The Role of Amide Proton Transfer (APT)-Weighted Imaging in Glioma: Assessment of Tumor Grading, Molecular Profile and Survival in Different Tumor Components
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Molecular Analysis
2.2.1. MGMT
2.2.2. IDH
2.3. MRI Acquisition
- 3D T1-weighted sequence after administration of contrast medium (Gadovist, gadobutrol 1 mmol/mL, Bayer AG®, Leverkusen, Germany) fast-field-echo (FFE): Repetition time/echo time (TR/TE) = 9.93 ms/4.5 ms; flip angle (FA) = 8°; slice thickness = 1 mm; no gap = 1 mm; matrix = 240 × 240 mm; field of view (FOV) = 240 × 240 mm;
- 3D FLAIR sequence: TR/TE = 4800 ms/333 ms; TI = 1650 ms; slice thickness = 1 mm; no gaps; matrix = 240 × 240 mm; FOV = 240 × 240 mm;
- APT: 3D TSE DIXON sequence, saturation radiofrequency (RF) pulse duration 2.0 s; B1 power 2.0 µT; 40 sync Gaussian pulses each of 50 ms; 7 off-resonance saturation pulses: ±3.1, ±3.5, ±3.9 and −1560 ppm (S0); 9 slices; FOV 212 × 183 × 40 mm; matrix = 116 × 116 reconstructed 224 × 224; voxel size 1.8 × 1.8 × 4.4 mm; TR = 3825 ms; TE = 6.2 ms; FA = 90°; refocusing angle 120°; Echo train length (ETL) 181; Spectral presaturation with inversion recovery (SPIR) fat suppression. Total acquisition time was 4 min and 30 s.
2.4. Image Procesing and Analysis
2.5. Statistical Analysis
3. Results
3.1. Between-Subject Variability of Mean APT Values in Tumor Compartments and Other Tissues
3.2. Correlation of APT-Derived Statistical Parameters with Tumor and Patient Characteristics
3.2.1. “Lesion” ROI
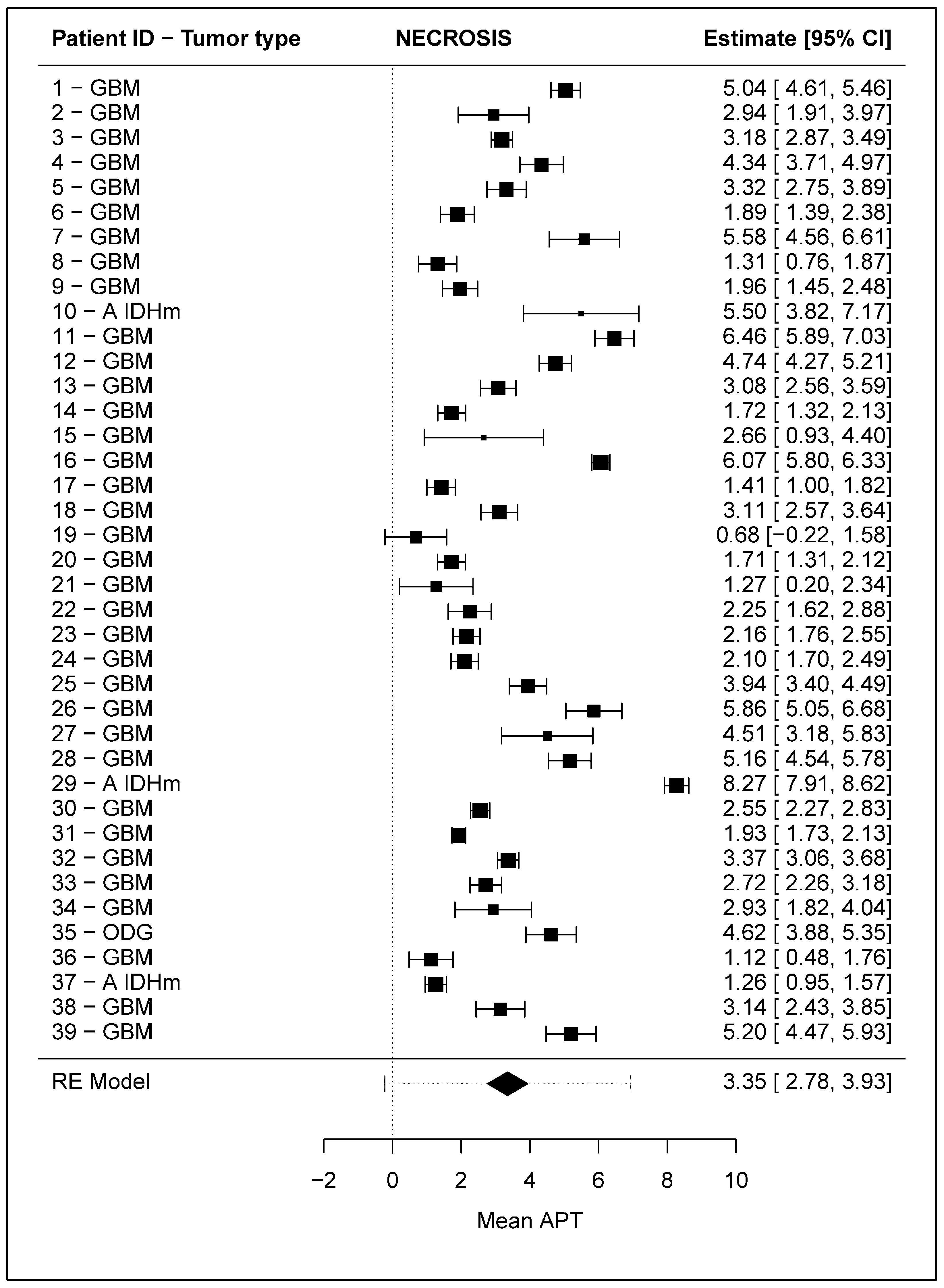
3.2.2. “Necrosis” ROI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Payen, J.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C.M. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Van Zijl, P.C.M.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reason. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Nakajo, M.; Yoneyama, T.; Takumi, K.; Kumagae, Y.; Fukukura, Y.; Yoshiura, T. Amide proton transfer imaging of tumors: Theory, clinical applications, pitfalls, and future directions. Jpn. J. Radiol. 2019, 37, 109–116. [Google Scholar] [CrossRef]
