Rare Clinical Symptoms in Hairy Cell Leukemia: An Overview
Abstract
Simple Summary
Abstract
1. Introduction
2. Skin Symptoms
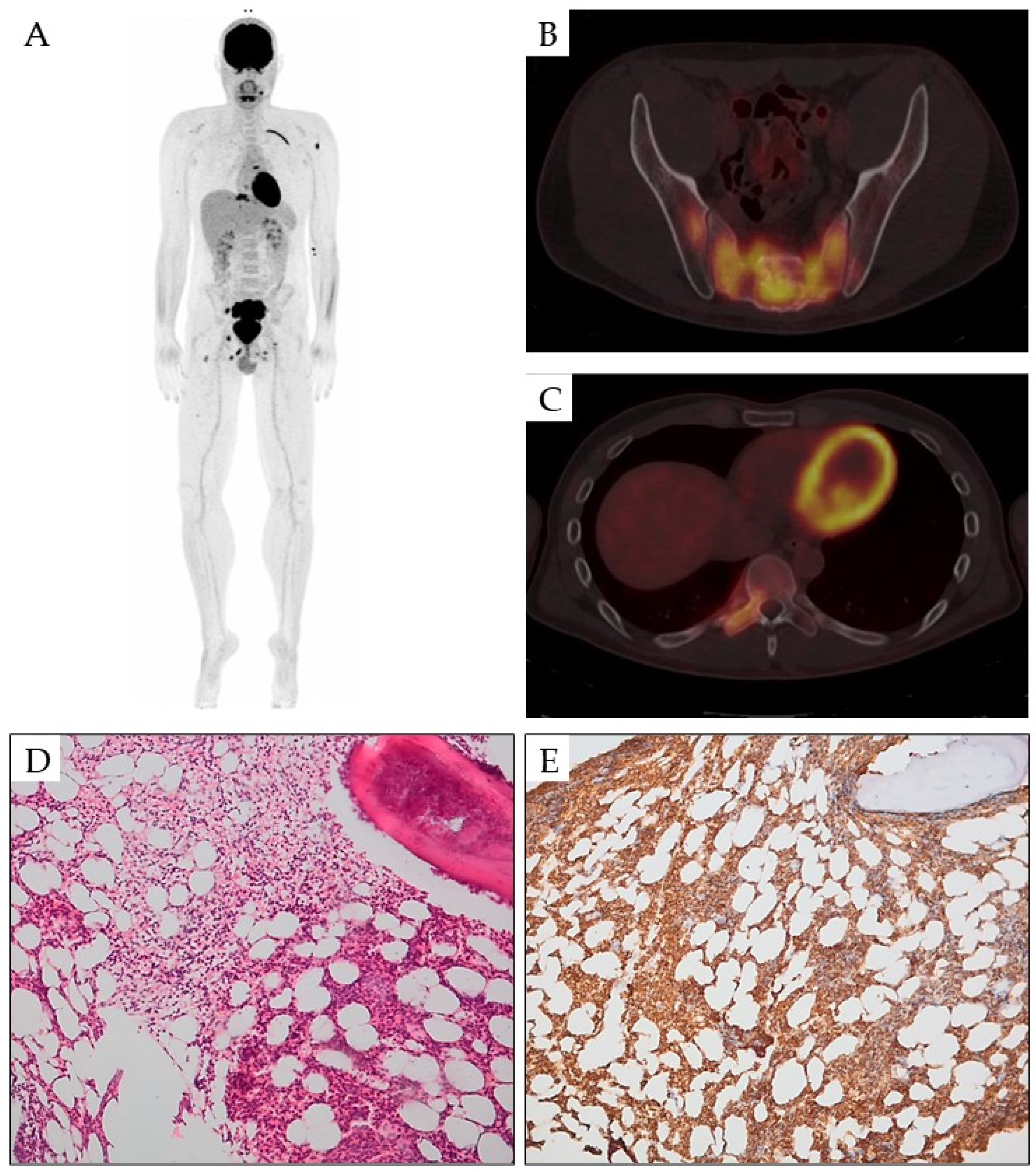
3. Bone Lesions
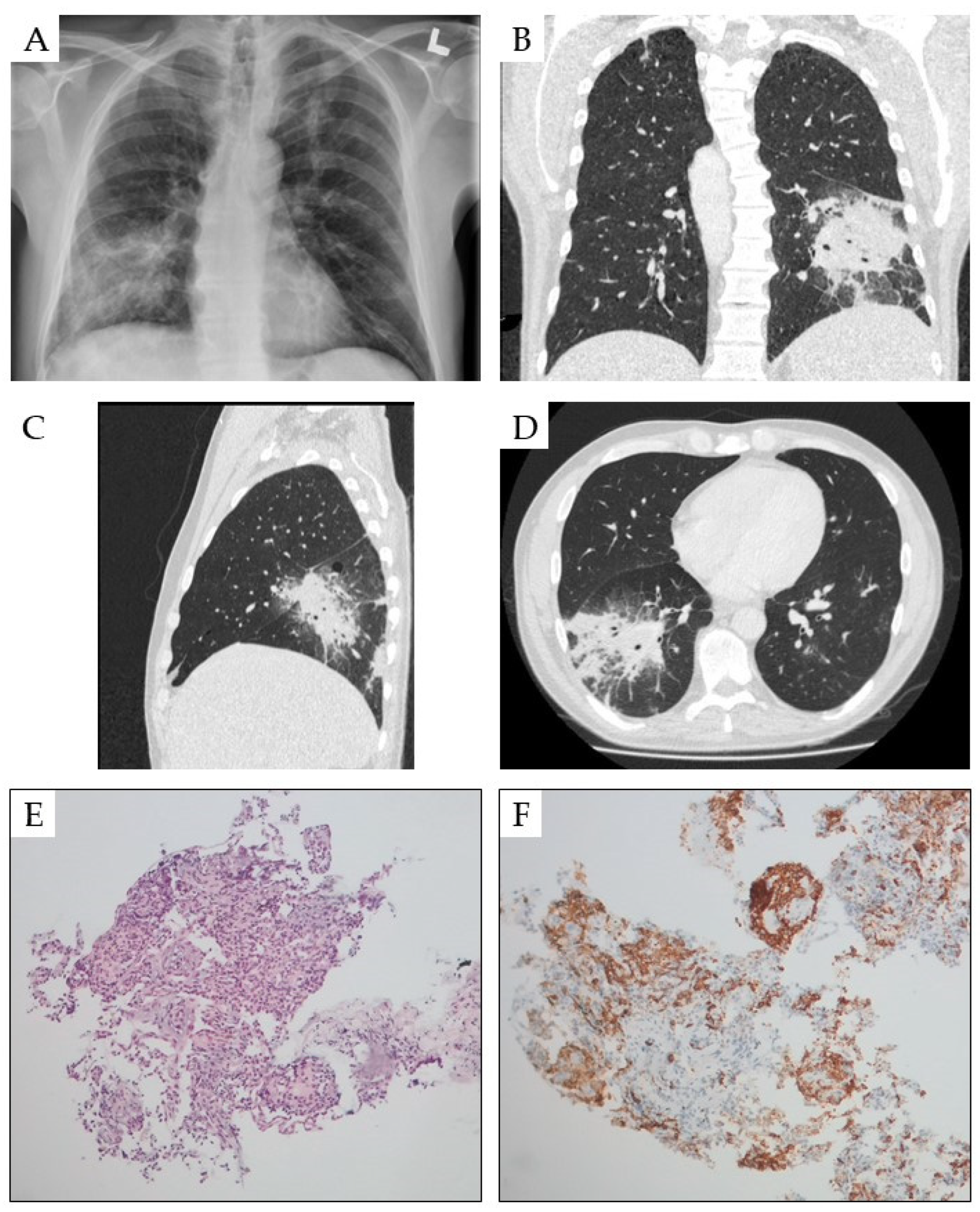
4. Pulmonary Symptoms
5. Neurological Manifestations
6. Ocular Symptoms
7. Hearing Loss
8. Liver and Gastrointestinal Tract Symptoms
9. Cardiac Manifestation
10. Rheumatological Manifestations
11. Soft Tissue Involvement
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grever, M.R.; Abdel-Wahab, O.; Andritsos, L.A.; Banerji, V.; Barrientos, J.; Blachly, J.S.; Call, T.G.; Catovsky, D.; Dearden, C.; Demeter, J.; et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood 2017, 129, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Matutes, E.; Catovsky, D.; Zinzani, P.L.; Buske, C.; ESMO Guidelines Committee. Hairy cell leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v100–v107. [Google Scholar] [CrossRef] [PubMed]
- Zakarija, A.; Peterson, L.C.; Tallman, M.S. Chapter 84-Hairy cell leukaemia. In Hematology: Basic Principles and Practice, 5th ed.; Hoffman, R., Benz, E.J., Shatil, S.J., Furie, B., Silberstein, L.E., McGlave, P., Heslop, H., Eds.; Churchill livingStone/Elsevier: Philadelphia, PA, USA, 2009; pp. 1349–1358. [Google Scholar]
- Puła, A.; Robak, T. Hairy cell leukemia: A brief update on current knowledge and treatment prospects. Curr. Opin. Oncol. 2021, 33, 412–419. [Google Scholar] [CrossRef]
- Gupta, R.; Yadav, S.; Mittal, N.; Rahman, K.; Sharma, A.; Gupta, A.; Nityan, S. Clinicopathological spectrum of hairy-cell leukemia: A single-center study with brief review of Indian literature. J. Cancer Res. Ther. 2020, 16, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Bosma, M.; Bartels, M. Hairy cell leukemia in a child?! Blood 2018, 132, 1216. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Tiacci, E.; Liso, A.; Basso, K.; Sabattini, E.; Pacini, R.; Foa, R.; Pulsoni, A.; Dalla Favera, R.; Pileri, S. Simple diagnostic assay for hairy cell leukaemia by immunocytochemical detection of annexin A1 (ANXA1). Lancet 2004, 363, 1869–1870. [Google Scholar] [CrossRef]
- Tiacci, E.; Trifonov, V.; Schiavoni, G.; Holmes, A.; Kern, W.; Martelli, M.P.; Pucciarini, A.; Bigerna, B.; Pacini, R.; Wells, V.A.; et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 2011, 364, 2305–2315. [Google Scholar] [CrossRef]
- Pattnaik, S.A.; Padhi, S.; Chhabra, G.; Panigrahi, M.K.; Das, P.K.; Bhola, R.K.; Mishra, S. Atypical presentation of hairy cell leukemia: A report and comprehensive review. Blood Res. 2020, 55, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Polliack, A. Hairy cell leukemia: Biology, clinical diagnosis, unusual manifestations and associated disorders. Rev. Clin. Exp. Hematol. 2002, 6, 366–388. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T.; Polliack, A. Hairy cell leukemia: Uncommon clinical features, unusual sites of involvement and some rare associations. Best Pract. Res. Clin. Haematol. 2015, 28, 193–199. [Google Scholar] [CrossRef]
- Rahman, K.; Kumari, S.; Singh, M.K.; Gupta, R.; Yadav, G.; Kumari, N.; Nityan, S. Atypical presentation of hairy cell leukemia: Significance of CD200 on flow cytometry. J. Cancer Res. Ther. 2018, 14, 1130–1134. [Google Scholar] [CrossRef]
- Troussard, X. Unusual clinical presentations of hairy cell leukemia. Acta Haematol. 2024, 147, 465–466. [Google Scholar] [CrossRef]
- Robak, E.; Jesionek-Kupnicka, D.; Robak, T. Skin changes in hairy cell leukemia. Ann. Hematol. 2021, 100, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Finan, M.C.; Su, W.P.; Li, C.Y. Cutaneous findings in hairy cell leukemia. J. Am. Acad. Dermatol. 1984, 11 Pt 1, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Robak, E.; Jesionek-Kupnicka, D.; Iskierka-Jazdzewska, E.; Janus, A.; Robak, T. Cutaneous leukocytoclastic vasculitis at diagnosis of hairy cell leukemia successfully treated with vemurafenib and rituximab. Leuk. Res. 2021, 104, 106571. [Google Scholar] [CrossRef] [PubMed]
