Contemporary Molecular Markers for Predicting Systemic Treatment Response in Urothelial Bladder Cancer: A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
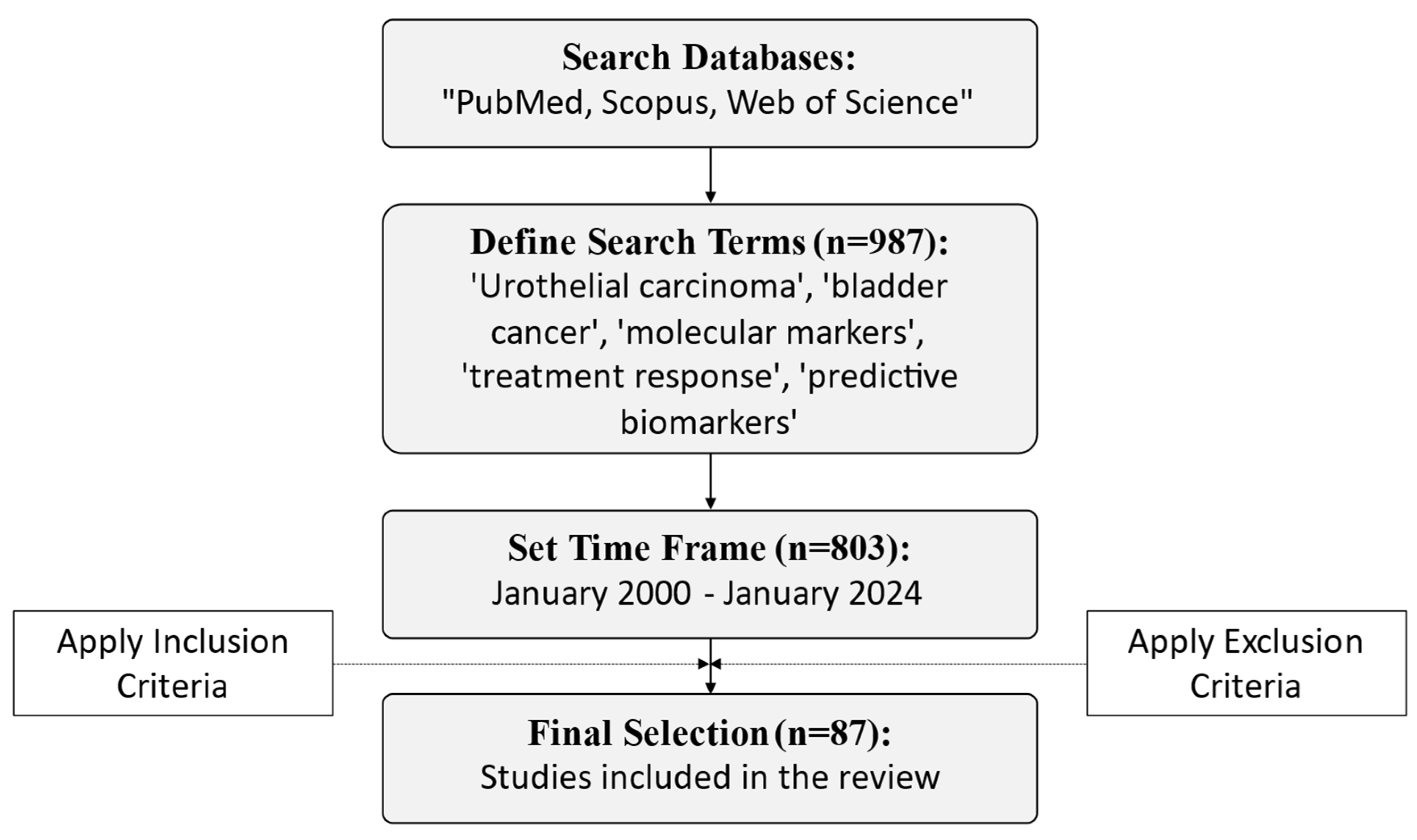
2. Methodology
- Studies that specifically addressed molecular biomarkers in UBC.
- Articles discussing biomarkers related to systemic treatment response or prediction of therapeutic outcomes.
- Research that provided significant clinical or experimental data supporting the role of these biomarkers.
- Publications in peer-reviewed journals, available in English.
- Exclusion criteria were as follows:
- Studies focused exclusively on non-muscle-invasive bladder cancer (NMIBC).
- Articles lacking sufficient experimental or clinical data to support their findings.
- Reviews, editorials, duplicates, and case reports without new data or substantial contribution to the field.