- Wen, Z.; Hu, S.; Huang, F.; Wang, X.; Guo, L.; Quan, X.; Wang, S.; Zhou, J. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. NeuroImage 2010, 51, 616–622. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, H.; Lim, M.; Blair, L.; Quinones-Hinojosa, A.; Messina, S.A.; Eberhart, C.G.; Pomper, M.G.; Laterra, J.; Barker, P.B.; et al. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J. Magn. Reason. Imaging 2013, 38, 1119–1128. [Google Scholar] [CrossRef]
- Su, C.; Liu, C.; Zhao, L.; Jiang, J.; Zhang, J.; Li, S.; Zhu, W.; Wang, J. Amide proton transfer imaging allows detection of glioma grades and tumor proliferation: Comparison with Ki-67 expression and proton MR spectroscopy imaging. AJNR Am. J. Neuroradiol. 2017, 38, 1702–1709. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Park, K.J.; Choi, C.G.; Kim, S.J. Histogram analysis of amide proton transfer imaging to identify contrast-enhancing low-grade brain tumor that mimics high-grade tumor: Increased accuracy of MR perfusion. Radiology 2015, 277, 151–161. [Google Scholar] [CrossRef]
- Togao, O.; Hiwatashi, A.; Yamashita, K.; Kikuchi, K.; Keupp, J.; Yoshimoto, K.; Kuga, D.; Yoneyama, M.; Suzuki, S.O.; Iwaki, T.; et al. Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: Comparisons with diffusion- and perfusion-weighted imaging. Eur. Radiol. 2017, 27, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Paech, D.; Dreher, C.; Regnery, S.; Meissner, J.-E.; Goerke, S.; Windschuh, J.; Oberhollenzer, J.; Schultheiss, M.; Deike-Hofmann, K.; Bickelhaupt, S.; et al. Relaxation-compensated amide proton transfer (APT) MRI signal intensity is associated with survival and progression in high-grade glioma patients. Eur. Radiol. 2019, 29, 4957–4967. [Google Scholar] [CrossRef]
- Joo, B.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kang, S.-G.; Kim, S.H.; Zhou, J.; Lee, S.-K. Amide proton transfer imaging might predict survival and IDH mutation status in high-grade glioma. Eur. Radiol. 2019, 29, 6643–6652. [Google Scholar] [CrossRef]
