrossRef]
- Yu, Z.; Qin, S.; Wang, H. Alter circulating cell-free DNA variables in plasma of ovarian cancer patients. J. Obstet. Gynaecol. Res. 2019, 45, 2237–2242. [Google Scholar] [CrossRef]
- Stamenkovic, S.; Cheng, J.; Surowy, H.; Burwinkel, B.; Gündert, M. Circulating cell-free DNA variables as marker of ovarian cancer patients: A pilot study. Cancer Biomark. Sect. A Dis. Markers 2020, 28, 159–167. [Google Scholar] [CrossRef]
- Dobilas, A.; Chen, Y.; Brueffer, C.; Leandersson, P.; Saal, L.H.; Borgfeldt, C. Preoperative ctDNA Levels Are Associated With Poor Overall Survival in Patients With Ovarian Cancer. Cancer Genom. Proteom. 2023, 20, 763–770. [Google Scholar] [CrossRef]
- Jamieson, A.; McConechy, M.K.; Lum, A.; Senz, J.; Dowhy, T.; Huntsman, D.G.; McAlpine, J.N. Selective utilization of circulating tumor DNA testing enables disease monitoring in endometrial and ovarian carcinomas. J. Gynecol. Oncol. 2024, 36, e5. [Google Scholar] [CrossRef]
- Kutz, O.; Drukewitz, S.; Krüger, A.; Aust, D.; William, D.; Oster, S.; Schröck, E.; Baretton, G.; Link, T.; Wimberger, P.; et al. Exploring evolutionary trajectories in ovarian cancer patients by longitudinal analysis of ctDNA. Clin. Chem. Lab. Med. 2024, 62. [Google Scholar] [CrossRef]
- Maritschnegg, E.; Wang, Y.; Pecha, N.; Horvat, R.; Van Nieuwenhuysen, E.; Vergote, I.; Heitz, F.; Sehouli, J.; Kinde, I.; Diaz, L.A., Jr.; et al. Lavage of the Uterine Cavity for Molecular Detection of Müllerian Duct Carcinomas: A Proof-of-Concept Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4293–4300. [Google Scholar] [CrossRef]
- Pikor, L.; Thu, K.; Lam, W. The detection and implication of genome instability in cancer. Cancer Metastasis Rev. 2013, 32, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.R.; Kovtun, I.V.; Smadbeck, J.; Multinu, F.; Jatoi, A.; Kosari, F.; Kalli, K.R.; Murphy, S.J.; Halling, G.C.; Johnson, S.H.; et al. Quantification of Somatic Chromosomal Rearrangements in Circulating Cell-Free DNA from Ovarian Cancers. Sci. Rep. 2016, 6, 29831. [Google Scholar] [CrossRef] [PubMed]
- Vanderstichele, A.; Busschaert, P.; Smeets, D.; Landolfo, C.; Van Nieuwenhuysen, E.; Leunen, K.; Neven, P.; Amant, F.; Mahner, S.; Braicu, E.I.; et al. Chromosomal Instability in Cell-Free DNA as a Highly Specific Biomarker for Detection of Ovarian Cancer in Women with Adnexal Masses. Clin. cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Braicu, E.I.; Bois, A.D.; Sehouli, J.; Beck, J.; Prader, S.; Kulbe, H.; Eiben, B.; Harter, P.; Traut, A.; Pietzner, K.; et al. Cell-Free-DNA-Based Copy Number Index Score in Epithelial Ovarian Cancer-Impact for Diagnosis and Treatment Monitoring. Cancers 2021, 14, 168. [Google Scholar] [CrossRef]
- Martins, F.C.; Couturier, D.-L.; de Santiago, I.; Sauer, C.M.; Vias, M.; Angelova, M.; Sanders, D.; Piskorz, A.; Hall, J.; Hosking, K.; et al. Clonal somatic copy number altered driver events inform drug sensitivity in high-grade serous ovarian cancer. Nat. Commun. 2022, 13, 6360. [Google Scholar] [CrossRef]
- Patch, A.-M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef]
- Köbel, M.; Piskorz, A.M.; Lee, S.; Lui, S.; LePage, C.; Marass, F.; Rosenfeld, N.; Masson, A.-M.M.; Brenton, J.D. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J. Pathol. Clin. Res. 2016, 2, 247–258. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609. [CrossRef]
- Park, Y.R.; Kim, Y.-M.; Lee, S.W.; Lee, H.Y.; Lee, G.E.; Lee, J.-E.; Kim, Y.-T. Optimization to detect TP53 mutations in circulating cell-free tumor DNA from patients with serous epithelial ovarian cancer. Obstet. Gynecol. Sci. 2018, 61, 328–336. [Google Scholar] [CrossRef]
