Amitotic Cell Division, Malignancy, and Resistance to Anticancer Agents: A Tribute to Drs. Walen and Rajaraman
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Amitotic Cell Division in Polyploid Mammalian Cells
1.2. Objectives
2. Polyploidy, Amitosis, and In Vitro Cell Transformation
3. Fate of Cancer Cells with Genome Instability
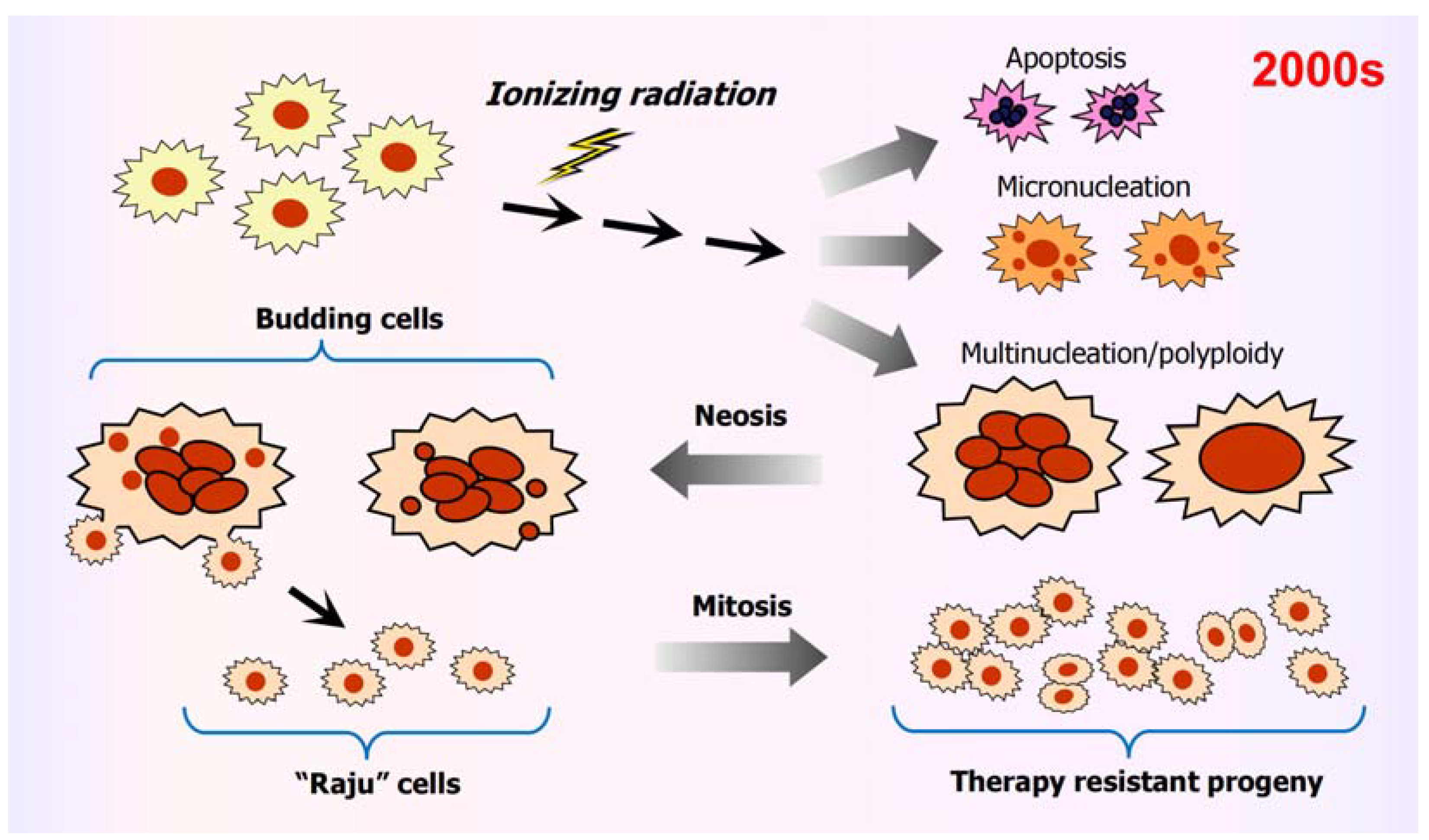
3.1. Amitosis in Solid Tumors/Tumor-Derived Cell Lines
3.2. Amitosis and System-Level Information Alteration
4. Targeting PGCCs in Cancer Therapy
- The formation of PGCCs and their tumor repopulating progeny (via neosis) can be blocked by the contraceptive drug mifepristone [70].
- PGCCs developed following cisplatin treatment have a high content of mitochondria and a distinct metabolic profile, which includes high levels of lipid droplets and cholesterol. These PGCCs could be targeted using zoledronic acid, a potent inhibitor of osteoclasts (multinucleated bone cells) [71], which is commonly used to treat osteoporosis and bone metastases [72,73].