- Koike, H.; Morikawa, M.; Ishimaru, H.; Ideguchi, R.; Uetani, M.; Miyoshi, M. Amide Proton Transfer-Chemical Exchange Saturation Transfer Imaging of Intracranial Brain Tumors and Tumor-like Lesions: Our Experience and a Review. Diagnostics 2023, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Kloosterhof, N.K.; Bralten, L.B.; Dubbink, H.J.; French, P.J.; van den Bent, M.J. Isocitrate dehydrogenase-1 mutations: A fundamentally new understanding of diffuse glioma? Lancet Oncol. 2011, 12, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.; Heo, H.-Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH mutation status in grade-II gliomas using amide proton transfer-weighted (APTw) MRI. Magn. Reason. Med. 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, T.; Guo, M.; Duan, Y.; Zhuo, Z.; Weng, J.; Xie, C.; Sun, J.; Li, J.; Cheng, D.; et al. Amide proton transfer-weighted imaging and derived radiomics in the classification of adult-type diffuse gliomas. Eur. Radiol. 2024, 34, 2986–2996. [Google Scholar] [CrossRef]
- Jiang, S.; Rui, Q.; Wang, Y.; Heo, H.-Y.; Zou, T.; Yu, H.; Zhang, Y.; Wang, X.; Du, Y.; Wen, X.; et al. Discriminating MGMT Promoter Methylation Status in Patients with Glioblastoma Employing Amide Proton Transfer-Weighted MRI Metrics. Eur. Radiol. 2018, 28, 2115–2123. [Google Scholar] [CrossRef]
- Paech, D.; Windschuh, J.; Oberhollenzer, J.; Dreher, C.; Sahm, F.; Meissner, J.-E.; Goerke, S.; Schuenke, P.; Zaiss, M.; Regnery, S.; et al. Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neuro-Oncology 2018, 20, 1661–1671. [Google Scholar] [CrossRef]
- Esteller, M.; Hamilton, S.R.; Burger, P.C.; Baylin, S.B.; Herman, J.G. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999, 59, 793–797. [Google Scholar]
- van de Ven, K.; Keupp, J. Amide proton transfer weighted imaging: Advancement in molecular tumor diagnosis. Philips White Pap. Ser. 2018. Available online: https://www.documents.philips.com/assets/20180614/1fa1f9ad18a74c2f9388a8ff008b3dcc.pdf (accessed on 20 August 2024).
