Using mHealth Technology to Evaluate Daily Symptom Burden among Adult Survivors of Childhood Cancer: A Feasibility Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Collection
2.3. PRO Assessment
2.4. Satisfaction Survey
2.5. Covariates
2.6. Statistical Analysis
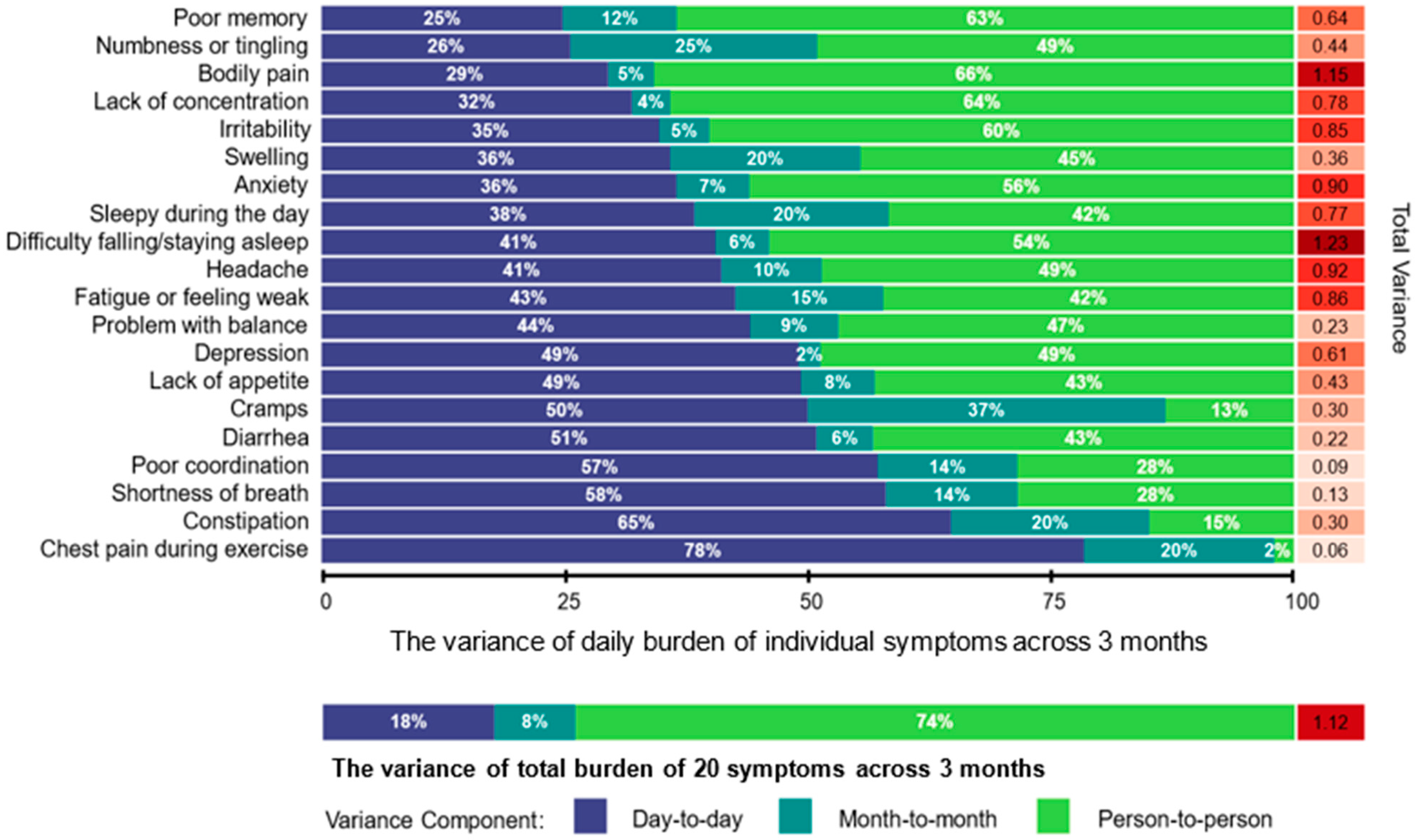
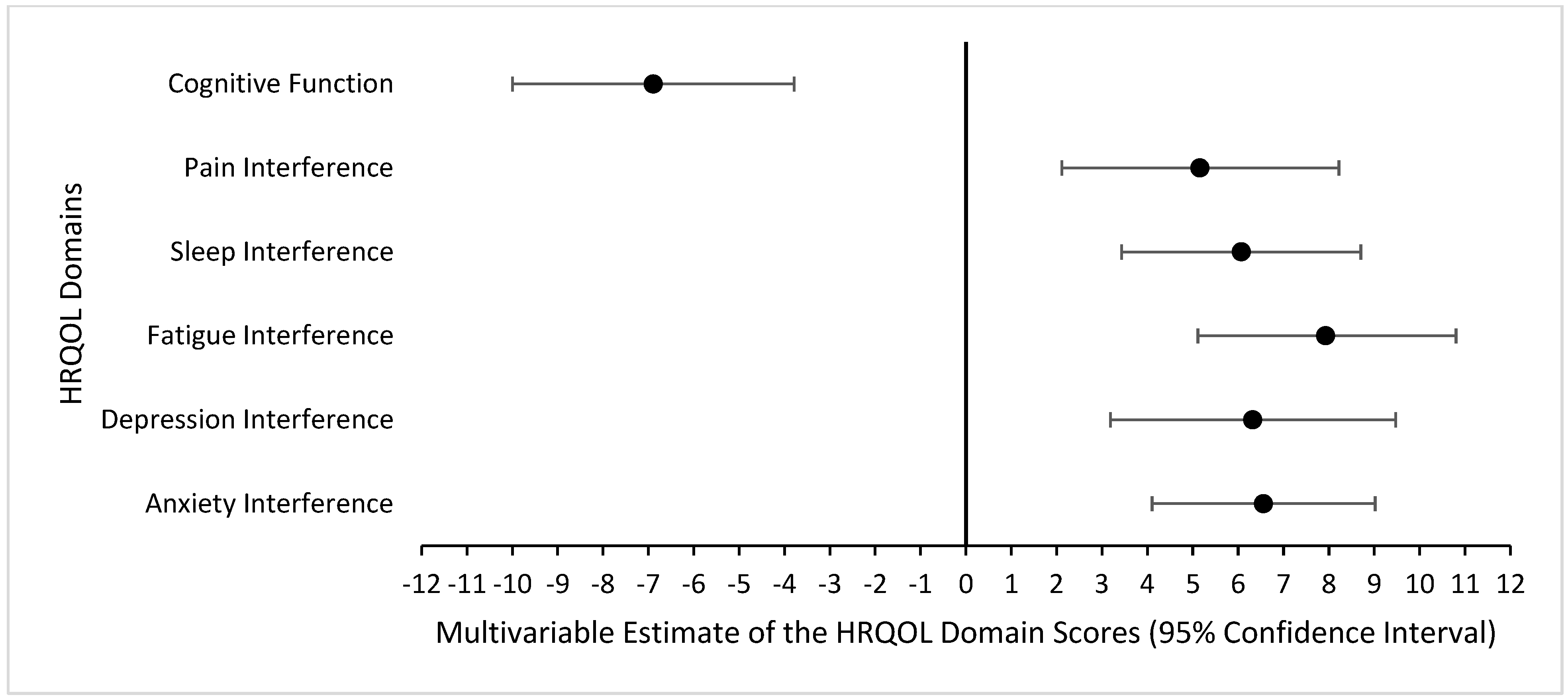
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, N.; Liu, Q.; Ness, K.K.; Baassiri, M.; Eissa, H.; Yeo, F.; Chemaitilly, W.; Ehrhardt, M.J.; Bass, J.; Bishop, M.W.; et al. The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017, 390, 2569–2582. [Google Scholar] [CrossRef]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L.; Neglia, J.P. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2010, 102, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.C.; Brinkman, T.M.; Kenzik, K.; Gurney, J.G.; Ness, K.K.; Lanctot, J.; Shenkman, E.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort study. J. Clin. Oncol. 2013, 31, 4242–4251. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.C.; Brinkman, T.M.; Armstrong, G.T.; Leisenring, W.; Robison, L.L.; Krull, K.R. Emotional distress impacts quality of life evaluation: A report from the Childhood Cancer Survivor Study. J. Cancer Surviv. 2017, 11, 309–319. [Google Scholar] [CrossRef]
- Shin, H.; Dudley, W.N.; Bhakta, N.; Horan, M.R.; Wang, Z.; Bartlett, T.R.; Srivastava, D.; Yasui, Y.; Baker, J.N.; Robison, L.L.; et al. Associations of Symptom Clusters and Health Outcomes in Adult Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2023, 41, 497–507. [Google Scholar] [CrossRef]
- Yeh, J.M.; Nekhlyudov, L.; Goldie, S.J.; Mertens, A.C.; Diller, L. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann. Intern. Med. 2010, 152, 409–417. [Google Scholar] [CrossRef]
- Xiao, C.; Polomano, R.; Bruner, D.W. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs. 2013, 36, E1–E16. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.F.; Aaronson, N.K.; Choucair, A.K.; Elliott, T.E.; Greenhalgh, J.; Halyard, M.Y.; Hess, R.; Miller, D.M.; Reeve, B.B.; Santana, M. Implementing patient-reported outcomes assessment in clinical practice: A review of the options and considerations. Qual. Life Res. 2012, 21, 1305–1314. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef]