- Parkinson, C.A.; Gale, D.; Piskorz, A.M.; Biggs, H.; Hodgkin, C.; Addley, H.; Freeman, S.; Moyle, P.; Sala, E.; Sayal, K.; et al. Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study. Public Libr. Sci. Med. Med. 2016, 13, e1002198. [Google Scholar] [CrossRef] [PubMed]
- Arend, R.C.; Londoño, A.I.; Montgomery, A.M.; Smith, H.J.; Dobbin, Z.C.; Katre, A.A.; Martinez, A.; Yang, E.S.; Alvarez, R.D.; Huh, W.K.; et al. Molecular Response to Neoadjuvant Chemotherapy in High-Grade Serous Ovarian Carcinoma. Mol. Cancer Res. MCR 2018, 16, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Lee, S.W.; Lee, Y.J.; Lee, H.Y.; Lee, J.E.; Choi, E.K. Prospective study of the efficacy and utility of TP53 mutations in circulating tumor DNA as a non-invasive biomarker of treatment response monitoring in patients with high-grade serous ovarian carcinoma. J. Gynecol. Oncol. 2019, 30, e32. [Google Scholar] [CrossRef]
- Otsuka, J.; Okuda, T.; Sekizawa, A.; Amemiya, S.; Saito, H.; Okai, T.; Kushima, M. Detection of p53 mutations in the plasma DNA of patients with ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2004, 14. [Google Scholar] [CrossRef]
- Swisher, E.M.; Wollan, M.; Mahtani, S.M.; Willner, J.B.; Garcia, R.; Goff, B.A.; King, M.-C. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am. J. Obstet. Gynecol. 2005, 193, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Dobrzycka, B.; Terlikowski, S.J.; Kinalski, M.; Kowalczuk, O.; Niklinska, W.; Chyczewski, L. Circulating free DNA and p53 antibodies in plasma of patients with ovarian epithelial cancers. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2011, 22, 1133–1140. [Google Scholar] [CrossRef]
- Kumar, S.S.; Swamy, S.N.; Premalatha, C.S.; Pallavi, V.R.; Gawari, R. Aberrant Promoter Hypermethylation of RASSF1a and BRCA1 in Circulating Cell-Free Tumor DNA Serves as a Biomarker of Ovarian Carcinoma. Asian Pacific J. Cancer Prev. APJCP 2019, 20, 3001–3005. [Google Scholar]
- Paluszczak, J.; Baer-Dubowska, W. Epigenetic diagnostics of cancer--the application of DNA methylation markers. J. Appl. Genet. 2006, 47, 365–375. [Google Scholar] [CrossRef]
- Constâncio, V.; Nunes, S.P.; Henrique, R.; Jerónimo, C. DNA Methylation-Based Testing in Liquid Biopsies as Detection and Prognostic Biomarkers for the Four Major Cancer Types. Cells 2020, 9, 624. [Google Scholar] [CrossRef]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Barton, C.A.; Hacker, N.F.; Clark, S.J.; O’Brien, P.M. DNA methylation changes in ovarian cancer: Implications for early diagnosis, prognosis and treatment. Gynecol. Oncol. 2008, 109, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Liggett, T.E.; Melnikov, A.; Yi, Q.; Replogle, C.; Hu, W.; Rotmensch, J.; Kamat, A.; Sood, A.K.; Levenson, V. Distinctive DNA methylation patterns of cell-free plasma DNA in women with malignant ovarian tumors. Gynecol. Oncol. 2011, 120, 113–120. [Google Scholar] [CrossRef] [PubMed]
- de Caceres, I.I.; Battagli, C.; Esteller, M.; Herman, J.G.; Dulaimi, E.; Edelson, M.I.; Bergman, C.; Ehya, H.; Eisenberg, B.L.; Cairns, P. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004, 64, 6476–6481. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, W.; Li, Y.; Chen, B.; Li, D. A Study of hTERT Promoter Methylation in Circulating Tumour DNAs of Patients with Ovarian Magnificent Tumour. OncoTargets Ther. 2020, 13, 12317–12323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, G.; Yang, Q.; Dong, R.; Xie, X.; Ma, D.; Shen, K.; Kong, B. A multiplex methylation-specific PCR assay for the detection of early-stage ovarian cancer using cell-free serum DNA. Gynecol. Oncol. 2013, 130, 132–139. [Google Scholar] [CrossRef]