- Robak, E.; Braun, M.; Robak, T. Leukemia cutis—The current view on pathogenesis, diagnosis, and treatment. Cancers 2023, 15, 5393. [Google Scholar] [CrossRef]
- Cho-Vega, J.H.; Medeiros, L.J.; Prieto, V.G.; Vega, F. Leukemia cutis. Am. J. Clin. Pathol. 2008, 129, 130–142. [Google Scholar] [CrossRef]
- Parsi, M.; Go, M.S.; Ahmed, A. Leukemia Cutis. [Updated 17 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541136/ (accessed on 2 August 2024).
- Lawrence, D.M.; Sun, N.C.; Mena, R.; Moss, R. Cutaneous lesions in hairy-cell leukemia. Case report and review of the literature. Arch. Dermatol. 1983, 119, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.R.; Flanagan, N.G.; Kelsey, P.R. Severe skin rash in two consecutive patients treated with 2-chlorodeoxyadenosine for hairy cell leukaemia at a single institution. Clin. Lab. Haematol. 2000, 22, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Gandolfi, L.; Pellegrini, C.; Agostinelli, C.; Argnani, L.; Zinzani, P.L. Leukocytoclastic vasculitis associated with hairy cell leukemia at diagnosis: A case report and review of the literature. Tumori 2016, 102 (Suppl. S2), S124–S127. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Conn, D.L.; Phyliky, R.L.; Scott, R.E. Vasculitis in hairy cell leukaemia: Review of literature and consideration of possible pathogenic mechanisms. J. Rheumatol. 1986, 13, 1167–1172. [Google Scholar] [PubMed]
- Fain, O.; Hamidou, M.; Cacoub, P.; Godeau, B.; Wechsler, B.; Pariès, J.; Stirnemann, J.; Morin, A.S.; Gatfosse, M.; Hanslik, T.; et al. Vasculitides associated with malignancies: Analysis of sixty patients. Arthritis Care Res. 2007, 57, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Moyers, J.T.; Liu, L.W.; Ossowski, S.; Goddard, L.; Kamal, M.O.; Cao, H. A rash in a hairy situation: Leukocytoclastic vasculitis at presentation of hairy cell Leukemia. Am. J. Hematol. 2019, 94, 1433–1434. [Google Scholar] [CrossRef]
- Hasler, P.; Kistler, H.; Gerber, H. Vasculitides in hairy cell leukemia. Semin. Arthritis Rheum. 1995, 25, 134–142. [Google Scholar] [CrossRef]
- Ventura, F.; Rocha, J.; Pereira, T.; Marques, H.; Pardal, F.; Brito, C. Sweet syndrome as the presenting symptom of hairy cell leukemia. Dermatol. Online J. 2009, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Junkins-Hopkins, J.M.; Turchi, J.J.; James, W.D. Sweet syndrome as the presenting symptom of relapsed hairy cell leukemia. Arch. Dermatol. 2002, 138, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Cailly, L.; Gruchet, C.; Maitre, E.; Guidez, S.; Delwail, V.; Systchenko, T.; Moya, N.; Sabirou, F.; Levy, A.; Bobin, A.; et al. Hairy cell leukemia with isolated bone lesions. Clin. Case Rep. 2023, 11, e7343. [Google Scholar] [CrossRef]
- Quesada, J.R.; Keating, M.J.; Libshitz, H.I.; Llamas, L. Bone involvement in hairy cell leukemia. Am. J. Med. 1983, 74, 228–231. [Google Scholar] [CrossRef]
- Robak, P.; Jesionek-Kupnicka, D.; Kupnicki, P.; Polliack, A.; Robak, T. Bone lesions in hairy cell leukemia: Diagnosis and treatment. Eur. J. Haematol. 2020, 105, 682–691. [Google Scholar] [CrossRef]
- Yonal-Hindilerden, I.; Hindilerden, F.; Bulut-Dereli, S.; Yıldız, E.; Dogan, I.O.; Nalcaci, M. Hairy cell leukemia presenting with isolated skeletal involvement successfully treated by radiation therapy and cladribine: A case report and review of the literature. Case Rep. Hematol. 2015, 2015, 803921. [Google Scholar] [CrossRef]
- Fasulo, S.M.; Narvaneni, S.; Kumar, V.; Manje Gowda, A.; Sultana, Y. Lytic Bone Lesion: An Unusual presentation of hairy cell leukemia. Cureus 2021, 13, e12959. [Google Scholar] [CrossRef] [PubMed]
- Notarfranchi, L.; Russo, F.; Re, F.; Mancini, C.; Martella, E.; Falini, B.; Aversa, F.; Tiacci, E. Hairy cell leukaemia mimicking multiple myeloma. Lancet Oncol. 2019, 20, e187. [Google Scholar] [CrossRef]