- Petersen, C. Patient-generated health data: A pathway to enhanced long-term cancer survivorship. J. Am. Med. Inform. Assoc. 2016, 23, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Jim, H.S.L.; Hoogland, A.I.; Brownstein, N.C.; Barata, A.; Dicker, A.P.; Knoop, H.; Gonzalez, B.D.; Perkins, R.; Rollison, D.; Gilbert, S.M.; et al. Innovations in research and clinical care using patient-generated health data. CA Cancer J. Clin. 2020, 70, 182–199. [Google Scholar] [CrossRef]
- Phillips, K.M.; Faul, L.A.; Small, B.J.; Jacobsen, P.B.; Apte, S.M.; Jim, H.S. Comparing the retrospective reports of fatigue using the Fatigue Symptom Index with daily diary ratings in women receiving chemotherapy for gynecologic cancer. J. Pain Symptom Manag. 2013, 46, 282–288. [Google Scholar] [CrossRef]
- Mobile Fact Sheet; Pew Research Center: Washington, DC, USA, 2019. Available online: https://www.pewresearch.org/internet/fact-sheet/mobile/ (accessed on 22 August 2024).
- Krebs, P.; Duncan, D.T. Health App Use Among US Mobile Phone Owners: A National Survey. JMIR mHealth uHealth 2015, 3, e101. [Google Scholar] [CrossRef]
- Carlson, E.B.; Field, N.P.; Ruzek, J.I.; Bryant, R.A.; Dalenberg, C.J.; Keane, T.M.; Spain, D.A. Advantages and psychometric validation of proximal intensive assessments of patient-reported outcomes collected in daily life. Qual. Life Res. 2016, 25, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Stone, A.A. Ambulatory and diary methods can facilitate the measurement of patient-reported outcomes. Qual. Life Res. 2016, 25, 497–506. [Google Scholar] [CrossRef]
- Abelson, J.S.; Kaufman, E.; Symer, M.; Peters, A.; Charlson, M.; Yeo, H. Barriers and benefits to using mobile health technology after operation: A qualitative study. Surgery 2017, 162, 605–611. [Google Scholar] [CrossRef]
- Atienza, A.A.; Zarcadoolas, C.; Vaughon, W.; Hughes, P.; Patel, V.; Chou, W.-Y.S.; Pritts, J. Consumer Attitudes and Perceptions on mHealth Privacy and Security: Findings From a Mixed-Methods Study. J. Health Commun. 2015, 20, 673–679. [Google Scholar] [CrossRef]
- Ancker, J.; Silver, M.; Miller, M.; Kaushal, R. Consumer experience with and attitudes toward health information technology: A nationwide survey. J. Am. Med. Inform. Assoc. 2012, 20, 152–156. [Google Scholar] [CrossRef][Green Version]