- Widschwendter, M.; Zikan, M.; Wahl, B.; Lempiäinen, H.; Paprotka, T.; Evans, I.; Jones, A.; Ghazali, S.; Reisel, D.; Eichner, J.; et al. The potential of circulating tumor DNA methylation analysis for the early detection and management of ovarian cancer. Genome Med. 2017, 9, 116. [Google Scholar] [CrossRef]
- Wang, B.; Yu, L.; Luo, X.; Huang, L.; Li, Q.-S.; Shao, X.-S.; Liu, Y.; Fan, Y.; Yang, G.-Z. Detection of OPCML methylation, a possible epigenetic marker, from free serum circulating DNA to improve the diagnosis of early-stage ovarian epithelial cancer. Oncol. Lett. 2017, 14, 217–223. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Lin, L.; Ma, X.-P.; Ma, Y.-C.; Liu, P.-S. Aberrant methylation of RASSF2A in tumors and plasma of patients with epithelial ovarian cancer. Asian Pacific J. Cancer Prev. APJCP 2014, 15, 1171–1176. [Google Scholar] [CrossRef]
- Giannopoulou, L.; Chebouti, I.; Pavlakis, K.; Kasimir-Bauer, S.; Lianidou, E.S. RASSF1A promoter methylation in high-grade serous ovarian cancer: A direct comparison study in primary tumors, adjacent morphologically tumor cell-free tissues and paired circulating tumor DNA. Oncotarget 2017, 8, 21429. [Google Scholar] [CrossRef]
- Giannopoulou, L.; Mastoraki, S.; Buderath, P.; Strati, A.; Pavlakis, K.; Kasimir-Bauer, S.; Lianidou, E.S. ESR1 methylation in primary tumors and paired circulating tumor DNA of patients with high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 150, 355–360. [Google Scholar] [CrossRef]
- Dvorská, D.; Braný, D.; Nagy, B.; Grendár, M.; Poka, R.; Soltész, B.; Jagelková, M.; Zelinová, K.; Lasabová, Z.; Zubor, P.; et al. Aberrant Methylation Status of Tumour Suppressor Genes in Ovarian Cancer Tissue and Paired Plasma Samples. Int. J. Mol. Sci. 2019, 20, 4119. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK collaborative trial of ovarian cancer screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B.; et al. Effect of screening on ovarian cancer mortality: The prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. J. Am. Med. Assoc. 2011, 305, 2295–2303. [Google Scholar] [CrossRef]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef]
- Zachariah, R.R.; Schmid, S.; Buerki, N.; Radpour, R.; Holzgreve, W.; Zhong, X. Levels of circulating cell-free nuclear and mitochondrial DNA in benign and malignant ovarian tumors. Obstet. Gynecol. 2008, 112, 843–850. [Google Scholar] [CrossRef]
- Kamat, A.A.; Baldwin, M.; Urbauer, D.; Dang, D.; Han, L.Y.; Godwin, A.; Karlan, B.Y.; Simpson, J.L.; Gershenson, D.M.; Coleman, R.L.; et al. Plasma cell-free DNA in ovarian cancer: An independent prognostic biomarker. Cancer 2010. [Google Scholar] [CrossRef]
- Morikawa, A.; Hayashi, T.; Shimizu, N.; Kobayashi, M.; Taniue, K.; Takahashi, A.; Tachibana, K.; Saito, M.; Kawabata, A.; Iida, Y.; et al. PIK3CA and KRAS mutations in cell free circulating DNA are useful markers for monitoring ovarian clear cell carcinoma. Oncotarget 2018, 9, 15266–15274. [Google Scholar] [CrossRef]
- Salani, R.; Davidson, B.; Fiegl, M.; Marth, C.; Müller-Holzner, E.; Gastl, G.; Huang, H.-Y.; Hsiao, J.-C.; Lin, H.-S.; Wang, T.-L.; et al. Measurement of cyclin E genomic copy number and strand length in cell-free DNA distinguish malignant versus benign effusions. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 5805–5809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, R.; Pu, W.; Zhang, S.; Chen, L.; Zhu, W.; Xiao, L.; Xing, C.; Li, K. Clinical value of ALU concentration and integrity index for the early diagnosis of ovarian cancer: A retrospective cohort trial. PLoS ONE 2018, 13, e0191756. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019S006C.pdf (accessed on 6 January 2024).