- Treatment with LCL521 or simvastatin disrupts cholesterol signaling and interferes with PGCC progeny formation [74].
- The ESCRT (endosomal complexes required for transport) proteins are involved in the budding of PGCCs [78]. Treatment of PGCCs with interferon, a modulator of ESCRT, prevented PGCC budding [78]. In that study, PGCCs were created following exposure to ionizing radiation and were referred to as “radiation-tolerant persister” cells.
5. The Role of PGCCs in Minimal Residual Disease and Tumor Repopulation Post-Therapy
- i.
- Treatment Phase. This first phase involves conventional therapeutic strategies (such as surgery, chemotherapy, and radiation therapy) to inhibit tumor growth and hopefully to prevent or at least mitigate metastasis. The majority (perhaps >95%) of anticancer studies have focused on this vital phase of cancer therapy.
- ii.
- Response. A proportion of cancer cells within a solid tumor responds to genotoxic insult (incurred from the treatment phase) by entering a state of dormancy (active sleep). This group includes PGCCs. In tissue culture studies, it may take ~10 days after treatment for PGCCs to be fully manifested (see, e.g., [78]). It is worth noting that ubiquitously used preclinical anticancer end-points (e.g., multiwell plate cell “viability” and tumor growth delay assays in live animals) are performed within this time frame, and thus they often overlook the impact of PGCCs or score them as “dead” (see, e.g., [22,26]).
- iii.
- Dormancy. Senescence-like dormancy [84] (also referred to as MRD [85]) is an extended latency period during which PGCCs undergo depolyploidization through a meiosis-like process as well as through amitosis-neosis. PGCCs are also known to have the ability to transfer a small portion of their nuclear material containing stem cell markers to neighboring cells via cytoplasmic tunnels [86].
- iv.
- Recurrence. Rapidly proliferating progenitor cells emerging from PGCCs repopulate the tumor. Such cells have stem cell characteristics and can show resistance to conventional therapies (used in the “treatment phase”). Whether cells subjected to horizontal gene transfer also exhibit these properties is not known.
6. Amitotic Cell Division Contributes to Intratumor Heterogeneity
7. Human Genetic Disorders Associated with Genome Instability and Cancer Predisposition: Does Amitosis-Neosis Play a Role?
8. Conclusions
Funding
Conflicts of Interest
Appendix A
References
- Benson, K. Study Discovers Potential Target for Treating Aggressive Cancer Cells. 2021. Available online: https://www.ikcest.org/articleS-552496.htm (accessed on 10 August 2024).
- Xuan, B.; Ghosh, D.; Jiang, J.; Shao, R.; Dawson, M.R. Vimentin filaments drive migratory persistence in polyploidal cancer cells. Proc. Natl. Acad. Sci. USA 2020, 117, 26756–26765. [Google Scholar] [CrossRef] [PubMed]
- Walen, K.H. The origin of transformed cells: Studies of spontaneous and induced cell transformation in cell cultures from marsupials, a snail, and human amniocytes. Cancer Genet. 2002, 133, 45–54. [Google Scholar] [CrossRef]
- Sundaram, M.; Guernsey, D.L.; Rajaraman, M.M.; Rajaraman, R. Neosis: A novel type of cell division in cancer. Cancer Biol. Ther. 2004, 3, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Walen, K.H. Spontaneous cell transformation: Karyoplasts derived from multinucleated cells produce new cell growth in senescent human epithelial cell cultures. Cell. Dev. Biol. Anim. 2004, 40, 150–158. [Google Scholar] [CrossRef]
- Walen, K.H. Budded karyoplasts from multinucleated fibroblast cells contain centrosomes and change their morphology to mitotic cells. Cell Biol. Int. 2005, 29, 1057–1065. [Google Scholar] [CrossRef]