- VanderMolen, L.A.; Urba, W.J.; Longo, D.L.; Lawrence, J.; Gralnick, H.; Steis, R.G. Diffuse osteosclerosis in hairy cell leukemia. Blood 1989, 74, 2066–2069. [Google Scholar] [CrossRef] [PubMed]
- Snell, K.S.; O’Brien, M.M.; Sendelbach, K.; Martino, R. Pathologic fracture occurring 22 years after diagnosis of hairy cell leukemia: Case report and literature review. West. J. Med. 1999, 170, 172–174. [Google Scholar] [PubMed]
- Hudson, J.; Cobby, M.; Yates, P.; Watt, I. Extensive infiltration of bone with marrow necrosis in a case of hairy cell leukaemia. Skeletal Radiol. 1995, 24, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Lembersky, B.C.; Ratain, M.J.; Golomb, H.M. Skeletal complications in hairy cell leukaemia: Diagnosis and therapy. J. Clin. Oncol. 1988, 6, 1280–1284. [Google Scholar] [CrossRef]
- Filippi, A.R.; Franco, P.; Marinone, C.; Tarella, C.; Ricardi, U. Treatment options in skeletal localizations of hairy cell leukemia: A systematic review on the role of radiation therapy. Am. J. Hematol. 2007, 82, 1017–1021. [Google Scholar] [CrossRef]
- Lal, A.; Tallman, M.S.; Soble, M.B.; Golubovich, I.; Peterson, L. Hairy cell leukemia presenting as localized skeletal involvement. Leuk. Lymphoma 2002, 43, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Karmali, R.; Farhat, M.; Leslie, W.; McIntire, M.G.; Gregory, S. Localized bone disease as a presentation of hairy cell leukemia. Clin. Adv. Hematol. Oncol. 2008, 6, 290–294. [Google Scholar] [PubMed]
- Robak, P.; Jesionek-Kupnicka, D.; Kupnicki, P.; Polliack, A.; Robak, T. Multifocal osteolytic lesions in hairy cell leukemia-the importance of PET/CT in diagnosis and assessment. Ann. Hematol. 2021, 100, 1641–1645. [Google Scholar] [CrossRef]
- Gray, M.T.; Rutherford, M.N.; Bonin, D.M.; Patterson, B.; Lopez, P.G. Hairy-cell leukemia presenting as lytic bone lesions. J. Clin. Oncol. 2013, 31, e410–e412. [Google Scholar] [CrossRef] [PubMed]
- Spedini, P.; Tajana, M.; Bergonzi, C. Unusual presentation of hairy cell leukemia. Haematologica 2000, 85, 548. [Google Scholar] [PubMed]
- Vardiman, J.W.; Golomb, H.M. Autopsy findings in hairy cell leukemia. Semin. Oncol. 1984, 11, 370–380. [Google Scholar] [PubMed]
- Williams, J.P.; Arcement, C.M.; Wong, R.; Robinson, A.E. Diffuse pulmonary disease associated with hairy cell leukemia. Acad. Radiol. 1995, 2, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Fields, B.N.; Harris, N.L. A 50-year-old man with hairy cell leukemia, recurrent fever, and variable pulmonary infiltrates. N. Engl. J. Med. 1982, 307, 1693–1700. [Google Scholar] [CrossRef]
- Hansen, T.; Constantin, C.; Weber, M.; Titze, U.; Hartmann, F. Bronchoalveolar lavage in hairy cell leukemia with pulmonary infiltration. Pathologe 2019, 40, 529–533. [Google Scholar] [CrossRef]
- Uzunhan, Y.; Cadranel, J.; Boissel, N.; Gardin, C.; Arnulf, B.; Bergeron, A. Lung involvement in lymphoid and lympho-plasmocytic proliferations (except lymphomas). Rev. Mal. Respir. 2010, 27, 599–610. [Google Scholar] [CrossRef]
- Ecsiova, D.; Kamaradova, K.; Nova, M.; Hoffmann, P.; Rozsivalova, P.; Simkovic, M.; Zak, P. Pulmonary damage in a patient with hairy cell leukemia-infectious involvement or hematological disease activity? Case report. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2023, 167, 385–389. [Google Scholar] [CrossRef]
- Lemiale, V.; Meignin, V.; Azoulay, É. Hairy cell leukemia with pulmonary infiltrates. In Pulmonary Involvement in Patients with Hematological Malignancies; Azoulay, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Kimmel, D.W.; Hermann, R.C., Jr.; O’Neill, B.P. Neurologic complications of hairy cell 176 leukemia. Arch. Neurol. 1984, 41, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Plenderleith, I.H. Hairy cell leukemia. Can. Med. Assoc. J. 1970, 102, 1056–1060. [Google Scholar] [PubMed]