- Zhou, J.; Zaiss, M.; Knutsson, L.; Sun, P.Z.; Ahn, S.S.; Aime, S.; Bachert, P.; Blakeley, J.O.; Cai, K.; Chappell, M.A.; et al. Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors. Magn. Reason. Med. 2022, 88, 546–574. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Hedges, L.V.; Vevea, J.L. Fixed-and Random-Effects Models in Meta-Analysis. Psychol. Methods 1998, 3, 486–504. [Google Scholar] [CrossRef]
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- Cochran, W.G. Some Methods for Strengthening the Common χ2 Tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved Tests for a Random Effects Meta-Regression with a Single Covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for Routinely Presenting Prediction Intervals in Meta-Analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, J.E.; Jung, S.C.; Jo, Y.; Kim, D.; Kim, H.S.; Choi, C.-G.; Kim, S.J.; Kang, D.-W. Repeatability of amide proton transfer-weighted signals in the brain according to clinical condition and anatomical location. Eur. Radiol. 2020, 30, 346–356. [Google Scholar] [CrossRef]
- Wu, Y.; Wood, T.C.; Derks, S.H.A.E.; Pruis, I.J.; van der Voort, S.; van Zanten, S.E.M.V.; Smits, M.; Warnert, E.A.H. Reproducibility of APT-weighted CEST MRI at 3T in healthy brain and tumor across sessions and scanners. Sci. Rep. 2023, 13, 18115. [Google Scholar] [CrossRef]
- Choi, Y.S.; Ahn, S.S.; Lee, S.-K.; Chang, J.H.; Kang, S.-G.; Kim, S.H.; Zhou, J. Amide proton transfer imaging to discriminate between low- and high-grade gliomas: Added value to apparent diffusion coefficient and relative cerebral blood volume. Eur. Radiol. 2017, 27, 3181–3189. [Google Scholar] [CrossRef]
- Jiang, S.; Eberhart, C.G.; Zhang, Y.; Heo, H.-Y.; Wen, Z.; Blair, L.; Qin, H.; Lim, M.; Quinones-Hinojosa, A.; Weingart, J.D.; et al. Amide proton transfer-weighted magnetic resonance image-guided stereotactic biopsy in patients with newly diagnosed gliomas. Eur. J. Cancer 2017, 83, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lin, Y.; Zhang, W.; Kong, L.; Wang, L.; Zuo, P.; Vallines, I.; Schmitt, B.; Tian, J.; Song, X.; et al. Noninvasive amide proton transfer magnetic resonance imaging in evaluating the grading and cellularity of gliomas. Oncotarget 2016, 8, 5834–5842. [Google Scholar] [CrossRef]
- Togao, O.; Yoshiura, T.; Keupp, J.; Hiwatashi, A.; Yamashita, K.; Kikuchi, K.; Suzuki, Y.; Suzuki, S.O.; Iwaki, T.; Hata, N.; et al. Amide proton transfer imaging of adult diffuse gliomas: Correlation with histopathological grades. Neuro Oncol. 2014, 16, 441–448. [Google Scholar] [CrossRef]
- Zou, T.; Yu, H.; Jiang, C.; Wang, X.; Jiang, S.; Rui, Q.; Mei, Y.; Zhou, J.; Wen, Z. Differentiating the histologic grades of gliomas preoperatively using amide proton transfer-weighted (APTW) and intravoxel incoherent motion MRI. NMR Biomed. 2018, 31, e3850. [Google Scholar] [CrossRef] [PubMed]
- Sakata, A.; Okada, T.; Yamamoto, A.; Kanagaki, M.; Fushimi, Y.; Okada, T.; Dodo, T.; Arakawa, Y.; Schmitt, B.; Miyamoto, S.; et al. Grading glial tumors with amide proton transfer MR imaging: Different analytical approaches. J. Neurooncol. 2015, 122, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.-W.; Xi, Y.-B.; Liu, T.-T.; Wang, N.; Zhu, Y.-Q.; Wang, X.-R.; Guo, F. Grading of glioma: Combined diagnostic value of amide proton transfer weighted, arterial spin labelling and diffusion weighted magnetic resonance imaging. BMC Med. Imaging 2020, 20, 50. [Google Scholar] [CrossRef]