- Walen, K.H. Genetic stability of senescence reverted cells: Genome reduction division of polyploidy cells, aneuploidy and neoplasia. Cell Cycle 2008, 7, 1623–1629. [Google Scholar] [CrossRef]
- Walen, K.H. Neoplastic-like cell changes of normal fibroblast cells associated with evolutionary conserved maternal and paternal genomic autonomous behavior (gonomery). J. Cancer Ther. 2014, 05, 860–877. [Google Scholar] [CrossRef]
- Walen, K.H. Genomic instability in cancer II: 4N-skewed (90°) reductive division via fragile sites to fitness increase for solid and hematological cancer beginnings. J. Cancer Ther. 2019, 10, 537–564. [Google Scholar] [CrossRef][Green Version]
- Walen, K.H. A traceable cancer model: DNA damage, fragile site-SMGs, mitotic slippage, 4n-genome-reduction to fitness-gained, initiating, 2n first cells. J. Cancer Ther. 2021, 12, 365–386. [Google Scholar] [CrossRef]
- Walen, K.H. Epigenetic enabled normal human cells, lead to first cell’s unique division system, driving tumorigenesis evolution. J. Cancer Ther. 2022, 13, 48–69. [Google Scholar] [CrossRef]
- Rajaraman, R.; Rajaraman, M.M.; Rajaraman, S.R.; Guernsey, R.L. Neosis—A paradigm of self-renewal in cancer. Cell Biol. Int. 2005, 29, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, R.; Guernsey, D.L.; Rajaraman, M.M.; Rajaraman, S.R. Stem cells, senescence, neosis and self-renewal in cancer. Cell Biol. Int. 2006, 29, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, R.; Guernsey, D.L.; Rajaraman, M.M.; Rajaraman, S.R. Neosis—A parasexual somatic reduction division in cancer. Int. J. Hum. Genet. 2007, 7, 29–48. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Mirzayans, R.; Waters, R. DNA damage and its repair in human normal or xeroderma pigmentosum fibroblasts treated with 4-nitroquinoline 1-oxide or its 3-methyl derivative. Carcinogenesis 1981, 2, 1359–1362. [Google Scholar] [CrossRef]
- Mirzayans, R.; Famulski, K.S.; Enns, L.; Fraser, M.; Paterson, M.C. Characterization of the signal transduction pathway mediating γ ray-induced inhibition of DNA synthesis in human cells: Indirect evidence for involvement of calmodulin but not protein kinase C nor p53. Oncogene 1995, 11, 1597–1605. [Google Scholar]
- Mirzayans, R.; Enns, L.; Dietrich, K.; Barley, R.D.; Paterson, M.C. Faulty DNA polymerase δ/ɛ-mediated excision repair in response to γ radiation or ultraviolet light in p53-deficient fibroblast strains from affected members of a cancer-prone family with Li-Fraumeni syndrome. Carcinogenesis 1996, 17, 691–698. [Google Scholar] [CrossRef]
- Barley, R.D.C.; Enns, L.; Paterson, M.C.; Mirzayans, R. Aberrant p21WAF1-dependent growth arrest as the possible mechanism of abnormal resistance to ultraviolet light cytotoxicity in Li-Fraumeni syndrome fifibroblast strains heterozygous for TP53 mutations. Oncogene 1998, 17, 533–543. [Google Scholar] [CrossRef]
- Murray, D.; Mirzayans, R.; Scott, A.L.; Allalunis-Turner, M.J. Influence of oxygen on the radiosensitivity of human glioma cell lines. Am. J. Clin. Oncol. 2003, 26, e169–e177. [Google Scholar] [CrossRef]
- Uversky, V.N. p53 proteoforms and intrinsic disorder: An illustration of the protein structure-function continuum concept. Int. J. Mol. Sci. 2016, 17, 1874. [Google Scholar] [CrossRef]
- Mirzayans, R. Changing the landscape of solid tumor therapy from apoptosis-promoting to apoptosis-inhibiting strategies. Curr. Issues Mol. Biol. 2024, 46, 5379–5396. [Google Scholar] [CrossRef]
- Weinberg, R.A. Coming full circle-from endless complexity to simplicity and back again. Cell 2014, 157, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kailen, W.G. Publish houses of brick, not mansions of straw. Nature 2017, 5454, 387. [Google Scholar]
- Heng, H.H. Genome Chaos: Rethinking Genetics, Evolution, and Molecular Medicine; Academic Press: Cambridge, MA, USA, 2019; p. 556. [Google Scholar]