3. Pathology of Bladder Tumors
4. Systemic Therapies in UBC
4.1. Platinum-Based Systemic Chemotherapy
4.2. Immune Checkpoint Inhibitors
4.3. Target Therapies
4.4. Combining Systemic Modalities
4.5. Limitations of Current Therapies and Patient Selection
5. Tissue-Based Biomarkers
5.1. Molecular Subtypes of UBC
5.2. DNA Damage Response and Repair (DDR) Genes
5.3. Driver Mutations
5.4. PD-L1/PD-1 Expression
5.5. Tumor-Mutation Burden
5.6. Biomarker Interaction
5.7. miRNAs
6. Biological Fluid-Based Biomarkers
Circulating Tumor Cells and cfDNA
7. Discussion
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, P.; Studer, U.E.; Skinner, E.C.; Thalmann, G.N.; Miranda, G.; Roth, B.; Cai, J.; Birkhäuser, F.D.; Mitra, A.P.; Burkhard, F.C.; et al. Unaltered Oncological Outcomes of Radical Cystectomy with Extended Lymphadenectomy over Three Decades. BJU Int. 2013, 112, E51–E58. [Google Scholar] [CrossRef]
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current Best Practice for Bladder Cancer: A Narrative Review of Diagnostics and Treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef]
- Mitra, A.P.; Jordà, M.; Cote, R.J. Pathological Possibilities and Pitfalls in Detecting Aggressive Bladder Cancer. Curr. Opin. Urol. 2012, 22, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Lamm, D.; Persad, R.; Brausi, M.; Buckley, R.; Witjes, J.A.; Palou, J.; Böhle, A.; Kamat, A.M.; Colombel, M.; Soloway, M. Defining Progression in Nonmuscle Invasive Bladder Cancer: It Is Time for a New, Standard Definition. J. Urol. 2014, 191, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kompier, L.C.; Lurkin, I.; van der Aa, M.N.; van Rhijn, B.W.; van der Kwast, T.H.; Zwarthoff, E.C. Fgfr3, Hras, Kras, Nras and Pik3ca Mutations in Bladder Cancer and Their Potential as Biomarkers for Surveillance and Therapy. PLoS ONE 2010, 5, e13821. [Google Scholar] [CrossRef] [PubMed]
- Lindskrog, S.V.; Prip, F.; Lamy, P.; Taber, A.; Groeneveld, C.S.; Birkenkamp-Demtröder, K.; Jensen, J.B.; Strandgaard, T.; Nordentoft, I.; Christensen, E.; et al. An Integrated Multi-Omics Analysis Identifies Prognostic Molecular Subtypes of Non-Muscle-Invasive Bladder Cancer. Nat. Commun. 2021, 12, 2301. [Google Scholar] [CrossRef]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; van de Putte, E.E.F.; Horenblas, S.; Drabick, J.J. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 2016, 21, 708–715. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; de Vere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- Griffiths, G.; Hall, R.; Sylvester, R.; Raghavan, D.; Parmar, M.K. International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (Now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Finnbladder; Norwegian Bladder Cancer Study Group; Club Urologico Espanol De Tratamiento Oncologico Group. International Phase Iii Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the Ba06 30894 Trial. J. Clin. Oncol. 2011, 29, 2171–2177. [Google Scholar]
- Thompson, R.H.; Boorjian, S.A.; Kim, S.P.; Cheville, J.C.; Thapa, P.; Tarrel, R.; Dronca, R.; Costello, B.; Frank, I. Eligibility for Neoadjuvant/Adjuvant Cisplatin-Based Chemotherapy among Radical Cystectomy Patients. BJU Int. 2014, 113, E17–E21. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Abern, M.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chan, K.; Chang, S.; Friedlander, T.; et al. Nccn Guidelines(R) Insights: Bladder Cancer, Version 2.2022. J. Natl. Compr. Canc Netw. 2022, 20, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Nováková, Z.V.; Kuniaková, M.; Žiaran, S.; Harsányi, Š. Molecular Biomarkers of Bladder Cancer: A Mini-Review. Physiol. Res. 2023, 72, S247–S256. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Lobo, A.; Mishra, S.K.; Cheng, L. Precision Medicine in Bladder Cancer: Present Challenges and Future Directions. J. Pers. Med. 2023, 13, 756. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, A.; Lv, W.; Wang, Q.; Jiang, S.; Pan, X.; Wang, F.; Yang, H.; Bolund, L.; Lin, C.; et al. Recent Development of Urinary Biomarkers for Bladder Cancer Diagnosis and Monitoring. Clin. Transl. Discov. 2023, 3, e183. [Google Scholar] [CrossRef]
- Vandekerkhove, G.; Todenhöfer, T.; Annala, M.; Struss, W.J.; Wong, A.; Beja, K.; Ritch, E.; Brahmbhatt, S.; Volik, S.V.; Hennenlotter, J.; et al. Circulating Tumor DNA Reveals Clinically Actionable Somatic Genome of Metastatic Bladder Cancer. Clin. Cancer Res. 2017, 23, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- Khetrapal, P.; Lee, M.W.L.; Tan, W.S.; Dong, L.; de Winter, P.; Feber, A.; Kelly, J.D. The Role of Circulating Tumour Cells and Nucleic Acids in Blood for the Detection of Bladder Cancer: A Systematic Review. Cancer Treat. Rev. 2018, 66, 56–63. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Escrig, J.L.D.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non–Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma In Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Varinot, J.; Moroch, J.; Eymerit-Morin, C.; Brimo, F. A Practical Guide to Bladder Cancer Pathology. Nat. Rev. Urol. 2018, 15, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.K.; Lobo, A.; Cheng, L. The 2022 Revision of the World Health Organization Classification of Tumors of the Urinary System And Male Genital Organs: Advances and Challenges. Hum. Pathol. 2023, 136, 123–143. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Jacobus, S.; Bellmunt, J.; Qu, A.; Appleman, L.J.; Tretter, C.; Bubley, G.J.; Stack, E.C.; Signoretti, S.; Walsh, M.; et al. Neoadjuvant Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin with Pegfilgrastim Support in Muscle-Invasive Urothelial Cancer: Pathologic, Radiologic, and Biomarker Correlates. J. Clin. Oncol. 2014, 32, 1889–1894. [Google Scholar] [CrossRef]