- Williams, M.T.; Lewthwaite, H.; Fraysse, F.; Gajewska, A.; Ignatavicius, J.; Ferrar, K. Compliance with Mobile Ecological Momentary Assessment of Self-Reported Health-Related Behaviors and Psychological Constructs in Adults: Systematic Review and Meta-analysis. J. Med. Internet Res. 2021, 23, e17023. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Remmerswaal, D.; Verveer, I.; Robinson, E.; Franken, I.H.A.; Wen, C.K.F.; Field, M. Compliance with ecological momentary assessment protocols in substance users: A meta-analysis. Addiction 2019, 114, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.F.; Jensen, S.E.; Schalet, B.D.; Beaumont, J.L.; Amtmann, D.; Czajkowski, S.; Dewalt, D.A.; Fries, J.F.; Pilkonis, P.A.; Reeve, B.B.; et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J. Clin. Epidemiol. 2016, 73, 89–102. [Google Scholar] [CrossRef]
- Gershon, R.C.; Lai, J.S.; Bode, R.; Choi, S.; Moy, C.; Bleck, T.; Miller, D.; Peterman, A.; Cella, D. Neuro-QOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Qual. Life Res. 2012, 21, 475–486. [Google Scholar] [CrossRef]
- Clark, R.A.; Mostoufi-Moab, S.; Yasui, Y.; Vu, N.K.; Sklar, C.A.; Motan, T.; Brooke, R.J.; Gibson, T.M.; Oeffinger, K.C.; Howell, R.M.; et al. Predicting acute ovarian failure in female survivors of childhood cancer: A cohort study in the Childhood Cancer Survivor Study (CCSS) and the St Jude Lifetime Cohort (SJLIFE). Lancet Oncol. 2020, 21, 436–445. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Statistical Software; Release 14.2; StataCorp LLC: College Station, TX, USA, 2016. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statitical Computing: Vienna, Austria, 2023. [Google Scholar]
- Team, P. RStudio: Integrated Development Environment for R.; Posit Software PBC: Boston, MA, USA, 2023. [Google Scholar]
- Burkett, V.S.; Cleeland, C.S. Symptom burden in cancer survivorship. J. Cancer Surviv. 2007, 1, 167–175. [Google Scholar] [CrossRef]
- Koo, M.M.; Swann, R.; McPhail, S.; Abel, G.A.; Elliss-Brookes, L.; Rubin, G.P.; Lyratzopoulos, G. Presenting symptoms of cancer and stage at diagnosis: Evidence from a cross-sectional, population-based study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef]
- Collaborative, N.E. Patient-Generated Health Data Technical Expert Panel: Final Report. 2013. Available online: https://www.healthit.gov/sites/default/files/pghi_tep_finalreport121713.pdf (accessed on 22 August 2024).
- Chung, A.E.; Basch, E.M. Potential and challenges of patient-generated health data for high-quality cancer care. J. Oncol. Pract. 2015, 11, 195–197. [Google Scholar] [CrossRef][Green Version]