- Wolfe, D.W.; Scopelliti, J.A.; Boselli, B.D. Leukemic meningitis in a patient with hairy cell leukemia. A case report. Cancer 1984, 54, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Knecht, H.; Budmiger, H.; Groscurth, P.; Streuli, R.A. Central nervous system involvement 182 in hairy cell leukemia. Klin. Wochenschr. 1985, 63, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Le Bezu, M.; Pinaudeau, Y.; Poirier, J.; Dreyfus, B. Involvement of the nervous system in hairy cell leukemia. Arch. Neurol. 1985, 42, 839. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, D.; Bodega, E. Leukemic meningitis in a patient with hairy cell leukemia. A 186 case report. Nouv. Rev. Fr. Hematol. 1987, 29, 247–249. [Google Scholar] [PubMed]
- Chandana, S.R.; Kotecha, R.; Al-Janadi, A.; Chang, H.T.; Conley, B.A. Rare case of hairy cell leukemia with brain parenchymal involvement: A diagnostic dilemma. J. Clin. Oncol. 2013, 31, e186–e188. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.M.; Zhu, X.; Agarwal, N.; Nikiforova, M.N.; Lieberman, F.S.; Drappatz, J. Response of relapsed central nervous system hairy cell leukemia to vemurafenib. Leuk. Lymphoma 2016, 57, 2952–2954. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.M.; Matsuda, K.; Wadhwa, V.; Hewitt, D.; Almiski, M.; Johnston, J.B.; Banerji, V. Multifocal brain involvement in a patient with hairy cell leukemia successfully treated with rituximab and cladribine. Blood Adv. 2017, 1, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.S.; Smith, S.; Gurbuxani, S.; Yamini, B. Extranodal hairy cell leukemia presenting in the lumbar spine. J. Neurosurg. Spine 2008, 9, 374–376. [Google Scholar] [CrossRef]
- Claves, F.; Carras, S.; Burroni, B.; Maitre, E.; Boutonnat, J.; Troussard, X.; Molina, L. Atypical meningeal localization of classical hairy cell leukemia with an impressive response to rituximab and cladribine association. A case report and literature review. eJHaem 2024, 5, 242–246. [Google Scholar] [CrossRef]
- Johnson, A.E.; Raju, A.R.; Jacob, A.; Hildebrandt, G.C. Case report: A case of classic hairy cell leukemia with CNS involvement treated with vemurafenib. Front. Oncol. 2023, 12, 1100577. [Google Scholar] [CrossRef]
- Robak, T.; Puła, A.; Braun, M.; Robak, E. Extramedullary and extranodal manifestations in chronic lymphocytic leukemia-an update. Ann. Hematol. 2024, 103, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Harada, K.; Uchino, Y.; Hirano, K.; Sekiguchi, N. A rare clinical case of secondary central nervous system involvement without transformation in hairy cell leukemia: A case report and literature review. Acta Haematol. 2024, 147, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Dietrich, S. Hairy Cell Leukemia. In Indolent Lymphomas. Hematologic Malignancies; Dreyling, M., Ladetto, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 179–194. [Google Scholar] [CrossRef]
- Ng, M.H.; Tsang, S.S.; Ng, H.K.; Sriskandavarman, V.; Feng, C.S. An unusual case of hairy cell leukemia: Death due to leukostasis and intracerebral hemorrhage. Hum. Pathol. 1991, 22, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Dinçol, G.; Kahraman, R. Cryptococcus neoformans meningitis in a patient with hairy cell leukemia. Am. J. Hematol. 2006, 81, 387. [Google Scholar] [CrossRef]
- Jiang, K.; Punja, K.G.; Hyrcza, M.D.; Simpson, S.M. Beyond the norm: Unusual orbital manifestation of hairy cell leukemia. Can. J. Ophthalmol. 2024, 59, e285–e287. [Google Scholar] [CrossRef] [PubMed]
- Deitch, R.D.; Wilson, F.M. Leukemic reticuloendotheliosis with presenting ocular complaints: Report of acase. Arch. Ophthalmol. 1963, 69, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Eting, E.; Zeidman, A.; Djaldetti, M.; Mittelman, M.; Savir, H. Ocular manifestation of hairy cell leukemia with dramatic response to 2-chlorodeoxyadenosine. Am. J. Ophthalmol. 1996, 121, 97–99. [Google Scholar] [CrossRef]