- Advanced Bladder Cancer Meta-analysis Collaborators Group. Adjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis of Individual Participant Data from Randomised Controlled Trials. Eur. Urol. 2022, 81, 50–61. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Valderrama, B.P. Treatment of Metastatic Urothelial Carcinoma of the Bladder and Urinary Tract; Lerner, S.P., Kluwer, W., Eds.; UpToDate: Waltham, MA, USA, 2024. [Google Scholar]
- Yoshida, T.; Kates, M.; Fujita, K.; Bivalacqua, T.J.; McConkey, D.J. Predictive Biomarkers for Drug Response in Bladder Cancer. Int. J. Urol. 2019, 26, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Ogbuji, V.; Paster, I.C.; Recio-Boiles, A.; Carew, J.S.; Nawrocki, S.T.; Chipollini, J. Current Landscape of Immune Checkpoint Inhibitors for Metastatic Urothelial Carcinoma: Is There a Role for Additional T-Cell Blockade? Cancers 2024, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.S.M.; Soares, A.; Mollica, V.; Leite, C.A.; Carneiro, A.P.C.D.; Rizzo, A.; Bourlon, M.T.; Sasse, A.D.; Santoni, M.; Gupta, S.; et al. Efficacy of Immune Checkpoint Inhibitors Combinations as First-Line Systemic Treatment in Patients with Advanced Urothelial Carcinoma: A Systematic Review and Network Meta-Analysis. Crit. Rev. Oncol./Hematol. 2024, 196, 104321. [Google Scholar] [CrossRef]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J.H.; Valderrama, B.P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1961–1971. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Peng, M.; Chu, X.; Peng, Y.; Li, D.; Zhang, Z.; Wang, W.; Zhou, X.; Xiao, D.; Yang, X. Targeted Therapies in Bladder Cancer: Signaling Pathways, Applications, and Challenges. MedComm 2023, 4, e455. [Google Scholar] [CrossRef]
- Chou, J.; Trepka, K.; Sjöström, M.; Egusa, E.A.; Chu, C.E.; Zhu, J.; Chan, E.; Gibb, E.A.; Badura, M.L.; Contreras-Sanz, A.; et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-Resistant Cells. Eur. Urol. Oncol. 2022, 5, 714–718. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.; Ravaud, A.; Shariat, S.; et al. ESMO Clinical Practice Guideline Interim Update on First-Line Therapy in Advanced Urothelial Carcinoma. Ann. Oncol. 2024, 35, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Ascani, S.; Zizzo, M.; Cocco, G.; Björnebo, L.; Lantz, A.; Falagario, U.G.; Cormio, L.; et al. Are We Ready to Implement Molecular Subtyping of Bladder Cancer in Clinical Practice? Part 1: General Issues and Marker Expression. Int. J. Mol. Sci. 2022, 23, 7819. [Google Scholar] [CrossRef] [PubMed]
- Font, A.; Domènech, M.; Benítez, R.; Rava, M.; Marqués, M.; Ramírez, J.L.; Pineda, S.; Domínguez-Rodríguez, S.; Gago, J.L.; Badal, J.; et al. Immunohistochemistry-Based Taxonomical Classification of Bladder Cancer Predicts Response to Neoadjuvant Chemotherapy. Cancers 2020, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-Invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Lerner, S.P.; McConkey, D.J.; Hoadley, K.A.; Chan, K.S.; Kim, W.Y.; Radvanyi, F.; Höglund, M.; Real, F.X. Bladder Cancer Molecular Taxonomy: Summary from a Consensus Meeting. Bladder Cancer 2016, 2, 37–47. [Google Scholar] [CrossRef]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.-L.; et al. Identification of Distinct Basal and Luminal Subtypes of Muscle-Invasive Bladder Cancer with Different Sensitivities to Frontline Chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef]
- McConkey, D.J.; Choi, W.; Shen, Y.U.; Lee, I.L.; Porten, S.; Matin, S.F.; Kamat, A.M.; Corn, P.; Millikan, R.E.; Dinney, C.; et al. A Prognostic Gene Expression Signature in the Molecular Classification of Chemotherapy-Naive Urothelial Cancer Is Predictive of Clinical Outcomes from Neoadjuvant Chemotherapy: A Phase 2 Trial of Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin with Bevacizumab in Urothelial Cancer. Eur. Urol. 2016, 69, 855–862. [Google Scholar] [PubMed]
- Seiler, R.; Ashab, H.A.D.; Erho, N.; van Rhijn, B.W.; Winters, B.; Douglas, J.; Van Kessel, K.E.; van de Putte, E.E.F.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef]
- Kardos, J.; Chai, S.; Mose, L.E.; Selitsky, S.R.; Krishnan, B.; Saito, R.; Iglesia, M.D.; Milowsky, M.I.; Parker, J.S.; Kim, W.Y.; et al. Claudin-Low Bladder Tumors Are Immune Infiltrated and Actively Immune Suppressed. J. Clin. Investig. 2016, 1, e85902. [Google Scholar] [CrossRef]
- Grivas, P.; Bismar, T.A.; Alva, A.S.; Huang, H.-C.; Liu, Y.; Seiler, R.; Alimohamed, N.; Cheng, L.; Hyndman, M.E.; Dabbas, B.; et al. Validation of a Neuroendocrine-like Classifier Confirms Poor Outcomes in Patients with Bladder Cancer Treated with Cisplatin-Based Neoadjuvant Chemotherapy. Urol. Oncol. Semin. Orig. Investig. 2019, 38, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Hensley, P.J.; Kyprianou, N.; Purdom, M.S.; He, D.; DiCarlo, V.; Wang, C.; James, A.C. Predictive Value of Phenotypic Signatures of Bladder Cancer Response to Cisplatin-Based Neoadjuvant Chemotherapy. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 572.e1–572.e11. [Google Scholar] [CrossRef] [PubMed]