- Xu, Z.; Ke, C.; Liu, J.; Xu, S.; Han, L.; Yang, Y.; Qian, L.; Liu, X.; Zheng, H.; Lv, X.; et al. Diagnostic performance between MR amide proton transfer (APT) and diffusion kurtosis imaging (DKI) in glioma grading and IDH mutation status prediction at 3T. Eur. J. Radiol. 2021, 134, 109466. [Google Scholar] [CrossRef]
- Guo, H.; Liu, J.; Hu, J.; Zhang, H.; Zhao, W.; Gao, M.; Zhang, Y.; Yang, G.; Cui, Y. Diagnostic performance of gliomas grading and IDH status decoding A comparison between 3D amide proton transfer APT and four diffusion-weighted MRI models. J. Magn. Reason. Imaging 2022, 56, 1834–1844. [Google Scholar] [CrossRef]
- Mostafa, M.A.; Abo-Elhoda, P.M.; Abdelrahman, A.S.; Elzoghby, A.M.; Elmahdy, M.M.; Abbas, Y.A. The added value of relative amide proton transfer (rAPT) to advanced multiparametric MR imaging for brain glioma characterization. Egypt. J. Radiol. Nucl. Med. 2023, 54, 182. [Google Scholar] [CrossRef]
- Sotirios, B.; Demetriou, E.; Topriceanu, C.C.; Zakrzewska, Z. The role of APT imaging in gliomas grading: A systematic review and meta-analysis. Eur. J. Radiol. 2020, 133, 109353. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Urisman, A.; Oses-Prieto, J.A.; Arnott, D.; Burlingame, A.L. Quantitative Proteomics Reveals Fundamental Regulatory Differences in Oncogenic HRAS and Isocitrate Dehydrogenase (IDH1) Driven Astrocytoma. Mol. Cell. Proteom. 2017, 16, 39–56. [Google Scholar] [CrossRef]
- Solomou, G.; Finch, A.; Asghar, A.; Bardella, C. Mutant IDH in gliomas: Role in Cancer and Treatment Options. Cancers 2023, 15, 2883. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, T.; Ma, W.; Wang, Y. Clinical strategies to manage adult glioblastoma patients without MGMT hypermethylation. J. Cancer 2022, 13, 354–363. [Google Scholar] [CrossRef]
- Zhou, J.; Heo, H.Y.; Knutsson, L.; van Zijl, P.C.M.; Jiang, S. APT-Weighted MRI: Techniques, Current Neuro Applications, and Challenging Issues. J. Magn. Reason. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Yan, K.; Zhu, H. A simple model for understanding the origin of the amide proton transfer MRI signal in tissue. Appl. Magn. Reason. 2012, 42, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.S.; Milbach, N.; Hurrell, S.L.; Cochran, E.; Connelly, J.; Bovi, J.A.; Schultz, C.J.; Mueller, W.M.; Rand, S.D.; Schmainda, K.M.; et al. Progressing Bevacizumab-Induced Diffusion Restriction Is Associated with Coagulative Necrosis Surrounded by Viable Tumor and Decreased Overall Survival in Patients with Recurrent Glioblastoma. AJNR Am. J. Neuroradiol. 2016, 37, 2201–2208. [Google Scholar] [CrossRef]
- Zakhari, N.; Taccone, M.; Torres, C.; Chakraborty, S.; Sinclair, J.; Woulfe, J.; Jansen, G.; Nguyen, T.B. Diagnostic Accuracy of Centrally Restricted Diffusion in the Differentiation of Treatment-Related Necrosis from Tumor Recurrence in High-Grade Gliomas. AJNR Am. J. Neuroradiol. 2018, 39, 260–264. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Xie, Y.-F.; Chang, X.; Zhang, L.; Wu, H.-Y.; Liu, J.-B.; Zhang, J.-X.; Sun, P. Type of Necrosis Influences Prognosis in Hepatocellular Carcinoma After the First Transarterial Chemoembolization. Med. Sci. Monit. 2021, 27, e929884. [Google Scholar] [CrossRef]