- Charalel, R.; Jain, A.; Rachakonda, L.; Gaynon, M. Visualdis-turbance as initial presentation of hairy cell leukemia. Eur. J. Ophthalmol. 2009, 19, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Di Maria, A.; Redaelli, C.; Canevari, A.; Pagnucco, G.; Martinetti, M.; Bianchi, P.E. Unilateral retinal vasculitis associated with hairy cell leukaemia: Immunogenetic study. Ophthalmologica 1998, 212, 355–357. [Google Scholar] [CrossRef]
- Davies, R. Limbal nodules in Sweet’s syndrome. Aust. N. Z. J. Ophthalmol. 1992, 20, 263–265. [Google Scholar] [CrossRef]
- Coupland, S.E.; Krause, L.; Delecluse, H.J.; Anagnostopoulos, I.; Foss, H.D.; Hummel, M.; Bornfeld, N.; Lee, W.R.; Stein, H. Lymphoproliferative lesions of the ocular adnexa: Analysis of 112 cases. Ophthalmology 1998, 105, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Zeidman, A.; Floru, S.; Robinson, A.; Polliack, A.; Djaldeti, M.; Savir, H.; Mittelman, M. Panuveitis responsive to 2-CdA: An unusual ocular presentation of hairy cell leukemia. Leuk. Lymphoma 1996, 20, 501–503. [Google Scholar] [CrossRef] [PubMed]
- North, V.S.; Jamerson, E.C.; Plum, W.; Tran, A.Q.; Kazim, M. Spontaneous subperiosteal orbital hematoma as a presenting sign of hairy cell leukemia in a patient with a long-standing orbital implant. Can. J. Ophthalmol. 2023, 58, e189–e191. [Google Scholar] [CrossRef] [PubMed]
- Bertram, B.; Schulte, K.; Wolf, S.; Glöckner, W.M.; Reim, M. Retinopathy as the first symptom of hairy cell leukemia. Klin. Monatsbl. Augenheilkd. 1991, 199, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Bertram, B.; Schulte, K.; Wolf, S.; Glöckner, W.M. A visual disorder as the initial symptom of hairy cell leukemia. Dtsch. Med. Wochenschr. 1991, 116, 157. [Google Scholar] [PubMed]
- Zák, P.; Chrobák, L.; Podzimek, K.; Hejcmanová, D.; Voglová, J.; Dulícek, P.; Mirová, S. An unusual course in hairy-cell leukemia with marked abdominal lymphadenopathy, leukemic infiltration of the cornea and skin changes. Vnitr. Lek. 1996, 42, 463–466. [Google Scholar] [PubMed]
- Hejcmanová, D.; Abulgasim, H.A.; Jebavá, R.; Chrobák, L.; Podzimek, K.; Zák, P. Leukemické infiltráty rohovky u nemocné leukémiís “vlasatými bunkami” [Leukemic infiltration of the cornea in a patient with hairy-cell leukemia]. Cesk Slov. Oftalmol. 1995, 51, 152–155. [Google Scholar] [PubMed]
- Sattar, M.A.; Cawley, M.I.D. Arthritis associated with hairy cell leukaemia. Ann. Rheum. Dis. 1982, 41, 289–391. [Google Scholar] [CrossRef] [PubMed]
- Harden, E.A.; Moore, J.O.; Haynes, R.F. Leukaemia associated arthritis: Indentification of leukaemic cells in synovial fluid using monoclonal and polyclonal antibodies. Arthritis Rheum. 1984, 27, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Crofts, M.A.; Sharp, J.C.; Joyner, M.Y. Rheumatoid arthritis and hairy-cell leukaemia. Lancet 1979, 314, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, C.A.; Golde, D.W. Autoimmune disease in hairy-cell leukaemia: Clinical syndromes and treatment. Br. J. Haematol. 1985, 61, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Facchini, A.; Mariani, E.; Ferrolli, A.; Mariani, A.R.; Gobbi, M.; Zizzi, F.; Frizziero, L. Hairy cell leukemia and rheumatoid arthritis: Cause or effect. Arthritis Rheum. 1981, 24, 1587. [Google Scholar] [CrossRef]
- Anil, V.; Ganipisetti, V.; Harisinghani, K.; Kanugula, A.K. Hairy cell leukemia (HCL) presenting with joint swelling: Case report and literature review of a rare rheumatological manifestation of a hematological disease. Cureus 2023, 15, e35912. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.G.; Davis, M.J.; Hothersall, T.E. Hairy cell leukaemia and rheumatoid arthritis. Br. J. Rheumatol. 1991, 30, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Cathey, M.A.; Hawley, D.J. Recombinant gamma interferon in rheumatoid arthritis. Arthritis Rheum. 1986, 30, S18. [Google Scholar]
- Raimbourg, J.; Cormier, G.; Stéphane, V.; Tanguy, G.; Bleher, Y.; Maisonneuve, H. Hairy-cell leukemia with inaugural joint manifestations. Jt. Bone Spine 2009, 76, 416–420. [Google Scholar] [CrossRef]