- Mouw, K.W. DNA Repair Pathway Alterations in Bladder Cancer. Cancers 2017, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukos, K.; Andrikopoulou, A.; Dedes, N.; Zagouri, F.; Bamias, A.; Dimopoulos, M.-A. Clinical Perspectives of ERCC1 in Bladder Cancer. Int. J. Mol. Sci. 2020, 21, 8829. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mouw, K.W.; Polak, P.; Braunstein, L.Z.; Kamburov, A.; Tiao, G.; Kwiatkowski, D.J.; Rosenberg, J.E.; Van Allen, E.M.; D’Andrea, A.D.; et al. Somatic ERCC2 Mutations Are Associated with a Distinct Genomic Signature in Urothelial Tumors. Nat. Genet. 2016, 48, 600–606. [Google Scholar] [CrossRef]
- Van Allen, E.M.; Mouw, K.W.; Kim, P.; Iyer, G.; Wagle, N.; Al-Ahmadie, H.; Zhu, C.; Ostrovnaya, I.; Kryukov, G.V.; O’Connor, K.W.; et al. Somatic ERCC2 Mutations Correlate with Cisplatin Sensitivity in Muscle-Invasive Urothelial Carcinoma. Cancer Discov. 2014, 4, 1140–1153. [Google Scholar] [CrossRef]
- Miron, B.; Hoffman-Censits, J.H.; Anari, F.; O’neill, J.; Geynisman, D.M.; Zibelman, M.R.; Kutikov, A.; Viterbo, R.; Greenberg, R.E.; Chen, D.; et al. Defects in DNA Repair Genes Confer Improved Long-term Survival after Cisplatin-Based Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2020, 3, 544–547. [Google Scholar] [CrossRef]
- Yap, K.L.; Kiyotani, K.; Tamura, K.; Antic, T.; Jang, M.; Montoya, M.; Campanile, A.; Yew, P.Y.; Ganshert, C.; Fujioka, T. Whole-Exome Sequencing of Muscle-Invasive Bladder Cancer Identifies Recurrent Mutations of Unc5c and Prognostic Importance of DNA Repair Gene Mutations on Survival. Clin. Cancer Res. 2014, 20, 6605–6617. [Google Scholar] [CrossRef]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit from Pd-1/Pd-L1 Blockade in Advanced Urothelial Cancers. J. Clin. Oncol. 2018, 36, 1685. [Google Scholar] [CrossRef]
- Geynisman, D.M.; Abbosh, P.; Ross, E.A.; Zibelman, M.R.; Ghatalia, P.; Anari, F.; Ansel, K.; Mark, J.R.; Stamatakis, L.; Hoffman-Censits, J.H.; et al. A Phase II Trial of Risk-Enabled Therapy after Initiating Neoadjuvant Chemotherapy for Bladder Cancer (RETAIN). J. Clin. Oncol. 2023, 41, 438. [Google Scholar] [CrossRef]
- Ascione, C.M.; Napolitano, F.; Esposito, D.; Servetto, A.; Belli, S.; Santaniello, A.; Scagliarini, S.; Crocetto, F.; Bianco, R.; Formisano, L. Role of FGFR3 in Bladder Cancer: Treatment Landscape and Future Challenges. Cancer Treat. Rev. 2023, 115, 102530. [Google Scholar] [CrossRef]
- Chen, D.; Ye, Y.; Guo, S.; Yao, K. Progress in the Research and Targeted Therapy of ErbB/HER Receptors in Urothelial Bladder Cancer. Front. Mol. Biosci. 2021, 8, 800945. [Google Scholar] [CrossRef] [PubMed]
- Naski, M.C.; Wang, Q.; Xu, J.; Ornitz, D.M. Graded Activation of Fibroblast Growth Factor Receptor 3 by Mutations Causing Achondroplasia and Thanatophoric Dysplasia. Nat. Genet. 1996, 13, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.Y.; Mota, J.M.; Whiting, K.A.; Li, H.A.; Funt, S.A.; Lee, C.-H.; Solit, D.B.; Al-Ahmadie, H.; Milowsky, M.I.; Balar, A.V.; et al. Fibroblast Growth Factor Receptor 3 Alteration Status Is Associated with Differential Sensitivity to Platinum-Based Chemotherapy in Locally Advanced and Metastatic Urothelial Carcinoma. Eur. Urol. 2020, 78, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Erbb Receptors and Cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [PubMed]
- Carlsson, J.; Wester, K.; De La Torre, M.; Malmström, P.U.; Gårdmark, T. EGFR-Expression in Primary Urinary Bladder Cancer and Corresponding Metastases and the Relation to Her2-Expression. On the Possibility to Target These Receptors with Radionuclides. Radiol. Oncol. 2015, 49, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mooso, B.A.; Vinall, R.L.; Mudryj, M.; Yap, S.A.; White, R.W.D.; Ghosh, P.M. The Role of EGFR Family Inhibitors in Muscle Invasive Bladder Cancer: A Review of Clinical Data and Molecular Evidence. J. Urol. 2015, 193, 19–29. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the Erbb Signalling Network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Oudard, S.; Culine, S.; Vano, Y.; Goldwasser, F.; Théodore, C.; Nguyen, T.; Voog, E.; Banu, E.; Vieillefond, A.; Priou, F.; et al. Multicentre Randomised Phase II trial of Gemcitabine+Platinum, with or without Trastuzumab, in Advanced or Metastatic Urothelial Carcinoma Overexpressing Her2. Eur. J. Cancer 2015, 51, 45–54. [Google Scholar] [CrossRef]
- Fleischmann, A.; Rotzer, D.; Seiler, R.; Studer, U.E.; Thalmann, G.N. Her2 Amplification is Significantly More Frequent in Lymph Node Metastases from Urothelial Bladder Cancer Than in the Primary Tumours. Eur. Urol. 2011, 60, 350–357. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Urothelial Bladder Carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Dueñas, M.; Martínez-Fernández, M.; García-Escudero, R.; Villacampa, F.; Marqués, M.; Saiz-Ladera, C.; Duarte, J.; Martínez, V.; Gómez, M.J.; Martín, M.L.; et al. PIK3CA Gene Alterations in Bladder Cancer Are Frequent and Associate with Reduced Recurrence in Non-Muscle Invasive Tumors. Mol. Carcinog. 2015, 54, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.J.; Campanile, A.; Antic, T.; Yap, K.L.; Fitzpatrick, C.A.; Wade, J.L.; Karrison, T.; Stadler, W.M.; Nakamura, Y.; O’donnell, P.H. Afatinib Activity in Platinum-Refractory Metastatic Urothelial Carcinoma in Patients with ERBB Alterations. J. Clin. Oncol. 2016, 34, 2165–2171. [Google Scholar] [CrossRef]