- Mirzayans, R.; Murray, D. What are the reasons for continuing failures in cancer therapy? Are misleading/inappropriate preclinical assays to be blamed? Might some modern therapies cause more harm than benefit? Int. J. Mol. Sci. 2022, 23, 13217. [Google Scholar] [CrossRef]
- Prehn, R.T. Cancers beget mutations versus mutations beget cancers. Cancer Res. 1994, 54, 5296–5300. [Google Scholar] [PubMed]
- Salmina, K.; Huna, A.; Kalejs, M.; Pjanova, D.; Scherthan, H.; Cragg, M.S.; Erenpreisa, J. The cancer aneuploidy paradox: In the light of evolution. Genes 2019, 10, 83. [Google Scholar] [CrossRef]
- Raza, A. The First Cell: And the Human Costs of Pursuing Cancer to the Last; Basic Books: New York, NY, USA, 2019; p. 368. [Google Scholar]
- Editorial. The ‘war on cancer’ isn’t yet won. Nature 2022, 601, 297. [Google Scholar] [CrossRef]
- Coward, J.; Harding, A. Size does matter: Why polyploid tumor cells are critical drug targets in the war on cancer. Front. Oncol. 2014, 4, 123. [Google Scholar] [CrossRef]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- Heng, J.; Heng, H.H. Genome chaos, information creation, and cancer emergence: Searching for new frameworks on the 50th anniversary of the “war on cancer”. Genes 2022, 13, 101. [Google Scholar] [CrossRef]
- Erenpreisa, J.; Salmina, K.; Huna, A.; Jackson, T.R.; Vazquez-Martin, A.; Cragg, M.S. The “virgin birth”, polyploidy, and the origin of cancer. Oncoscience 2015, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Niu, N.; Zhang, J.; Qi, L.; Shen, W.; Donkena, K.V.; Feng, Z.; Liu, J. Polyploid giant cancer cells (PGCCs): The evil roots of cancer. Curr. Cancer Drug Targets 2019, 19, 360–367. [Google Scholar] [CrossRef]
- Pienta, K.J.; Hammarlund, E.U.; Austin, R.H.; Axelrod, R.; Brown, J.S.; Amend, S.R. Cancer cells employ an evolutionarily conserved polyploidization program to resist therapy. Semin. Cancer Biol. 2022, 81, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Saini, G.; Joshi, S.; Garlapati, C.; Li, H.; Kong, J.; Krishnamurthy, J.; Reid, M.D.; Aneja, R. Polyploid giant cancer cell characterization: New frontiers in predicting response to chemotherapy in breast cancer. Semin. Cancer Biol. 2022, 81, 220–231. [Google Scholar] [CrossRef]
- Liu, J.; Niu, N.; Li, X.; Zhang, X.; Sood, A.K. The life cycle of polyploid giant cancer cells and dormancy in cancer: Opportunities for novel therapeutic interventions. Semin. Cancer Biol. 2022, 81, 132–144. [Google Scholar] [CrossRef]
- Sikora, E.; Czarnecka-Herok, J.; Bojko, A.; Sunderland, P. Therapy-induced polyploidization and senescence: Coincidence or interconnection? Semin. Cancer Biol. 2022, 81, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiao, Q.; Xu, H.; Zhou, R.; Liu, X. Human cell polyploidization: The good and the evil. Semin. Cancer Biol. 2022, 81, 54–63. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, M.; Zheng, M.; Tian, S.; Yang, X.; Ning, Y.; Li, Y.; Zhang, S. Polyploid giant cancer cells and cancer progression. Front. Cell Dev. Biol. 2022, 10, 1017588. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Pharmacological modulation of p53 function in cancer therapy. Curr. Signal. Transduct. Ther. 2008, 3, 183–194. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Human genetic disorders associated with genome instability, premature aging and cancer predisposition. Open Cancer J. 2008, 2, 42–52. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Cellular Senescence: Implications for Cancer Therapy; Monograph Garvey, R.B., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; p. 130. [Google Scholar]
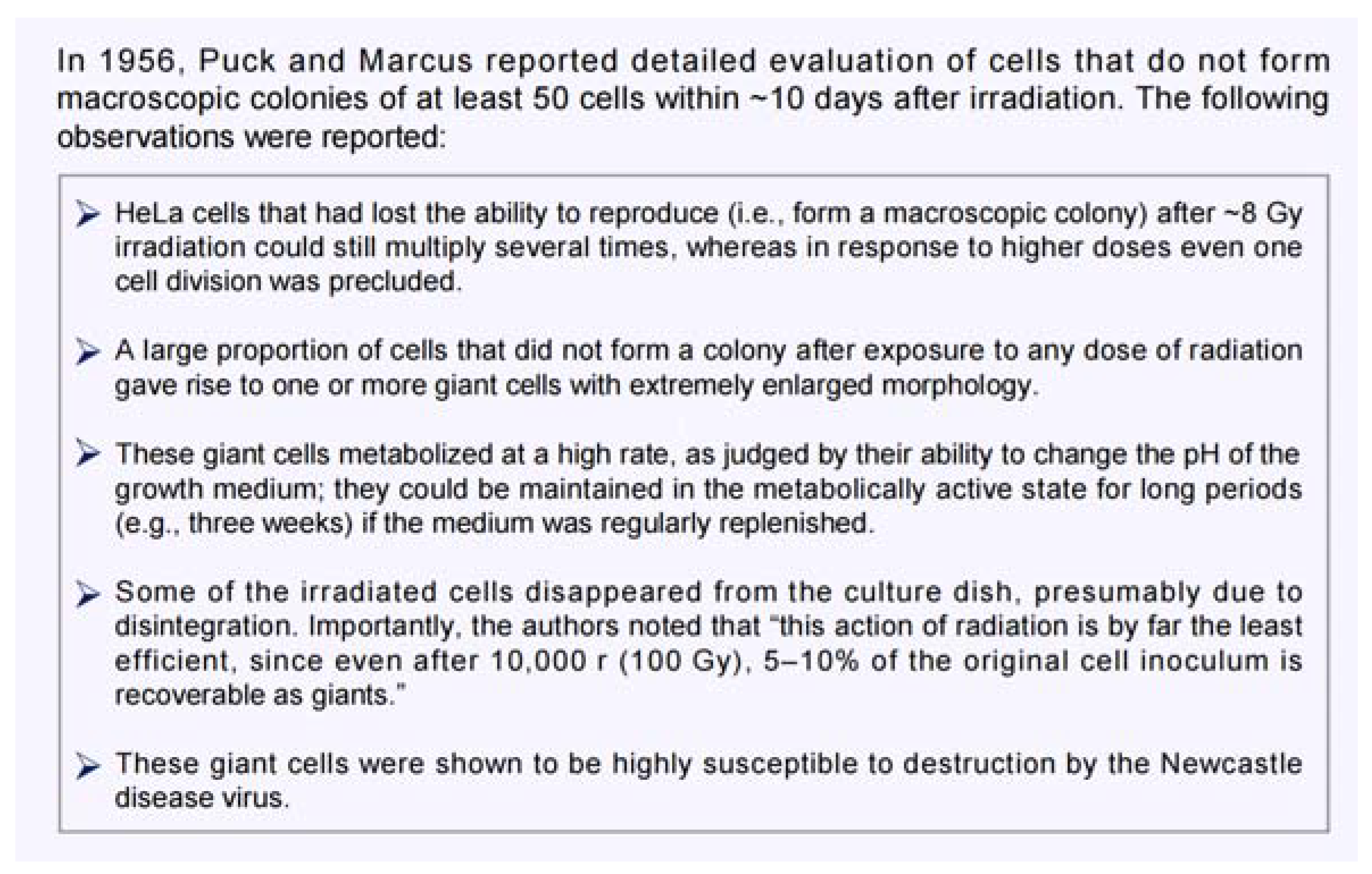