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Chang, X.; Ye, X.; Dong, L.; Cheng, H.; Cheng, Y.; Zhu, L.; Liao, Q.; Zhao, Y.; Tian, L.; Fu, T.; et al. Human epididymis protein 4 (HE4) as a serum tumor biomarker in patients with ovarian carcinoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2011, 21, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Zorn, K.K.; Tian, C.; McGuire, W.P.; Hoskins, W.J.; Markman, M.; Muggia, F.M.; Rose, P.G.; Ozols, R.F.; Spriggs, D.; Armstrong, D.K. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: A Gynecologic Oncology Group study. Cancer 2009, 115, 1028–1035. [Google Scholar] [CrossRef]
- Zargari, A.; Du, Y.; Heidari, M.; Thai, T.C.; Gunderson, C.C.; Moore, K.; Mannel, R.S.; Liu, H.; Zheng, B.; Qiu, Y. Prediction of chemotherapy response in ovarian cancer patients using a new clustered quantitative image marker. Phys. Med. Biol. 2018, 63, 155020. [Google Scholar] [CrossRef] [PubMed]
- Asante, D.-B.; Calapre, L.; Ziman, M.; Meniawy, T.M.; Gray, E.S. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time? Cancer Lett. 2020, 468, 59–71. [Google Scholar] [CrossRef]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid biopsy of cancer: A multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef] [PubMed]
- Perakis, S.; Speicher, M.R. Emerging concepts in liquid biopsies. BMC Med. 2017, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- No, J.H.; Kim, K.; Park, K.H.; Kim, Y.-B. Cell-free DNA level as a prognostic biomarker for epithelial ovarian cancer. Anticancer Res. 2012, 32, 3467–3471. [Google Scholar]
- Heo, J.; Kim, Y.-N.; Shin, S.; Lee, K.; Lee, J.-H.; Lee, Y.J.; Choi, Z.; Park, J.; Min, S.; Kim, S.W.; et al. Serial Circulating Tumor DNA Analysis with a Tumor-Naïve Next-Generation Sequencing Panel Detects Minimal Residual Disease and Predicts Outcome in Ovarian Cancer. Cancer Res. 2024, 84, 468–478. [Google Scholar] [CrossRef]
- Sharbatoghli, M.; Fattahi, F.; Es, H.A.; Akbari, A.; Akhavan, S.; Ebrahimi, M.; Asadi-Lari, M.; Totonchi, M.; Madjd, Z. Copy Number Variation of Circulating Tumor DNA (ctDNA) Detected Using NIPT in Neoadjuvant Chemotherapy-Treated Ovarian Cancer Patients. Front. Genet. 2022, 13, 938985. [Google Scholar] [CrossRef]
- Quigley, D.; Alumkal, J.J.; Wyatt, A.W.; Kothari, V.; Foye, A.; Lloyd, P.; Aggarwal, R.; Kim, W.; Lu, E.; Schwartzman, J.; et al. Analysis of Circulating Cell-Free DNA Identifies Multiclonal Heterogeneity of BRCA2 Reversion Mutations Associated with Resistance to PARP Inhibitors. Cancer Discov. 2017, 7, 999–1005. [Google Scholar] [CrossRef]
- Christie, E.L.; Fereday, S.; Doig, K.; Pattnaik, S.; Dawson, S.-J.; Bowtell, D.D.L. Reversion of BRCA1/2 Germline Mutations Detected in Circulating Tumor DNA From Patients With High-Grade Serous Ovarian Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Comino-Méndez, I.; de Bruijn, I.; Tian, L.; Meisel, J.L.; García-Murillas, I.; Fribbens, C.; Cutts, R.; Martelotto, L.G.; Ng, C.K.Y.; et al. Diverse BRCA1 and BRCA2 Reversion Mutations in Circulating Cell-Free DNA of Therapy-Resistant Breast or Ovarian Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6708–6720. [Google Scholar] [CrossRef] [PubMed]