- Meeks, J.J.; Black, P.C.; Galsky, M.; Grivas, P.; Hahn, N.M.; Hussain, S.A.; Milowsky, M.I.; Steinberg, G.D.; Svatek, R.S.; Rosenberg, J.E. Checkpoint Inhibitors in Urothelial Carcinoma—Future Directions and Biomarker Selection. Eur. Urol. 2023, 84, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as Neoadjuvant Therapy before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical Efficacy and Biomarker Analysis of Neoadjuvant Atezolizumab in Operable Urothelial Carcinoma in the ABACUS Trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Meghani, K.; Cooley, L.F.; McLaughlin, K.A.; Fall, L.A.; Yu, Y.; Castro, M.A.A.; Groeneveld, C.S.; de Reyniès, A.; Nazarov, V.I.; et al. Expression-Based Subtypes Define Pathologic Response to Neoadjuvant Immune-Checkpoint Inhibitors in Muscle-Invasive Bladder Cancer. Nat. Commun. 2023, 14, 2126. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Tapia, J.C.; Bosma, F.; Gavira, J.; Sanchez, S.; Molina, M.A.; Sanz-Beltran, J.; Martin-Lorente, C.; Anguera, G.; Maroto, P. Treatment Patterns and Survival Outcomes before and after Access to Immune Checkpoint Inhibitors for Patients with Metastatic Urothelial Carcinoma: A Single-Center Retrospective Study from 2004 to 2021. Clin. Genitourin. Cancer 2024, 22, 102047. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Li, Y.; Gou, X. EP300 Mutation Is Associated with Tumor Mutation Burden and Promotes Antitumor Immunity in Bladder Cancer Patients. Aging 2020, 12, 2132–2141. [Google Scholar] [CrossRef]
- Pichler, R.; Lindner, A.K.; Compérat, E.; Obrist, P.; Schäfer, G.; Todenhöfer, T.; Horninger, W.; Culig, Z.; Untergasser, G. Amplification of 7p12 Is Associated with Pathologic Nonresponse to Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Cancer. Am. J. Pathol. 2020, 190, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Manzano, R.G.; Catalan-Latorre, A.; Brugarolas, A. RB1 and TP53 Co-Mutations Correlate Strongly with Genomic Biomarkers of Response to Immunity Checkpoint Inhibitors in Urothelial Bladder Cancer. BMC Cancer 2021, 21, 432. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Damish, A.W.; Frazier, Z.; Liu, D.; Reznichenko, E.; Kamburov, A.; Bell, A.; Zhao, H.; Jordan, E.J.; Gao, S.P.; et al. Ercc2 Helicase Domain Mutations Confer Nucleotide Excision Repair Deficiency and Drive Cisplatin Sensitivity in Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2019, 25, 977–988. [Google Scholar] [CrossRef]
- Vidotto, T.; Nersesian, S.; Graham, C.; Siemens, D.R.; Koti, M. DNA Damage Repair Gene Mutations and Their Association with Tumor Immune Regulatory Gene Expression in Muscle Invasive Bladder Cancer Subtypes. J. Immunother. Cancer 2019, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Puyang, X.; Jeremy Wu, Z.; Seiler, R.; Furman, C.; Oo, H.Z.; Seiler, M.; Irwin, S.; Subramanian, V.; Julie Joshi, J.; et al. Evasion of Immunosurveillance by Genomic Alterations of Ppargamma/Rxralpha in Bladder Cancer. Nat. Commun. 2017, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Hayden, J.; Sullivan, T.; Rieger-Christ, K. The Roles of miRNAs in Predicting Bladder Cancer Recurrence and Resistance to Treatment. Int. J. Mol. Sci. 2023, 24, 964. [Google Scholar] [CrossRef] [PubMed]
- Claps, F.; Mir, M.C.; Zargar, H. Molecular Markers of Systemic Therapy Response in Urothelial Carcinoma. Asian J. Urol. 2021, 8, 376–390. [Google Scholar] [CrossRef]
- Wang, C.; Tang, Z.; Zhang, Z.; Liu, T.; Zhang, J.; Huang, H.; Li, Y. MiR-7-5p Suppresses Invasion via Downregulation of the Autophagy-Related Gene ATG7 and Increases Chemoresistance to Cisplatin in BCa. Bioengineered 2022, 13, 7328–7339. [Google Scholar] [CrossRef]
- Hwang, T.I.S.; Chen, P.C.; Tsai, T.F.; Lin, J.F.; Chou, K.Y.; Ho, C.Y.; Chen, H.E.; Chang, A.C. Hsa-miR-30a-3p Overcomes the Acquired Protective Autophagy of Bladder Cancer in Chemotherapy and Suppresses Tumor Growth and Muscle Invasion. Cell Death Dis. 2022, 13, 390. [Google Scholar] [CrossRef]
- Xu, T.; Qin, L.; Zhu, Z.; Wang, X.; Liu, Y.; Fan, Y.; Zhong, S.; Wang, X.; Zhang, X.; Xia, L.; et al. MicroRNA-31 Functions as a Tumor Suppressor and Increases Sensitivity to Mitomycin-C in Urothelial Bladder Cancer by Targeting Integrin α5. Oncotarget 2016, 7, 27445–27457. [Google Scholar] [CrossRef]
- Vinall, R.L.; Ripoll, A.Z.; Wang, S.; Pan, C.; White, R.W.D. MiR-34a Chemosensitizes Bladder Cancer Cells to Cisplatin Treatment Regardless of p53-Rb Pathway Status. Int. J. Cancer 2012, 130, 2526–2538. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Fang, Y.; Cao, Y.; Chen, Q.; Liu, Y. Enforced Expression of miR-101 Enhances Cisplatin Sensitivity in Human Bladder Cancer Cells by Modulating the Cyclooxygenase-2 Pathway. Mol. Med. Rep. 2014, 10, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xie, D.; Zhang, H. MicroRNA-101-3p Advances Cisplatin Sensitivity in Bladder Urothelial Carcinoma through Targeted Silencing EZH2. J. Cancer 2019, 10, 2628–2634. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Q.; Wu, G.; Li, S.; Wang, Q. miR-129-5p Inhibits Gemcitabine Resistance and Promotes Cell Apoptosis of Bladder Cancer Cells by Targeting Wnt5a. Int. Urol. Nephrol. 2018, 50, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Nordentoft, I.; Birkenkamp-Demtroder, K.; Agerbæk, M.; Theodorescu, D.; Ostenfeld, M.S.; Hartmann, A.; Borre, M.; Ørntoft, T.F.; Dyrskjøt, L. miRNAs Associated with Chemo-Sensitivity in Cell Lines and in Advanced Bladder Cancer. BMC Med. Genom. 2012, 5, 40. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, R.; Wang, J.; Han, J.; Yang, X.; Yu, H.; Lu, H.; Zhang, X.; Li, P.; Tao, J.; et al. Circular RNA Cdr1as Sensitizes Bladder Cancer to Cisplatin by Upregulating APAF1 Expression through miR-1270 Inhibition. Mol. Oncol. 2019, 13, 1559–1576. [Google Scholar] [CrossRef]