- Puck, T.T.; Marcus, P.I. Action of X-rays on mammalian cells. J. Exp. Med. 1956, 103, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Intratumor heterogeneity and treatment resistance of solid tumors with a focus on polyploid/senescent giant cancer cells (PGCCs). Int. J. Mol. Sci. 2023, 24, 11534. [Google Scholar] [CrossRef]
- Eastman, A. Improving anticancer drug development begins with cell culture: Misinformation perpetuated by the misuse of cytotoxicity assays. Oncotarget 2017, 8, 8854–8866. [Google Scholar] [CrossRef]
- Erenpreisa, J.A.; Cragg, M.S.; Fringes, B.; Sharakhov, I.; Illidge, T.M. Release of mitotic descendants by giant cells from irradiated Burkitt’s lymphoma cell lines. Cell Biol. Int. 2000, 24, 635–648. [Google Scholar] [CrossRef]
- Illidge, T.M.; Cragg, M.S.; Fringes, B.; Olive, P.; Erenpresia, J.A. Polyploid giant cells provide a survival mechanism of p53 mutant cells after DNA damage. Cell Biol. Int. 2000, 24, 621–633. [Google Scholar] [CrossRef]
- Erenpreisa, J.; Cragg, M.S. MOS, aneuploidy and the ploidy cycle of cancer cells. Oncogene 2010, 29, 5447–5451. [Google Scholar] [CrossRef] [PubMed]
- Navolanic, P.M.; Akula, S.M.; McCubrey, J.A. Neosis and its potential role in cancer development and chemoresistance. Cancer Biol. Ther. 2004, 3, 219–220. [Google Scholar] [CrossRef]
- Zhang, S.; Mercado-Uribe, I.; Xing, Z.; Sun, B.; Kuang, J.; Liu, J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene 2014, 33, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, X.; Yang, Z.; Fei, F.; Li, S.; Qu, J.; Zhang, M.; Li, Y.; Zhang, X.; Zhang, S. Daughter cells and erythroid cells budding from PGCCs and their clinicopathological significances in colorectal cancer. J. Cancer 2017, 8, 469–478. [Google Scholar] [CrossRef]
- White-Gilbertson, S.; Lu, P.; Norris, J.S.; Voelkel-Johnson, C. Genetic and pharmacological inhibition of acid ceramidase prevents asymmetric cell division by neosis. J. Lipid Res. 2019, 60, 1225–1235. [Google Scholar] [CrossRef]
- Fei, F.; Liu, K.; Li, C.; Du, J.; Wei, Z.; Li, B.; Li, Y.; Zhang, Y.; Zhang, S. Molecular mechanisms by which S100A4 regulates the migration and invasion of PGCCs with their daughter cells in human colorectal cancer. Front. Oncol. 2020, 10, 182. [Google Scholar] [CrossRef]
- White-Gilbertson, S.; Lu, P.; Jones, C.M.; Chiodini, S.; Hurley, D.; Das, A.; Delaney, J.R.; Norris, J.S.; Voelkel-Johnson, C. Tamoxifen is a candidate first-in-class inhibitor of acid ceramidase that reduces amitotic division in polyploid giant cancer cells—Unrecognized players in tumorigenesis. Cancer Med. 2020, 9, 3142–3152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, X.; Deng, Z.; Cheng, J.; Wang, Y.; Zhao, M.; Zhao, Y.; He, S.; Huang, Q. Irradiation-induced polyploid giant cancer cells are involved in tumor cell repopulation via neosis. Mol. Oncol. 2021, 15, 2219–2234. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, K.; Li, Z.; Zhang, H.; Fu, F.; Fu, J.; Zheng, M.; Zhang, S. High migration and invasion ability of PGCCs and their daughter cells associated with the nuclear localization of S100A10 modified by SUMOylation. Front. Cell Dev. Biol. 2021, 9, 696871. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Chen, L.; Yang, X.; Fan, L.; Zhang, M.; Chen, S.; Zheng, M.; Gao, M.; Zhang, S. PLK4 is a key molecule in the formation of PGCCs and promotes invasion and migration of progeny cells derived from PGCCs. J. Cancer 2022, 13, 2954–2969. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y.; Wu, M.; Liu, J.; Wu, H.; Xu, C.; Chen, L. Hypoxia-induced polypoid giant cancer cells in glioma promote the transformation of tumor-associated macrophages to a tumor-supportive phenotype. CNS Neurosci. Ther. 2022, 28, 1326–1338. [Google Scholar] [CrossRef]