- Brat, D.J.; Van Meir, E.G. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab. Investig. 2004, 84, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.Y.; Zhang, Y.; Jiang, S.; Lee, D.H.; Zhou, J. Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semisolid magnetization transfer reference (EMR) signals: II. Comparison of three EMR models and application to human brain glioma at 3 T. Magn. Reason. Med. 2016, 75, 1630–1639. [Google Scholar]
- Zaiss, M.; Windschuh, J.; Paech, D.; Meissner, J.-E.; Burth, S.; Schmitt, B.; Kickingereder, P.; Wiestler, B.; Wick, W.; Bendszus, M.; et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage 2015, 112, 180–188. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Value |
|---|---|
| Age, median (range) years | 56 (23–76) |
| Sex (M/F) | 45/16 |
| WHO grade (2/3/4) | 3/10/48 |
| Tumor volume, median (range) mm3 | 30, 187 (67–167, 636) |
| IDH mutation (Yes/No) | 15/46 |
| MGMT promoter methylation (Yes/No) 1 | 34/25 |
| Survival status after 1 year (Alive/Dead) | 39/22 |
| Survival status after 2 years (Alive/Dead) 2 | 19/38 |
| APT Parameter | WHO Grade | IDH Status | MGMT Promoter Status | |||
|---|---|---|---|---|---|---|
| 2–3 (n = 13) | 4 (n = 48) | Mutant (n = 15) | Wildtype (n = 46) | Methylated (n = 34) | Unmethylated (n = 25) | |
| Mean | 1.58 * (0.50) | 2.04 * (0.56) | 1.69 (0.55) | 2.02 (0.57) | 1.91 (0.53) | 1.93 (0.63) |
| Median | 1.55 * (0.52) | 1.99 * (0.57) | 1.65 (0.54) | 1.98 (0.58) | 1.85 (0.53) | 1.90 (0.64) |
| 10th percentile | 0.74 * (0.29) | 1.08 * (0.42) | 0.79 (0.31) | 1.08 (0.43) | 0.99 (0.39) | 1.01 (0.45) |
| 90th percentile | 2.41 * (0.72) | 3.07 * (0.84) | 2.64 (0.89) | 3.03 (0.83) | 2.91 (0.84) | 2.88 (0.87) |
| Skewness | 0.35 (0.46) | 0.27 (0.46) | 0.41 (0.49) | 0.25 (0.44) | 0.37 (0.48) | 0.20 (0.43) |
| Kurtosis | 3.45 (1.17) | 3.03 (0.63) | 3.43 (1.16) | 3.01 (0.60) | 3.21 (0.90) | 3.03 (0.61) |
| APT Parameter | Survival Status after 1 Year | Survival Status after 2 Years | Overall Survival | |||
|---|---|---|---|---|---|---|
| Alive (n = 39) | Dead (n = 22) | Alive (n = 19) | Dead (n = 38) | HR (95% CI) | p-Value | |
| Mean | 1.81 * (0.58) | 2.17 * (0.51) | 1.78 (0.61) | 2.01 (0.54) | 1.55 (0.95–2.54) | 0.081 |
| Median | 1.76 * (0.58) | 2.13 * (0.51) | 1.74 (0.63) | 1.96 (0.54) | 1.55 (0.94–2.54) | 0.085 |
| 10th percentile | 0.93 (0.44) | 1.15 (0.34) | 0.89 (0.42) | 1.04 (0.38) | 1.54 (0.76–3.11) | 0.227 |
| 90th percentile | 2.74 * (0.84) | 3.27 * (0.79) | 2.73 (0.92) | 3.04 (0.79) | 1.33 (0.98–1.82) | 0.070 |
| Skewness | 0.31 (0.42) | 0.25 (0.53) | 0.31 (0.43) | 0.26 (0.48) | 0.90 (0.44–1.84) | 0.768 |
| Kurtosis | 3.02 (0.84) | 3.29 (0.66) | 3.10 (1.05) | 3.14 (0.66) | 1.13 (0.83–1.53) | 0.444 |
| Factor | Overall Survival (Univariate) | Overall Survival (Multivariate) | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| APT Mean | 0.86 (0.51–1.46) | 0.576 | - | - | |
| APT Median | 0.89 (0.53–1.48) | 0.640 | - | - | |
| APT 10th percentile | 0.71 (0.35–1.43) | 0.335 | - | - | |