- Ratajska, M.; Koczkowska, M.; Żuk, M.; Gorczyński, A.; Kuźniacka, A.; Stukan, M.; Biernat, W.; Limon, J.; Wasąg, B. Detection of BRCA1/2 mutations in circulating tumor DNA from patients with ovarian cancer. Oncotarget 2017, 8, 101325. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, K.D.; Madsen, C.V.; Andersen, R.F.; Waldstrøm, M.; Adimi, P.; Jakobsen, A. Prognostic importance of cell-free DNA in chemotherapy resistant ovarian cancer treated with bevacizumab. Eur. J. Cancer Off. J. Eur. Organ. Res. Treat. Cancer Eur. Assoc. Cancer Res. (EACR) 2014, 50, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.M.; Savolainen, K.; Virtanen, T.; Ryyppö, L.; Selin, H.; Martikainen, P.; Staff, S.; Kivinummi, K.; Sipola, J.; Vuorinen, J.; et al. Sensitive circulating tumor DNA-based residual disease detection in epithelial ovarian cancer. Life Sci. Alliance 2024, 7, e202402658. [Google Scholar] [CrossRef]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.Y.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 2012, 4, 136ra68. [Google Scholar] [CrossRef]
- Pereira, E.; Camacho-Vanegas, O.; Anand, S.; Sebra, R.; Camacho, S.C.; Garnar-Wortzel, L.; Nair, N.; Moshier, E.; Wooten, M.; Uzilov, A.; et al. Personalized Circulating Tumor DNA Biomarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers. PLoS ONE 2015, 10, e0145754. [Google Scholar] [CrossRef]
- Vitale, S.R.; Groenendijk, F.H.; van Marion, R.; Beaufort, C.M.; Helmijr, J.C.; Jan Dubbink, H.; Dinjens, W.N.M.; Ewing-Graham, P.C.; Smolders, R.; van Doorn, H.C.; et al. TP53 Mutations in Serum Circulating Cell-Free Tumor DNA As Longitudinal Biomarker for High-Grade Serous Ovarian Cancer. Biomolecules 2020, 10, 415. [Google Scholar] [CrossRef]
- Zhu, J.W.; Wong, F.; Szymiczek, A.; Ene, G.E.V.; Zhang, S.; May, T.; Narod, S.A.; Kotsopoulos, J.; Akbari, M.R. Evaluating the Utility of ctDNA in Detecting Residual Cancer and Predicting Recurrence in Patients with Serous Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 14388. [Google Scholar] [CrossRef]
- Xu, J.; Dai, Y.; Gao, Y.; Chai, R.; Lu, C.; Yu, B.; Kang, Y.; Xu, C. RAD51D Secondary Mutation-Mediated Resistance to PARP-Inhibitor-Based Therapy in HGSOC. Int. J. Mol. Sci. 2023, 24, 14476. [Google Scholar] [CrossRef]
- Tivey, A.; Church, M.; Rothwell, D.; Dive, C.; Cook, N. Circulating tumour DNA—Looking beyond the blood. Nat. Rev. Clin. Oncol. 2022, 19, 600–612. [Google Scholar] [CrossRef] [PubMed]
| Type of Cancer | Application. | DOI |
|---|---|---|
| Endometrial cancer | ctDNA can be used as a tool for risk stratification and disease monitoring. | [20] |
| Cervical cancer | ctDNA may be used as a complementary diagnostic tool rather than the sole decisive biomarker in cervical cancer. | [21] |
| One helpful marker for predicting the return of cervical cancer is the detection of HPV ctDNA. | [22] | |
| Breast cancer | ctDNA analysis in early breast cancer is helpful for screening, treatment evaluation, and detection of minimal residual disease (MRD). | [23] |
| Colorectal cancer | Postoperative ctDNA screening identifies patients at very high risk of relapse and offers proof of remaining illness. | [24] |
| Ovarian cancer | Elevated levels of cfDNA are observed in patients with ovarian cancer. | [25] |
| When paired with additional molecular markers, cfDNA variables have the potential to serve as diagnostic biomarkers for ovarian cancer. | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golara, A.; Kozłowski, M.; Cymbaluk-Płoska, A. The Role of Circulating Tumor DNA in Ovarian Cancer. Cancers 2024, 16, 3117. https://doi.org/10.3390/cancers16183117
Golara A, Kozłowski M, Cymbaluk-Płoska A. The Role of Circulating Tumor DNA in Ovarian Cancer. Cancers. 2024; 16(18):3117. https://doi.org/10.3390/cancers16183117
Chicago/Turabian StyleGolara, Anna, Mateusz Kozłowski, and Aneta Cymbaluk-Płoska. 2024. "The Role of Circulating Tumor DNA in Ovarian Cancer" Cancers 16, no. 18: 3117. https://doi.org/10.3390/cancers16183117
APA StyleGolara, A., Kozłowski, M., & Cymbaluk-Płoska, A. (2024). The Role of Circulating Tumor DNA in Ovarian Cancer. Cancers, 16(18), 3117. https://doi.org/10.3390/cancers16183117