- Peña, C.M.; Skipper, T.A.; Hsu, J.; Schechter, I.; Ghosh, D.; Dawson, M.R. Metronomic and single high-dose paclitaxel treatments produce distinct heterogenous chemoresistant cancer cell populations. Sci. Rep. 2023, 13, 19232. [Google Scholar]
- Zhou, M.; Ma, Y.; Chiang, C.C.; Rock, E.C.; Butler, S.C.; Anne, R.; Yatsenko, S.; Gong, Y.; Chen, Y.C. Single-cell morphological and transcriptome analysis unveil inhibitors of polyploid giant breast cancer cells in vitro. Commun. Biol. 2023, 6, 1301. [Google Scholar] [CrossRef]
- Fan, L.; Zheng, M.; Zhou, X.; Yu, Y.; Ning, Y.; Fu, W.; Xu, J.; Zhang, S. Molecular mechanism of vimentin nuclear localization associated with the migration and invasion of daughter cells derived from polyploid giant cancer cells. J. Transl. Med. 2023, 21, 719. [Google Scholar] [CrossRef]
- Ye, C.J.; Sharpe, Z.; Alemara, S.; Mackenzie, S.; Liu, G.; Abdallah, B.; Horne, S.; Regan, S.; Heng, H.H. Micronuclei and genome chaos: Changing the system inheritance. Genes 2019, 10, 366. [Google Scholar] [CrossRef]
- Noble, D. Cellular Darwinism: Regulatory networks, stochasticity, and selection in cancer development. Prog. Biophys. Mol. Biol. 2021, 165, 66–71. [Google Scholar] [CrossRef]
- Gecow, A.; Iantovics, L.B.; Tez, M. Cancer and chaos and the complex network model of a multicellular organism. Biology 2022, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.C.; Heng, H.H. The new era of cancer cytogenetics and cytogenomics. Methods Mol. Biol. 2024, 2825, 3–37. [Google Scholar] [PubMed]
- Kasperski, A.; Heng, H.H. The spiral model of evolution: Stable life forms of organisms and unstable life forms of cancers. Int. J. Mol. Sci. 2024, 25, 9163. [Google Scholar] [CrossRef]
- Xuan, B.; Ghosh, D.; Cheney, E.M.; Clifton, E.M.; Dawson, M.R. Dysregulation in actin cytoskeletal organization drives increased stiffness and migratory persistence in polyploidal giant cancer cells. Sci. Rep. 2018, 8, 11935. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Y.; Li, X.; Niu, N.; Liu, Y.; Hajek, R.A.; Peng, G.; Westin, S.; Sood, A.K.; Liu, J. Targeting polyploid giant cancer cells potentiates a therapeutic response and overcomes resistance to PARP inhibitors in ovarian cancer. Sci. Adv. 2023, 9, eadf7195. [Google Scholar] [CrossRef]
- Adibi, R.; Moein, S.; Gheisari, Y. Zoledronic acid targets chemo-resistant polyploid giant cancer cells. Sci. Rep. 2023, 13, 419. [Google Scholar] [CrossRef]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Drake, M.T.; Ebetino, F.H.; Bilezikian, J.P.; Russell, R.G.G. Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. 2019, 85, 1052–1062. [Google Scholar] [CrossRef]
- White-Gilbertson, S.; Lu, P.; Esobi, I.; Echesabal-Chen, J.; Mulholland, P.J.; Gooz, M.; Ogretmen, B.; Stamatikos, A.; Voelkel-Johnson, C. Polyploid giant cancer cells are dependent on cholesterol for progeny formation through amitotic division. Sci. Rep. 2022, 12, 8971. [Google Scholar] [CrossRef]
- White-Gilbertson, S.; Lu, P.; Saatci, O.; Sahin, Z.; Delaney, J.R.; Ogretmen, B.; Voelkel-Johnson, C. Transcriptome analysis of polyploid giant cancer cells and their progeny reveals a functional role for p21 in polyploidization and depolyploidization. J. Biol. Chem. 2024, 300, 107136. [Google Scholar] [CrossRef]
- Wettersten, H.I.; Hwang, S.H.; Li, C.; Shiu, E.Y.; Wecksler, A.T.; Hammock, B.D.; Weiss, R.H. A novel p21 attenuator which is structurally related to sorafenib. Cancer Biol. Ther. 2013, 14, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhu, X. UC2288 induces cell apoptosis of nasopharyngeal carcinoma cells via inhibiting EGFR/ERK pathway. Cancer 2021, 12, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, T.; Song, Y.; Wen, Y.; Deng, Z.; Fan, J.; Zhao, M.; Zhao, R.; Luo, Y.; Xiem, J.; et al. Cancer cells enter an adaptive persistence to survive radiotherapy and repopulate tumor. Adv. Sci. 2023, 10, 2204177. [Google Scholar] [CrossRef] [PubMed]