- Bellmunt, J.; Zhou, C.W.; Mullane, S.A.; Werner, L.; Taplin, M.-E.; Fay, A.P.; Choueiri, T.K.; Orsola, A.; Takeda, D.Y.; Hahn, W.C.; et al. Association of Tumour microRNA Profiling with Outcomes in Patients with Advanced Urothelial Carcinoma Receiving First-Line Platinum-Based Chemotherapy. Br. J. Cancer 2016, 115, 12–19. [Google Scholar] [CrossRef]
- Xiao, J.; Niu, S.; Zhu, J.; Lv, L.; Deng, H.; Pan, D.; Shen, D.; Xu, C.; Shen, Z.; Tao, T. miR-22-3p Enhances Multi-Chemoresistance by Targeting NET1 in Bladder Cancer Cells. Oncol. Rep. 2018, 39, 2731–2740. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Wang, H.; Wang, Y.; Li, Z.; Pan, Y.; Liu, Q.; Yang, M. Repression of the miR-93-Enhanced Sensitivity of Bladder Carcinoma to Chemotherapy Involves the Regulation of LASS2. OncoTargets Ther. 2016, 9, 1813–1822. [Google Scholar] [CrossRef][Green Version]
- Luan, T.; Fu, S.; Huang, L.; Zuo, Y.; Ding, M.; Li, N.; Chen, J.; Wang, H.; Wang, J. MicroRNA-98 Promotes Drug Resistance and Regulates Mitochondrial Dynamics by Targeting LASS2 in Bladder Cancer Cells. Exp. Cell Res. 2018, 373, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, H. Knockdown of microRNA-130b Improves Doxorubicin Sensitivity in Bladder Urothelial Carcinoma by Negatively Regulating Cylindromatosis Expression. Arch. Med. Sci. 2021, 17, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Li, Y.; Deng, H.; Zhang, C.; Pu, Y.; Qian, L.; Xiao, J.; Zhao, W.; Liu, Q.; Zhang, D.; et al. MiR-193a-3p Promotes the Multi-Chemoresistance of Bladder Cancer by Targeting the HOXC9 Gene. Cancer Lett. 2015, 357, 105–113. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, X.; Fang, A.; Li, P.; Li, Z.; Liu, T.; Yang, Y.; Du, L.; Wang, C. MicroRNA-203 Is a Prognostic Indicator in Bladder Cancer and Enhances Chemosensitivity to Cisplatin via Apoptosis by Targeting Bcl-W and Survivin. PLoS ONE 2015, 10, e0143441. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Barone, B.; Ferro, M.; Busetto, G.M.; La Civita, E.; Buonerba, C.; Di Lorenzo, G.; Terracciano, D.; Schalken, J.A. Liquid Biopsy in Bladder Cancer: State of the Art and Future Perspectives. Crit. Rev. Oncol./Hematol. 2022, 170, 103577. [Google Scholar] [CrossRef]
- Ferro, M.; La Civita, E.; Liotti, A.; Cennamo, M.; Tortora, F.; Buonerba, C.; Crocetto, F.; Lucarelli, G.; Busetto, G.M.; Del Giudice, F.; et al. Liquid Biopsy Biomarkers in Urine: A Route towards Molecular Diagnosis and Personalized Medicine of Bladder Cancer. J. Pers. Med. 2021, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid Biopsy: A Step Closer to Transform Diagnosis, Prognosis and Future of Cancer Treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.T.; Coumans, F.A.W.; Attard, G.; Cassidy, A.M.; de Bono, J.S.; Terstappen, L.W.M.M. Unbiased and Automated Identification of a Circulating Tumour Cell Definition That Associates with Overall Survival. PLoS ONE 2011, 6, e27419. [Google Scholar] [CrossRef]
- Beije, N.; de Kruijff, I.; de Jong, J.; Klaver, S.; de Vries, P.; Jacobs, R.; Somford, D.; Slaa, E.T.; van der Heijden, A.; Witjes, J.A.; et al. Circulating Tumour Cells to Drive the Use of Neoadjuvant Chemotherapy in Patients with Muscle-Invasive Bladder Cancer. ESMO Open 2022, 7, 100416. [Google Scholar] [CrossRef]
- Nicolazzo, C.; de Berardinis, E.; Gazzaniga, P. Liquid Biopsy for Predicting Bacillus Calmette-Guérin Unresponsiveness in Non–Muscle-Invasive Bladder Cancer. Eur. Urol. Oncol. 2020, 4, 124–125. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Bisagni, A.; Zizzo, M.; Ascani, S.; Pedicillo, M.C.; Cormio, A.; Falagario, U.G.; Carrieri, G.; et al. HER2 Expression in Bladder Cancer: A Focused View on Its Diagnostic, Prognostic, and Predictive Role. Int. J. Mol. Sci. 2023, 24, 3720. [Google Scholar] [CrossRef]
- Luceno, C.F.; Jeon, W.J.; Samaeekia, R.; Shin, J.; Sonpavde, G.P. Precision Medicine to Treat Urothelial Carcinoma—The Way Forward. Cancers 2023, 15, 3024. [Google Scholar] [CrossRef] [PubMed]
- Tsoneva, D.K.; Ivanov, M.N.; Conev, N.V.; Manev, R.; Stoyanov, D.S.; Vinciguerra, M. Circulating Histones to Detect and Monitor the Progression of Cancer. Int. J. Mol. Sci. 2023, 24, 942. [Google Scholar] [CrossRef] [PubMed]
- Crupi, E.; de Padua, T.C.; Marandino, L.; Raggi, D.; Dyrskjøt, L.; Spiess, P.E.; Sonpavde, G.P.; Kamat, A.M.; Necchi, A. Circulating tumor DNA as a Predictive and Prognostic Biomarker in the Perioperative Treatment of Muscle-invasive Bladder Cancer: A Systematic Review. Eur. Urol. Oncol. 2024, 7, 44–52. [Google Scholar] [CrossRef]
- Laukhtina, E.; Hassler, M.R.; Pradere, B.; Yanagisawa, T.; Quhal, F.; Rajwa, P.; Motlagh, R.S.; König, F.; Pallauf, M.; Kawada, T.; et al. Circulating Tumour DNA Is a Strong Predictor of Outcomes in Patients Treated with Systemic Therapy for Urothelial Carcinoma. Eur. Urol. Focus. 2022, 8, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Pal, S.K.; Hahn, A.W.; Nussenzveig, R.H.; Pond, G.R.; Gupta, S.V.; Wang, J.; Bilen, M.A.; Naik, G.; Ghatalia, P.; et al. Characterization of Metastatic Urothelial Carcinoma via Comprehensive Genomic Profiling of Circulating Tumor DNA. Cancer 2018, 124, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Birkenkamp-Demtröder, K.; Sethi, H.; Shchegrova, S.; Salari, R.; Nordentoft, I.; Wu, H.-T.; Knudsen, M.; Lamy, P.; Lindskrog, S.V.; et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients with Urothelial Bladder Carcinoma. J. Clin. Oncol. 2019, 37, 1547–1557. [Google Scholar] [CrossRef]