| APT 90th percentile | 0.94 (0.66–1.33) | 0.709 | - | - | |
| APT Skewness | 0.99 (0.46–2.11) | 0.977 | - | - | |
| APT Kurtosis | 1.66 (1.07–2.56) | 0.023 * | 1.60 (1.02–2.52) | 0.040 * | |
| Surgery type | Biopsy (n = 11) | ref | - | ref | - |
| GTR (n = 23) | 0.31 (0.13–0.72) | 0.006 * | 0.29 (0.12–0.68) | 0.005 * | |
| Partial (n = 13) | 0.90 (0.39–2.08) | 0.810 | 0.76 (0.32–1.81) | 0.536 | |
| Age at surgery | 1.02 (0.99–1.05) | 0.256 | - | - | |
| APT Parameter | MGMT Promoter Status | Survival Status after 1 Year | Survival Status after 2 Years | |||
|---|---|---|---|---|---|---|
| Methylated (n = 19) | Unmethylated (n = 18) | Alive (n = 21) | Dead (n = 18) | Alive (n = 9) | Dead (n = 28) | |
| Mean | 3.69 (1.89) | 3.13 (1.67) | 3.05 (1.79) | 3.72 (1.73) | 3.63 (2.38) | 3.28 (1.56) |
| Median | 3.72 (1.92) | 3.13 (1.69) | 3.06 (1.81) | 3.74 (1.77) | 3.64 (2.39) | 3.29 (1.59) |
| 10th percentile | 3.20 (1.85) | 2.75 (1.65) | 2.70 (1.79) | 3.19 (1.70) | 3.30 (2.38) | 2.81 (1.51) |
| 90th percentile | 4.15 (1.97) | 3.51 (1.67) | 3.41 (1.81) | 4.22 (1.74) | 3.95 (2.36) | 3.75 (1.61) |
| Skewness | −0.24 (0.54) | 0.01 (0.42) | −0.10 (0.47) | −0.12 (0.52) | −0.08 (0.53) | −0.12 (0.50) |
| Kurtosis | 2.55 (0.62) | 2.40 (0.75) | 2.48 (0.71) | 2.41 (0.65) | 2.41 (0.57) | 2.51 (0.71) |
| APT Parameter | Overall Survival | |
|---|---|---|
| HR (95% CI) | p-Value | |
| Mean | 0.98 (0.81–1.19) | 0.828 |
| Median | 0.98 (0.81–1.18) | 0.823 |
| 10th percentile | 0.95 (0.77–1.16) | 0.600 |
| 90th percentile | 1.01 (0.84–1.21) | 0.932 |
| Skewness | 0.90 (0.44–1.83) | 0.768 |
| Kurtosis | 1.14 (0.71–1.83) | 0.578 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges de Almeida, G.; Pascuzzo, R.; Mambrin, F.; Aquino, D.; Verri, M.; Moscatelli, M.; Del Bene, M.; DiMeco, F.; Silvani, A.; Pollo, B.; et al. The Role of Amide Proton Transfer (APT)-Weighted Imaging in Glioma: Assessment of Tumor Grading, Molecular Profile and Survival in Different Tumor Components. Cancers 2024, 16, 3014. https://doi.org/10.3390/cancers16173014
Borges de Almeida G, Pascuzzo R, Mambrin F, Aquino D, Verri M, Moscatelli M, Del Bene M, DiMeco F, Silvani A, Pollo B, et al. The Role of Amide Proton Transfer (APT)-Weighted Imaging in Glioma: Assessment of Tumor Grading, Molecular Profile and Survival in Different Tumor Components. Cancers. 2024; 16(17):3014. https://doi.org/10.3390/cancers16173014
Chicago/Turabian StyleBorges de Almeida, Gonçalo, Riccardo Pascuzzo, Francesca Mambrin, Domenico Aquino, Mattia Verri, Marco Moscatelli, Massimiliano Del Bene, Francesco DiMeco, Antonio Silvani, Bianca Pollo, and et al. 2024. "The Role of Amide Proton Transfer (APT)-Weighted Imaging in Glioma: Assessment of Tumor Grading, Molecular Profile and Survival in Different Tumor Components" Cancers 16, no. 17: 3014. https://doi.org/10.3390/cancers16173014
APA StyleBorges de Almeida, G., Pascuzzo, R., Mambrin, F., Aquino, D., Verri, M., Moscatelli, M., Del Bene, M., DiMeco, F., Silvani, A., Pollo, B., Grisoli, M., & Doniselli, F. M. (2024). The Role of Amide Proton Transfer (APT)-Weighted Imaging in Glioma: Assessment of Tumor Grading, Molecular Profile and Survival in Different Tumor Components. Cancers, 16(17), 3014. https://doi.org/10.3390/cancers16173014