- Weissmann, J.; Mueller, A.; Messinger, D.; Parkin, C.G.; Amann-Zalan, I. Improving the Quality of Outpatient Diabetes Care Using an Information Management System: Results From the Observational VISION Study. J. Diabetes Sci. Technol. 2015, 10, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, A.D.; Pfeiffer, S.M.; Wilson, C. Cancer-related cognitive impairment in children. Curr. Opin. Support. Palliat. Care 2017, 11, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Docking, K.M.; Knijnik, S.R. Prospective longitudinal decline in cognitive-communication skills following treatment for childhood brain tumor. Brain Inj. 2021, 35, 1472–1479. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | N (%) or Mean (SD; Min, Max) |
|---|---|
| Age at study enrollment (in years) | 34.0 (5.3; 25.7, 47.1) |
| Age at study enrollment (%) | |
| 18–29.9 years | 10 (24) |
| 30–39.9 years | 24 (59) |
| ≥40 years | 7 (17) |
| Time since cancer diagnosis (in years) | 26.1 (3.7; 19.5, 32.4) |
| Time since cancer diagnosis (%) | |
| 10–19 years | 3 (7) |
| 20–29 years | 28 (68) |
| ≥30 years | 10 (24) |
| Sex (%) | |
| Male | 14 (34) |
| Female | 27 (66) |
| Race/Ethnicity (%) | |
| White non-Hispanic | 30 (73) |
| Black non-Hispanic | 11 (27) |
| Educational Attainment | |
| High school/GED or less | 6 (15) |
| Some college or post-high school training | 21 (51) |
| College graduate or post-graduate level | 14 (34) |
| Cancer diagnosis (%) | |
| Central nervous system tumor | 15 (37) |
| Acute lymphoblastic leukemia | 8 (20) |
| Wilms tumor | 5 (12) |
| Non-Hodgkin lymphoma | 5 (12) |
| Hodgkin lymphoma | 2 (5) |
| Neuroblastoma | 2 (5) |
| Rhabdomyosarcoma | 2 (5) |
| Osteosarcoma | 1 (2) |
| Ewing sarcoma | 1 (2) |
| Cancer Treatment | |
| Chemotherapy (%) | 25 (61) |
| Radiation (%) | 11 (27) |
| Invasive surgery (%) | 32 (78) |
| PROMIS-29 Profile reported in Month 3 | |
| Anxiety Interference | 56.3 (9.6; 40.3, 73.4) |
| Depression Interference | 55.7 (10.4; 41.0, 79.3) |
| Fatigue Interference | 54.5 (11.8; 33.7, 75.8) |
| Sleep Interference | 56.0 (9.5; 36.9, 73.3) |
| Pain Interference | 54.0 (10.9; 41.6, 75.6) |
| Neuro-QOL reported in Month 3 | |
| Cognitive Function | 47.1 (11.2; 22.8, 64.2) |
| Questions | Strongly Agree/Agree | Neutral | Strongly Disagree/Disagree |
|---|---|---|---|
| It was easy for me to complete brief daily symptom evaluations (e.g., a few days in a week) over the past 3 months | 35 (89.7%) | 2 (5.1%) | 2 (5.1%) |