- Patel, K.; Van Der Vos, K.E.; Smith, C.G.; Mouliere, F.; Tsui, D.; Morris, J.; Chandrananda, D.; Marass, F.; Van Den Broek, D.; Neal, D.; et al. Association of Plasma and Urinary Mutant DNA with Clinical Outcomes in Muscle Invasive Bladder Cancer. Sci. Rep. 2017, 7, 5554. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, Y.; Feng, Q.; Xu, L.; Jiang, Y.; Meng, F.; Shu, X. A Novel Prognostic Biomarker for Muscle Invasive Bladder urothelial Carcinoma Based on 11 DNA Methylation Signature. Cancer Biol. Ther. 2020, 21, 1119–1127. [Google Scholar] [CrossRef]
- Stubendorff, B.; Wilhelm, K.; Posselt, K.; Catto, J.; Hartmann, A.; Bertz, S.; Füssel, S.; Novotny, V.; Toma, M.; Gajda, M.; et al. A Three-Gene Methylation Marker Panel for the Nodal Metastatic Risk Assessment of Muscle-Invasive Bladder Cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 811–820. [Google Scholar] [CrossRef]
- Xu, Z.; Gujar, H.; Fu, G.; Ahmadi, H.; Bhanvadia, S.; Weisenberger, D.J.; Jin, B.; Gill, P.S.; Gill, I.; Daneshmand, S.; et al. A Novel DNA Methylation Signature as an Independent Prognostic Factor in Muscle-Invasive Bladder Cancer. Front. Oncol. 2021, 11, 614927. [Google Scholar] [CrossRef]
- Lu, Y.T.; Plets, M.; Morrison, G.; Cunha, A.T.; Cen, S.Y.; Rhie, S.K.; Siegmund, K.D.; Daneshmand, S.; Quinn, D.I.; Meeks, J.J.; et al. Cell-Free DNA Methylation as a Predictive Biomarker of Response to Neoadjuvant Chemotherapy for Patients with Muscle-Invasive Bladder Cancer in Swog S1314. Eur. Urol. Oncol. 2023, 6, 516–524. [Google Scholar] [CrossRef] [PubMed]
| Differentiation | Urothelial/Luminal | Mixed | Basal | Neuroendocrine | ||
|---|---|---|---|---|---|---|
| Class name | Papillary | Non-specified | Unstable | Stromal-rich | Basal/squamous | Neuroendocrine-like |
| % of MIBC | 24% | 8% | 15% | 15% | 35% | 3% |
| Oncogenic mechanism | FGF3 + PPARG + CDKN2A + | PPARG + | PPARG + E2F3 + ERBB2 + Genomic instability Cell cycle + | - | EGFR + | TP53 – RB1 – Cell cycle + |
| Mutations | FGFR3 (~40%) KDM6A (~40%) | ELF3 (~35%) | TP53 (~75%) ERCC2 (~20%) TMB + APOBEC + | - | TP53 (~60%) RB1 (~25%) | TP53 (~95%) RB1 (~40%) |
| Stromal infiltrate | - | Fibroblasts | - | Smooth muscle Fibroblasts Myofibroblasts | Fibroblasts Myofibroblasts | - |
| Immune infiltrate | - | - | - | B-cells | CD8 T-cells NK cells | - |
| Histology | Papillary morphology (~60%) | Micropapillary morphology (~35%) | - | - | Squamous differentiation (~40%) | Neuroendocrine differentiation (~70%) |
| Clinical | T2 stage | Older patients (>80) | - | - | Women T3/4 stage | - |
| Median overall survival (yr) | 4 | 1.8 | 2.9 | 3.8 | 1.2 | 1 |
| Biomarker | Condition | Frequency | Pathway | Summary |
|---|---|---|---|---|
| ERCC2 [46] | Tissue testing from biopsied UBC before cisplatin-based NACT | ~15% | Nucleotide excision repair | Alterations correlate with better response rates, pDS, and OS. |
| ATM [48] | ~5% | Double-strand break repair | Mutations correlate with pCR and improved PFS, DSS, and OS. | |
| RB1 [48] | ~15% | Cell-cycle control | Alterations predict better response rates, PFS, DSS, and OS. | |
| FANCC [48] | ~10% | Homologous recombination repair | Mutations predict better response rates, PFS, DSS, and OS. | |
| >1 gene [49] | Cisplatin-based NACT followed by cystectomy for MIBC | Varies | Double-strand break repair | Alterations in FANCD2, PALB2, BRCA1, or BRCA2 are associated with increased PFS in MIBC. |
| Biomarker | Condition | Frequency | Pathway | Summary |
|---|---|---|---|---|
| ERBB2 | Tissue testing from biopsied UBC before cisplatin-based NACT | ~5% | MAPK, PI3K | Mutations predicted pCR, pDS, and better CSS. |
| FGFR3 | Tissue testing from biopsied UBC before cisplatin-based NACT | ~50% overexpression; 15% alteration | MAPK, PI3K | Alterations correlated with pDS and worse PFS. |
| Tissue testing from biopsied UBC before cisplatin-based adjuvant therapy | Mutations linked to worse PFS. | |||
| PIK3Ca | Tissue testing from biopsied UBC before cisplatin-based NACT | 13–27% | PI3K | Alterations correlated with pDS. |
| HUS1 | Tissue testing from biopsied UBC before cisplatin-based NACT | ~1% | Mismatch repair | Amplification predicted non-response and worse PFS. |
| ABCA13 | Tissue testing from biopsied UBC before cisplatin-based NACT | ~5% | Mediation across cell membrane | Amplification predicted non-response and worse PFS. |
| EGFR | Tissue testing from biopsied UBC before cisplatin-based NACT | ~70% | MAPK, PI3K | Alterations predicted non-response and worse PFS. |
| FIGNL1 | Tissue testing from biopsied UBC before cisplatin-based NACT | ~5% | Homologous recombination repair | Amplification predicted non-response and worse PFS. |
| IKZF1 | Tissue testing from biopsied UBC before cisplatin-based NACT | ~5% | Zinc finger transcription factor | Amplification predicted non-response and worse PFS. |
| Trial | ICI | Setting | Response Rate in ITT | OS in ITT | PFS in ITT | PD1/PD-L1 Predictive Value |
|---|---|---|---|---|---|---|
| IMvigor210 | Atezolizumab | 1L, Cisplatin-ineligible | 15% | 15.9 months | 2.7 months | Higher response rates (26%) in PD-L1-high tumors; IC2/3 ≥ 5% |
| Keynote-045 | Pembrolizumab | 2L, Post-platinum | 21.1% | 10.3 months | 2 months | No significant difference in OS (8 months) or PFS (2.1 months) based on CPS ≥ 10% |
| IMvigor211 | Atezolizumab | 2L, Post-platinum | 13% | 8.6 months | 2.1 months | No significant difference in OS (11.1 months) based on IC2/3 ≥5% |