- Sirois, I.; Aguilar-Mahecham, A.; Lafleur, J.; Fowler, M.; Vu, V.; Scriver, M.; Buchanan, M.; Chabot, C.; Ramanathan, A.; Balachandran, B.; et al. A unique morphological phenotype in chemoresistant triple-negative breast cancer reveals metabolic reprogramming and PLIN4 expression as a molecular vulnerability. Mol. Cancer Res. 2019, 17, 2492–2507. [Google Scholar] [CrossRef]
- Funk, E. Targeting Monster Cancer Cells Could Reduce Recurrence Rates after Cancer Therapy. 2024. Available online: https://web.musc.edu/about/news-center/2024/05/28/monster-cancer-cells (accessed on 10 August 2024).
- Rappe, M. First Study on Physical Properties of Giant Cancer Cells May Inform New Treatments. 2018. Available online: https://www.brown.edu/news/2018-08-13/giantcells (accessed on 10 August 2024).
- Puig, P.E.; Guilly, M.N.; Bouchot, A.; Droin, N.; Cathelin, D.; Bouyer, F.; Favier, L.; Ghiringhelli, F.; Kroemer, G.; Solary, E.; et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol. Int. 2008, 32, 1031–1043. [Google Scholar] [CrossRef]
- Weng, C.H.; Wu, C.S.; Wu, J.C.; Kung, M.L.; Wu, M.H.; Tai, M.H. Cisplatin-induced giant cells formation is involved in chemoresistance of melanoma cells. Int. J. Mol. Sci. 2020, 21, 7892. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020, 5, 228. [Google Scholar] [CrossRef]
- Zhao, Y.; He, S.; Zhao, M.; Huang, Q. Surviving the storm: The role of poly- and depolyploidization in tissues and tumors. Adv. Sci. 2024, 11, 2306318. [Google Scholar] [CrossRef]
- Díaz-Carballo, D.; Saka, S.; Klein, J.; Rennkamp, T.; Acikelli, A.H.; Malak, S.; Jastrow, H.; Wennemuth, G.; Tempfer, C.; Schmitz, I.; et al. A distinct oncogenerative multinucleated cancer cell serves as a source of stemness and tumor heterogeneity. Cancer Res. 2018, 78, 2318–2331. [Google Scholar] [CrossRef]
- Yang, L.; Fang, J.; Chen, J. Tumor cell senescence response produces aggressive variants. Cell Death Discov. 2017, 3, 17049. [Google Scholar] [CrossRef] [PubMed]
- Tonnessen-Murray, C.A.; Frey, W.D.; Rao, S.G.; Shahbandi, A.; Ungerleider, N.A.; Olayiwola, J.O.; Murray, L.B.; Vinson, B.T.; Chrisey, D.B.; Lord, C.J.; et al. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J. Cell Biol. 2019, 218, 3827–3844. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Czarnecka, J.; Kominek, A.; Barszcz, K.; Bernas, T.; Piwocka, K.; Kaminska, B. Some chemotherapeutics-treated colon cancer cells display a specific phenotype being a combination of stem-like and senescent cell features. Cancer Biol. Ther. 2018, 19, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Herok, J.; Sliwinska, M.A.; Herok, M.; Targonska, A.; Strzeszewska-Potyrala, A.; Bojko, A.; Wolny, A.; Mosieniak, G.; Sikora, E. Therapy-induced senescent/polyploid cancer cells undergo atypical divisions associated with altered expression of meiosis, spermatogenesis and EMT genes. Int. J. Mol. Sci. 2022, 23, 8288. [Google Scholar] [CrossRef]
- Kalkavan, H.; Rühl, S.; Shaw, J.J.P.; Green, D.R. Non-lethal outcomes of engaging regulated cell death pathways in cancer. Nat. Cancer 2023, 4, 795–806. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Peng, H.; Liu, L.; Heymann, D.; Robert, C.; Vallette, F.; Shen, S. Drug-tolerant persister cells in cancer: The cutting edges and future directions. Nat. Rev.Clin. Oncol. 2023, 20, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Nano, M.; Montell, D.J. Apoptotic signaling: Beyond cell death. Semin. Cell Dev. Biol. 2024, 156, 22–34. [Google Scholar] [CrossRef]