- L’Hirondel, J.L.; Troussard, X.; Macro, M.; Courtheoux, F.; Guaydier-Souquières, G.; Mandard, J.C.; Loyau, G. Polyarthrite révélatrice d’une leucémie à tricholeucocytes. Rev. Rhum. Mal. Osteoartic. 1992, 59, 749. [Google Scholar] [PubMed]
- Robak, T. Hairy-cell leukemia variant: Recent view on diagnosis, biology and treatment. Cancer Treat. Rev. 2011, 37, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Troussard, X.; Maitre, E. Untangling hairy cell leukaemia (HCL) variant and other HCL-like disorders: Diagnosis and treatment. J. Cell Mol. Med. 2024, 28, e18060. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Leticia Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Grever, M.; Andritsos, L.; Anghelina, M.; Arons, E.; Banerji, V.; Barrientos, J.; Bhat, S.A.; Blachly, J.; Broccoli, A.; Call, T.; et al. Hairy cell leukemia variant and WHO classification correspondence Re: 5th edition WHO classification haematolymphoid tumors: Lymphoid neoplasms. Leukemia 2024, 38, 1642–1644. [Google Scholar] [CrossRef] [PubMed]
- Janus, A.; Robak, T. Hairy Cell Leukemia. In Leukemia [Internet]; Li, W., Ed.; Exon Publications: Brisbane, Australia, 2022; Volume 3, pp. 33–52. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Purohit, A.; Aggarwal, M.; Manivannan, P.; Tyagi, S.; Mahapatra, M.; Pati, H.P.; Saxena, R. Unusual presentation of hairy cell leukemia: A case series of four clinically unsuspected cases. Indian J. Hematol. Blood Transfus. 2014, 30 (Suppl. S1), 413–417. [Google Scholar] [CrossRef]
- Falini, B.; De Carolis, L.; Tiacci, E. How I treat refractory/relapsed hairy cell leukemia with BRAF inhibitors. Blood 2022, 139, 2294–2305. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Robak, P. Refractory and relapsed hairy-cell leukemia (HCL): Casting light on promising experimental drugs in clinical trials. Expert Opin. Investig. Drugs 2023, 32, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J. Hairy cell leukemia: Present and future directions. Leuk. Lymphoma 2019, 60, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.P.; Newman, G.; Saperia, D. Pyoderma gangrenosum and hairy cell leukemia. J. Dermatol. Surg. Oncol. 1987, 1, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Lad, D.; Prakash, G.; Khadwal, A.; Malhotra, P.; Bal, A.; Mallik, N.; Kumar, N.; Varma, S. Bullous pyoderma gangrenosum associated with hairy cell leukemia and its complete response to cladribine therapy. Indian. J. Hematol. Blood Transfus. 2017, 33, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Tombak, A.; Aygun, S.; Serinsoz, E.; Tiftik, E.N. Complete recovery of pyoderma gangrenosum after successful treatment of underlying hairy cell leukemia with cladribine. Korean J. Intern. Med. 2015, 30, 739–741. [Google Scholar] [CrossRef]
- Elkon, K.B.; Hughes, G.R.; Catovsky, D.; Clauvel, J.P.; Dumont, J.; Seligmann, M.; Tannenbaum, H.; Esdaile, J. Hairy-cell leukaemia with polyarteritis nodosa. Lancet 1979, 2, 280–282. [Google Scholar] [CrossRef]
- Zervas, J.; Vayopoulos, G.; Kaklamanis, P.H.; Zerva, C.H.; Pfessas, P.H. Hairy-cell leukaemia-associated polyarthritis: A report of two cases. Br. J. Rheumatol. 1991, 30, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.J.; Wittich, G.R.; Schwarzinger, I.; Haller, J.; Chott, A.; Mostbeck, G.; Hajek, P.C. Skeletal involvement in hairy cell leukemia. Skelet. Radiol. 1988, 17, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Doma, A.; Škerget, M.; Žagar, I. 18F-FDG PET/CT for staging and evaluation of therapy in a patient with unusual hairy cell leukemia presentation. Clin. Nucl. Med. 2019, 44, e458–e460. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Vardiman, J.; Golomb, H. Disseminated atypical mycobacterial infection in patients with hairy cell leukemia. Am. J. Med. 1986, 80, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.A.; Golomb, H.M.; Grumet, G.; Gelmann, E.; Schechter, G.P. Hairy cell leukemia: Association with disseminated atypical mycobacterial infection. Cancer 1981, 48, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Stanton, D.J.; Quadri, N.Z.; Tanabe, M.B. Concomitantly diagnosed disseminated M kansasii infection and hairy cell leukemia with review of pathophysiology. J. Investig. Med. High. Impact Case Rep. 2024, 12, 23247096241253343. [Google Scholar] [CrossRef]