| JAVELIN Bladder 100 | Avelumab | 1L, Maintenance post-chemo | 16.1% | 21.4 months | 3.7 months | Improved OS (not reached) in PD-L1-positive tumors; expression in ≥25% of tumor cells |
| CheckMate 275 | Nivolumab | 2L, Post-platinum | 19.6% | 8.6 months | 2.0 months | Higher response rates (28.4%) and superior OS (11.3 months) in PD-L1-positive tumors; PD-L1 expression ≥1% |
| Keynote-361 | Pembrolizumab | 1L | 25.9% | 15.6 months | Not reported | No significant difference in OS (16.1) based on CPS ≥ 10% |
| Keynote-361 | Pembrolizumab | 1L, Combination with chemo | 25.9% | 17 months | 8.3 months | No significant difference in OS (17.0) based on CPS ≥ 10% |
| IMvigor130 | Atezolizumab | 1L | 13% | 15.2 months | 2.7 months | Higher response rates (23%) and a trend toward improved OS (18.6 months) in IC2/3 ≥ 5% |
| CheckMate 032 | Nivolumab | 2L, Post-platinum | 25.6% | 9.7 months | 2.7 months | No significant difference in response rates (26.9%) and OS (11.3 months) in PD-L1-positive tumors; PD-L1 expression ≥1% |
| CheckMate 032 | Nivolumab + Ipilimumab | 2L, Post-platinum | 26.9% | 7.3 months | 2.6 months | Higher response rates (38%) and improved OS (15.3 months) in PD-L1-positive tumors; PD-L1 expression ≥1% |
| PURE01 | Pembrolizumab | Neoadjuvant | 42% (pCR) | 36-month OS was 83.8% | 36-month EFS was 74.4% | Higher pCR rates (54.3%) in PD-L1-positive tumors; CPS ≥ 10% |
| DANUBE | Durvalumab + Tremelimumab | 1L, Combination with chemo | 24% | 12.9 months | 5.5 months | No significant OS benefit in (14.4 months) TC ≥ 25% compared to ITT population |
| EV-302 | Pembrolizumab + Enfortumab Vedotin | 1L | 67.7% (29.1% pCR) | 31.5 months | 12.5 months | Higher response rates (73.3%) and improved OS (not reached) in PD-L1-positive tumors; CPS ≥ 10% |
| Category | miRNA | Target/Regulator | Function |
|---|---|---|---|
| Promoting Chemosensitivity | miR-7-5p [79] | ATG7 | Inhibits invasive characteristics and enhances chemosensitivity. |
| miR-30a-3p [80] | MMP2 and MMP9 | Improves apoptosis and reduces cell viability when combined with cisplatin and decreases migration and invasion. | |
| miR-31 [81] | ITGA5 | Enhances chemosensitivity to mitomycin-C and inhibits proliferation, migration, and invasion. | |
| miR-34a [82] | TCF1, LEF1, Cdk6, SRT-1, CD44 | Enhances sensitivity to epirubicin and cisplatin while repressing metastatic characteristics. | |
| MiR-101[83] | COX2 | Promotes chemosensitivity to cisplatin. | |
| MiR-101-3p [84] | EZH2, affects MRP1 expression | Enhances sensitivity to gemcitabine. | |
| miR-129-5p [85] | Wnt5a | Promotes response to gemcitabine. | |
| miR-27a [86] | SLC7A11 | Increases cisplatin sensitivity. | |
| miR-642 [86] | Unknown | Increases cisplatin sensitivity. | |
| miR-34a [82] | Unknown | Increases cisplatin and epirubicin sensitivity. | |
| Cdr1as [87] | Unknown | Increases cisplatin sensitivity through the miR-1270/APAF1 axis. | |
| Promoting Chemoresistance | miR-21 [88] | PTEN | Promotes resistance to doxorubicin and inhibits doxorubicin-induced apoptosis. |
| miR-22-3p [89] | NET1 | Enhances chemoresistance by increasing cell viability and colony formation while reducing apoptosis. | |
| miR-93 [90] | Unknown | Promotes chemoresistance without direct binding to LASS2. | |
| miR-98 [91] | LASS2 | Leads to increased proliferation, resistance to cisplatin and doxorubicin, and reduced apoptosis. | |
| miR-130b [92] | CYLD | Promotes chemoresistance. | |
| miR-193a-3p [93] | Unknown | Promotes multi-chemoresistance. | |
| Correlated with Better Response and Survival | miR-886-3p [86] | Unknown | Associated with complete response (CR) and better overall survival (OS) in metastatic cases treated with MVAC or Gem-Cis. |
| miR-923 [86] | Unknown | ||
| miR-944 [86] | Unknown | ||
| miR-203 [94] | Unknown | Low expression is correlated with worse progression-free survival (PFS) and OS. | |
| Correlated with Worse Response and Survival | miR-372 [88] | Unknown | High expression is linked to worse PFS. |
| miR-21 [88] | Unknown | High expression is associated with shorter PFS in metastatic cases treated with MVAC or Gem-Cis. | |
| Mixed Responses Based on Expression Levels | miR-138 [86] | Unknown | Decreasing expression increases cisplatin sensitivity. |
| miR-101 [83] | Unknown | Downregulation induces cisplatin resistance through the COX-2 axis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrov, G.; Mangaldzhiev, R.; Slavov, C.; Popov, E. Contemporary Molecular Markers for Predicting Systemic Treatment Response in Urothelial Bladder Cancer: A Narrative Review. Cancers 2024, 16, 3056. https://doi.org/10.3390/cancers16173056
Dimitrov G, Mangaldzhiev R, Slavov C, Popov E. Contemporary Molecular Markers for Predicting Systemic Treatment Response in Urothelial Bladder Cancer: A Narrative Review. Cancers. 2024; 16(17):3056. https://doi.org/10.3390/cancers16173056
Chicago/Turabian StyleDimitrov, George, Radoslav Mangaldzhiev, Chavdar Slavov, and Elenko Popov. 2024. "Contemporary Molecular Markers for Predicting Systemic Treatment Response in Urothelial Bladder Cancer: A Narrative Review" Cancers 16, no. 17: 3056. https://doi.org/10.3390/cancers16173056
APA StyleDimitrov, G., Mangaldzhiev, R., Slavov, C., & Popov, E. (2024). Contemporary Molecular Markers for Predicting Systemic Treatment Response in Urothelial Bladder Cancer: A Narrative Review. Cancers, 16(17), 3056. https://doi.org/10.3390/cancers16173056