- Xue, Y.; Luis, B.S.; Lane, D.P. Intratumour heterogeneity of p53 expression; causes and consequences. J. Pathol. 2019, 249, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Andrais, B.; Scott, A.; Wang, Y.W.; Murray, D. Ionizing radiation-induced responses in human cells with differing TP53 status. Int. J. Mol. Sci. 2013, 14, 22409–22435. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Scott, A.; Paterson, M.C.; Murray, D. Single-cell analysis of p16INK4a and p21WAF1 expression suggests distinct mechanisms of senescence in normal human and Li-Fraumeni Syndrome fibroblasts. J. Cell. Physiol. 2010, 223, 57–67. [Google Scholar] [CrossRef]
- Vitale, I.; Pietrocola, F.; Guilbaud, E.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostini, M.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 2023, 30, 1097–1154. [Google Scholar] [PubMed]
- Biswas, U.; Roy, R.; Ghosh, S.; Chakrabarti, G. The interplay between autophagy and apoptosis: Its implication in lung cancer and therapeutics. Cancer Lett. 2024, 585, 216662. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kin, T.; Beck, W.T. Impact of complex apoptotic signaling pathways on cancer cell sensitivity to therapy. Cancers 2024, 16, 984. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Kayagaki, N.; Webster, J.D.; Newton, K. Control of cell death in health and disease. Annu. Rev. Pathol. 2024, 19, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Tuval, A.; Strandgren, C.; Heldin, A.; Palomar-Siles, M.; Wiman, K.G. Pharmacological reactivation of p53 in the era of precision anticancer medicine. Nat. Rev. Clin. Oncol. 2024, 21, 106–120. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Levine, A.J. Targeting the p53 protein for cancer therapies: The translational impact of p53 research. Cancer Res. 2022, 82, 362–364. [Google Scholar] [CrossRef]
- Brown, D.W.; Beatty, P.H.; Lewis, J.D. Molecular targeting of the most functionally complex gene in precision oncology: P53. Cancers 2022, 14, 5176. [Google Scholar] [CrossRef]
- Murai, J.; Pommier, Y. BRCAness, homologous recombination deficiencies, and synthetic lethality. Cancer Res. 2023, 83, 1173–1174. [Google Scholar] [CrossRef]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]



| Disorder | Mode of Transmission | Defective Protein | Defective Function |
|---|---|---|---|
| Xeroderma pigmentosum | Autosomal recessive | XPA through XPG, DNA polymerase η | Nucleotide excision repair (XPA through XPG), postreplication repair (XPV) |
| Ataxia telangiectasia | Autosomal recessive | ATM | ATM signaling |
| Li-Fraumeni syndrome | Autosomal dominant | p53; Chk2 | p53/Chk2 signaling |
| Nijmegen breakage syndrome | Autosomal recessive | NBS1 | DSB repair |
| Werner syndrome | Autosomal recessive | WRN | DNA helicase |
| Bloom syndrome | Autosomal recessive | BS | DNA helicase |
| Rothmund-Thompson syndrome | Autosomal recessive | RTS | DNA helicase |
| Fanconi anemia | Autosomal recessive | FANCA, B, C, D1, D2, E, F, G, I, J, L and M | DNA helicase, DNA cross-link repair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzayans, R.; Murray, D. Amitotic Cell Division, Malignancy, and Resistance to Anticancer Agents: A Tribute to Drs. Walen and Rajaraman. Cancers 2024, 16, 3106. https://doi.org/10.3390/cancers16173106
Mirzayans R, Murray D. Amitotic Cell Division, Malignancy, and Resistance to Anticancer Agents: A Tribute to Drs. Walen and Rajaraman. Cancers. 2024; 16(17):3106. https://doi.org/10.3390/cancers16173106
Chicago/Turabian StyleMirzayans, Razmik, and David Murray. 2024. "Amitotic Cell Division, Malignancy, and Resistance to Anticancer Agents: A Tribute to Drs. Walen and Rajaraman" Cancers 16, no. 17: 3106. https://doi.org/10.3390/cancers16173106
APA StyleMirzayans, R., & Murray, D. (2024). Amitotic Cell Division, Malignancy, and Resistance to Anticancer Agents: A Tribute to Drs. Walen and Rajaraman. Cancers, 16(17), 3106. https://doi.org/10.3390/cancers16173106