- Schlick, K.; Troch, M.; Placher-Sorko, G.; Faber, V.; Neureiter, D.; Berghoff, A.S.; Preusser, M.; Greil, R.; Hopfinger, G. A patient diagnosed with BRAF-mutated non-small cell lung cancer and hairy cell leukemia: At last, which entity is really carrying the BRAF mutation? Ann. Hematol. 2015, 94, 345–346. [Google Scholar] [CrossRef]
- Mitsogianni, M.; Mitsimponas, N.; Crespo, F.; Hartmann, K.A.; Klosterhalfen, B.; Haase, S.; Giagounidis, A. Concomitant non-small cell lung cancer and hairy cell leukemia in a patient harboring BRAF-V600E mutation in both tissues: A case report. Case Rep. Oncol. 2018, 11, 109–113. [Google Scholar] [CrossRef]
- AlEnazi, A.; Alhedaithy, R.; Alfayez, A.; Alghonaim, Y. Acute profound sensorineural hearing loss as the initial manifestation of hairy cell leukemia, case report and literature review. Int. J. Surg. Case Rep. 2019, 60, 200–203. [Google Scholar] [CrossRef]
- Nageris, B.; Or, R.; Hardan, I.; Polliack, A. Sudden onset deafness as a presenting manifestation of chronic lymphocytic leukemia. Leuk. Lymphoma 1993, 9, 269–271. [Google Scholar] [CrossRef]
- Genden, E.M.; Bahadori, R.S. Bilateral sensorineural hearing loss as a first symptom of chronic myelogenous leukemia. Otolaryngol. Head. Neck Surg. 1995, 113, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Acar, G.O.; Acioğlu, E.; Enver, O.; Ar, C.; Sahin, S. Unilateral sudden hearing loss as the first sign of chronic myeloid leukemia. Eur. Arch. Otorhinolaryngol. 2007, 264, 1513–2516. [Google Scholar] [CrossRef] [PubMed]
- Babakhanlou, R.; Nader, M.E.; Alvarado, Y. A case of sudden hearing loss in a patient with chronic myelomonocytic leukemia. Ann. Hematol. 2023, 102, 3427–3430. [Google Scholar] [CrossRef]
- Naraev, B.G.; Linthicum, F.H., Jr. Rapid hearing loss in chronic lymphocytic leukemia. Otol. Neurotol. 2006, 27, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Yam, L.T.; Janckila, A.J.; Chan, C.H.; Li, C.Y. Hepatic involvement in hairy cell leukemia. Cancer 1983, 51, 1497–1504. [Google Scholar] [CrossRef]
- Dhanesar, G.K.; Livingston, J.; Maroules, M.; Lee, S.H. Hairy cell leukemia: Hepatic affinity status post splenectomy. Cureus 2023, 15, e39830. [Google Scholar] [CrossRef] [PubMed]
- Sahar, N.; Schiby, G.; Davidson, T.; Kneller, A.; Apter, S.; Farfel, Z. Hairy cell leukemia presenting as multiple discrete hepatic lesions. World J. Gastroenterol. 2009, 15, 4453–4456. [Google Scholar] [CrossRef] [PubMed]
- Al-Saheli, Z.I.; Bazzi, T.; Barthel, B. Oncological management dilemma: A rare presentation of hairy cell leukaemia with hepatic involvement presenting concomitantly with pancreatic adenocarcinoma. BMJ Case Rep. 2022, 15, e252423. [Google Scholar] [CrossRef]
- Sen, P.; Shaaban, H.; Modi, T.; Kumar, A.; Guron, G. Hairy Cell Leukemia Presenting with Duodenal Involvement Duodenum: A Case Report. N. Am. J. Med. Sci. 2015, 7, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Bin Hamid, M.A.; Rahman, N.; Oleary, L.; Wong, K.; Sehbai, A. Unusual presentation of gastric cancer during treatment of hairy cell leukemia: Exploring the etiological basis of this rare phenomenon. Cancer Pathog. Ther. 2023, 1, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Iwashige, A.; Hirosawa, M.; Tsukada, J. Hairy cell leukemia presenting with progressive pericarditis and pleuritis. Ann. Hematol. 2018, 97, 2527–2528. [Google Scholar] [CrossRef]