| I would be willing to take part in symptom evaluations on a regular basis to help doctors understand more | 35 (89.7%) | 3 (7.7%) | 1 (2.6%) |
| I would be willing to take part in symptom evaluations 2–3 times per day to help doctors learn the symptom changes on a daily basis | 26 (66.7%) | 9 (23.1%) | 4 (10.3%) |
| I am interested in taking part in a clinical trial to help doctors use my symptom data for advancing treatment strategies | 31 (79.5%) | 8 (20.5%) | 0 (0%) |
| In future studies, I would be interested in receiving a report after my symptom evaluations are done | 32 (82.1%) | 5 (12.8%) | 2 (5.1%) |
| I am interested in discussing problematic symptoms with my oncologists or primary care physicians | 30 (76.9%) | 9 (23.1%) | 0 (0%) |
| I am interested in learning skills for self-managing my problematic symptoms | 34 (87.2%) | 5 (12.8%) | 0 (0%) |
| I believe that effective symptom control may improve my quality of life | 31 (79.5%) | 7 (17.9%) | 1 (2.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, K.E.; Baedke, J.L.; Bagherzadeh, F.; McDonald, A.; Nathan, P.C.; Ness, K.K.; Hudson, M.M.; Armstrong, G.T.; Yasui, Y.; Huang, I.-C. Using mHealth Technology to Evaluate Daily Symptom Burden among Adult Survivors of Childhood Cancer: A Feasibility Study. Cancers 2024, 16, 2984. https://doi.org/10.3390/cancers16172984
Howell KE, Baedke JL, Bagherzadeh F, McDonald A, Nathan PC, Ness KK, Hudson MM, Armstrong GT, Yasui Y, Huang I-C. Using mHealth Technology to Evaluate Daily Symptom Burden among Adult Survivors of Childhood Cancer: A Feasibility Study. Cancers. 2024; 16(17):2984. https://doi.org/10.3390/cancers16172984
Chicago/Turabian StyleHowell, Kristen E., Jessica L. Baedke, Farideh Bagherzadeh, Aaron McDonald, Paul C. Nathan, Kirsten K. Ness, Melissa M. Hudson, Gregory T. Armstrong, Yutaka Yasui, and I-Chan Huang. 2024. "Using mHealth Technology to Evaluate Daily Symptom Burden among Adult Survivors of Childhood Cancer: A Feasibility Study" Cancers 16, no. 17: 2984. https://doi.org/10.3390/cancers16172984
APA StyleHowell, K. E., Baedke, J. L., Bagherzadeh, F., McDonald, A., Nathan, P. C., Ness, K. K., Hudson, M. M., Armstrong, G. T., Yasui, Y., & Huang, I.-C. (2024). Using mHealth Technology to Evaluate Daily Symptom Burden among Adult Survivors of Childhood Cancer: A Feasibility Study. Cancers, 16(17), 2984. https://doi.org/10.3390/cancers16172984