- Koczwara, B.; Spangenthal, E.; Bernstein, S.H. The development of congestive cardiac failure in a patient with hairy cell leukemia treated with 2-chlorodeoxyadenosine. Leuk. Lymphoma 1997, 26, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.A.; Lowenstein, J.M. Inhibition of phosphorylation of troponin in rat heart by adenosine and 5-chloro-5-deoxyadenosine. Biochem. Pharmacol. 1991, 42, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, C.; Dubashi, B.; Rekha, J.S.; Basu, D.; Jain, A.; Dutta, T.K. Hairy cell leukemia masquerading as infective endocarditis. Indian. J. Hematol. Blood Transfus. 2013, 29, 84–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sucak, G.T.; Haznedar, R.; Yalçin, R. Reversible cardiomyopathy in a patient with hairy cell leukaemia. Postgrad. Med. J. 1998, 74, 313–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, L.A.; Engels, E.A. Autoimmune conditions and hairy cell leukemia: An exploratory case-control study. J. Hematol. Oncol. 2010, 3, 35. [Google Scholar] [CrossRef]
- Pilichowska, M.; Shariftabrizi, A.; Mukand-Cerro, I.; Miller, K. Primary hairy cell leukemia/lymphoma of the breast: A case report and review of the literature. Case Rep. Pathol. 2014, 2014, 497027. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Neupane, R.; Thorburn, C.; Chisti, M.; Jamil, L.H. Hairy cell leukemia: A rare differential for a pancreatic mass. Am. J. Gastroenterol. 2023, 118, 1562–1563. [Google Scholar] [CrossRef]
| Symptoms | Etiology | Clinical Characteristics | Diagnostic Procedures | References |
|---|---|---|---|---|
| Skin changes | Leukemic infiltration, autoimmune reactions, infections, secondary neoplasms and drug-induced symptoms | Disseminated erythematous maculopapules and nodules, vasculitis, neutrophilic dermatoses and periarteritis nodosa | Skin biopsy | [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] |
| Bone lesions | Leukemic skeletal infiltrations | Localized pain, multifocal osteolytic and osteoblastic lesions and severe osteoporosis | X-rays, MRI, CT, PET and core biopsy | [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] |
| Pulmonary changes | Leukemic infiltration, mediastinal infiltrations and infections | Pulmonary symptoms: cough, dyspnea, chest pain and hemoptysis | Chest X-rays, CT and lung biopsy if antibiotics and antifungal treatment are not effective | [45,46,47,48,49,50,51] |
| Neurologic manifestations | Leukemic infiltration and infections | Confusion, aphasia, headache, meningeal syndrome, motor ataxia, dizziness, weakness, slurred speech, fatigue and blurry vision | Imaging studies (NMR, CT, PET) and lumbar puncture | [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] |
| Ocular symptoms | Leukemic infiltration, bleeding and infections | Ocular or orbital manifestations, panuveitis, conjunctivitis, leukemic corneal infiltration, retinopathy and visual disturbance | Ophthalmological examination, CT and biopsy of ocular mass | [69,70,71,72,73,74,75,76,77,78,79,80,81] |
| Rheumatological symptoms | Immune-mediated or direct, non-immune mechanism | Athritis, joint pain and swelling, erythema and tenderness | Serologic tests, X-rays and cytologic evaluation of synovial fluid | [82,83,84,85,86,87,88,89,90,91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robak, T.; Braun, M.; Janus, A.; Guminska, A.; Robak, E. Rare Clinical Symptoms in Hairy Cell Leukemia: An Overview. Cancers 2024, 16, 3054. https://doi.org/10.3390/cancers16173054
Robak T, Braun M, Janus A, Guminska A, Robak E. Rare Clinical Symptoms in Hairy Cell Leukemia: An Overview. Cancers. 2024; 16(17):3054. https://doi.org/10.3390/cancers16173054
Chicago/Turabian StyleRobak, Tadeusz, Marcin Braun, Agnieszka Janus, Anna Guminska, and Ewa Robak. 2024. "Rare Clinical Symptoms in Hairy Cell Leukemia: An Overview" Cancers 16, no. 17: 3054. https://doi.org/10.3390/cancers16173054
APA StyleRobak, T., Braun, M., Janus, A., Guminska, A., & Robak, E. (2024). Rare Clinical Symptoms in Hairy Cell Leukemia: An Overview. Cancers, 16(17), 3054. https://doi.org/10.3390/cancers16173054







