Mitochondrial VDAC1 Silencing in Urethane-Induced Lung Cancer Inhibits Tumor Growth and Alters Cancer Oncogenic Properties
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Cell Culture
2.3. si-RNA Transfection
2.4. Preparation of si-RNA Loaded–PLGA-PEI Nanoparticles
2.5. Peptide Solution Preparation
2.6. Urethane-Induced Lung Tumor Mice Model
2.7. MRI Tumor Monitoring
2.8. Xenograft Mouse Model
2.9. Immunohistochemistry and Immunofluorescence of Tumor Tissue Sections
2.10. Protein Extraction, Gel Electrophoresis, and Immunoblotting
2.11. Statistical Analysis
3. Results
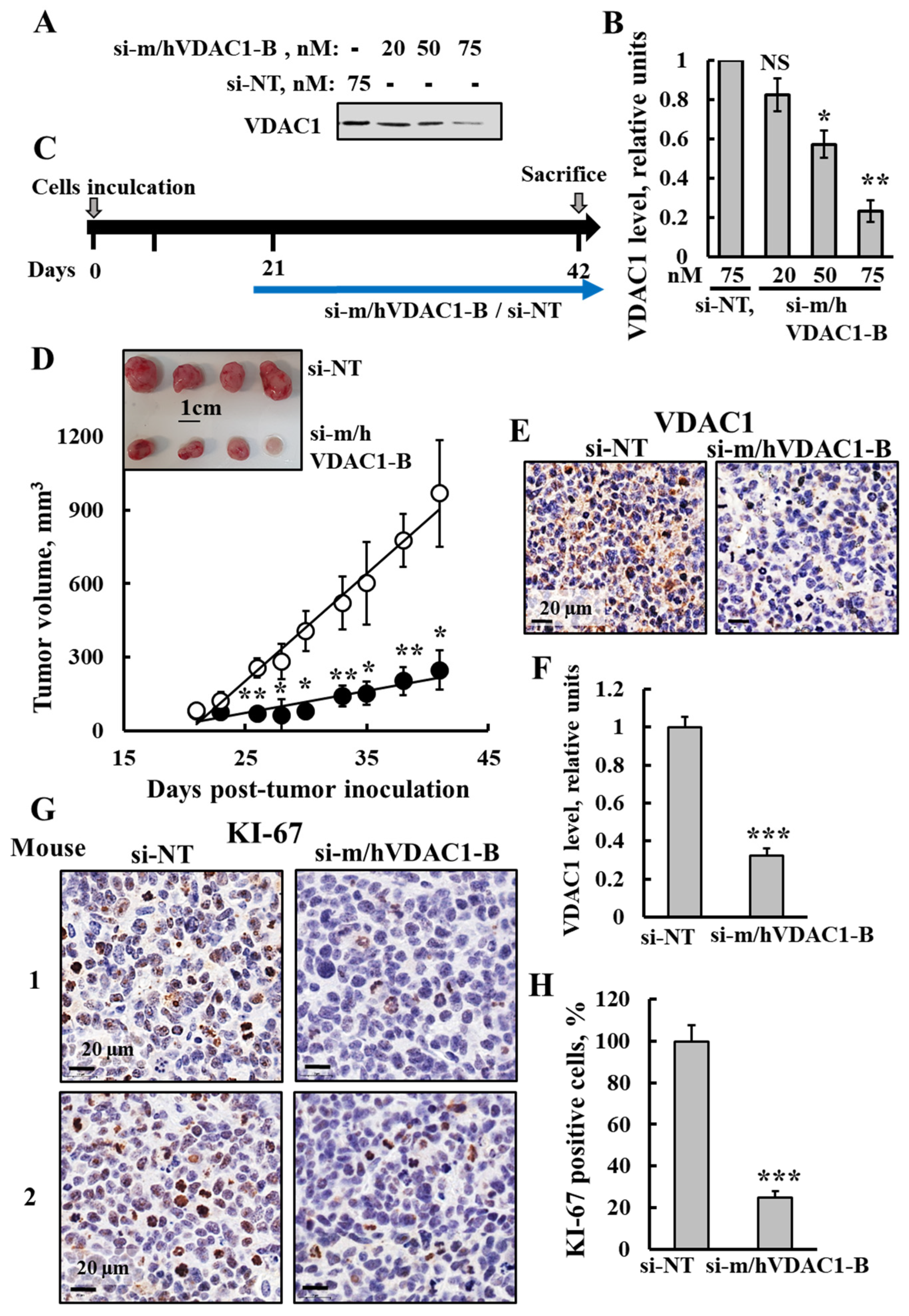
3.1. VDAC1 Overexpression in Human Lung Cancer Tissue and Silencing Its Expression by si-m/hVDAC1-B in Human and Mouse Lung Cancer Cell Lines
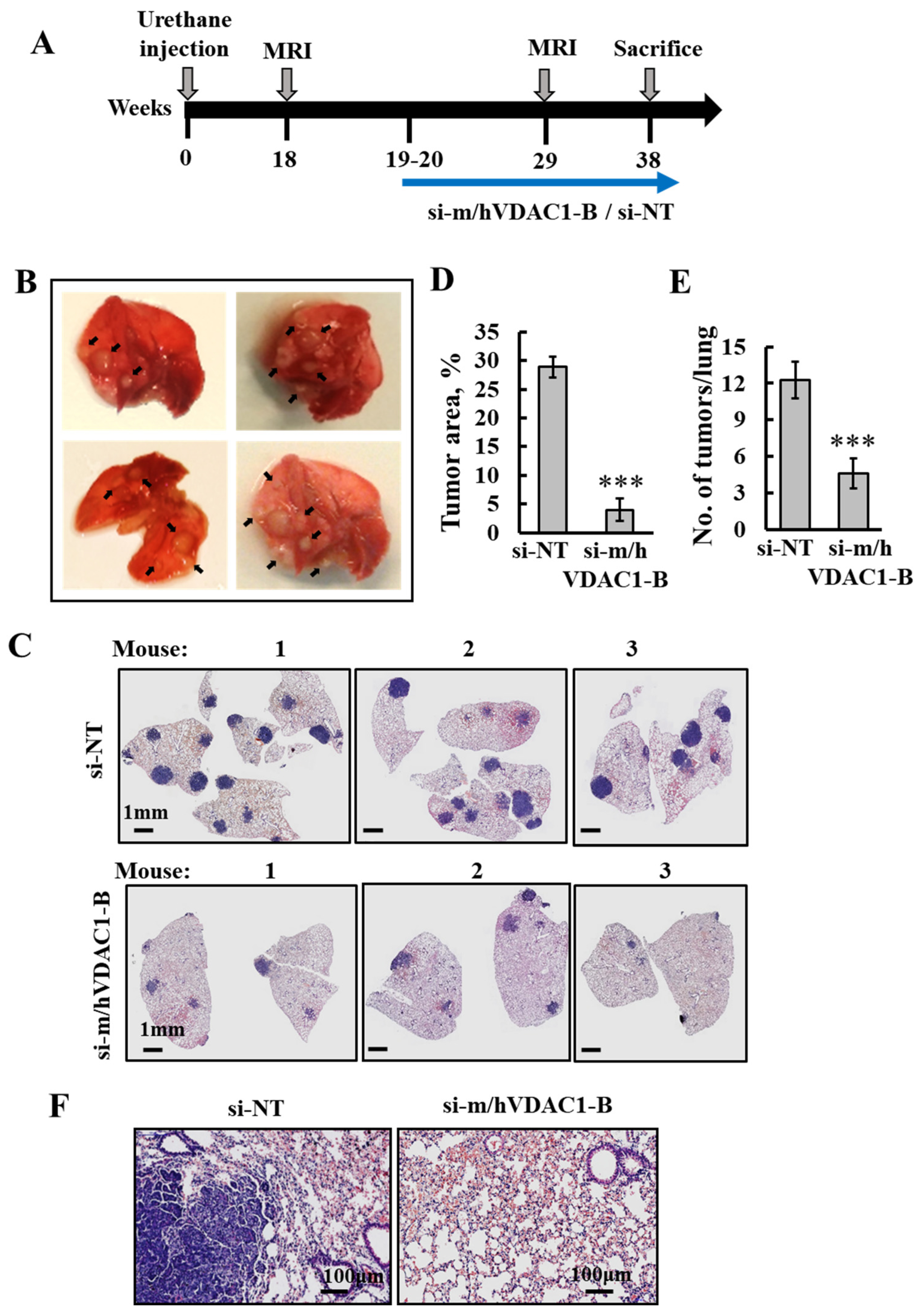
3.2. PLGA-PEI-si-m/hVDAC1-B Inhibits Urethane-Induced Lung Cancer in A/J Mice
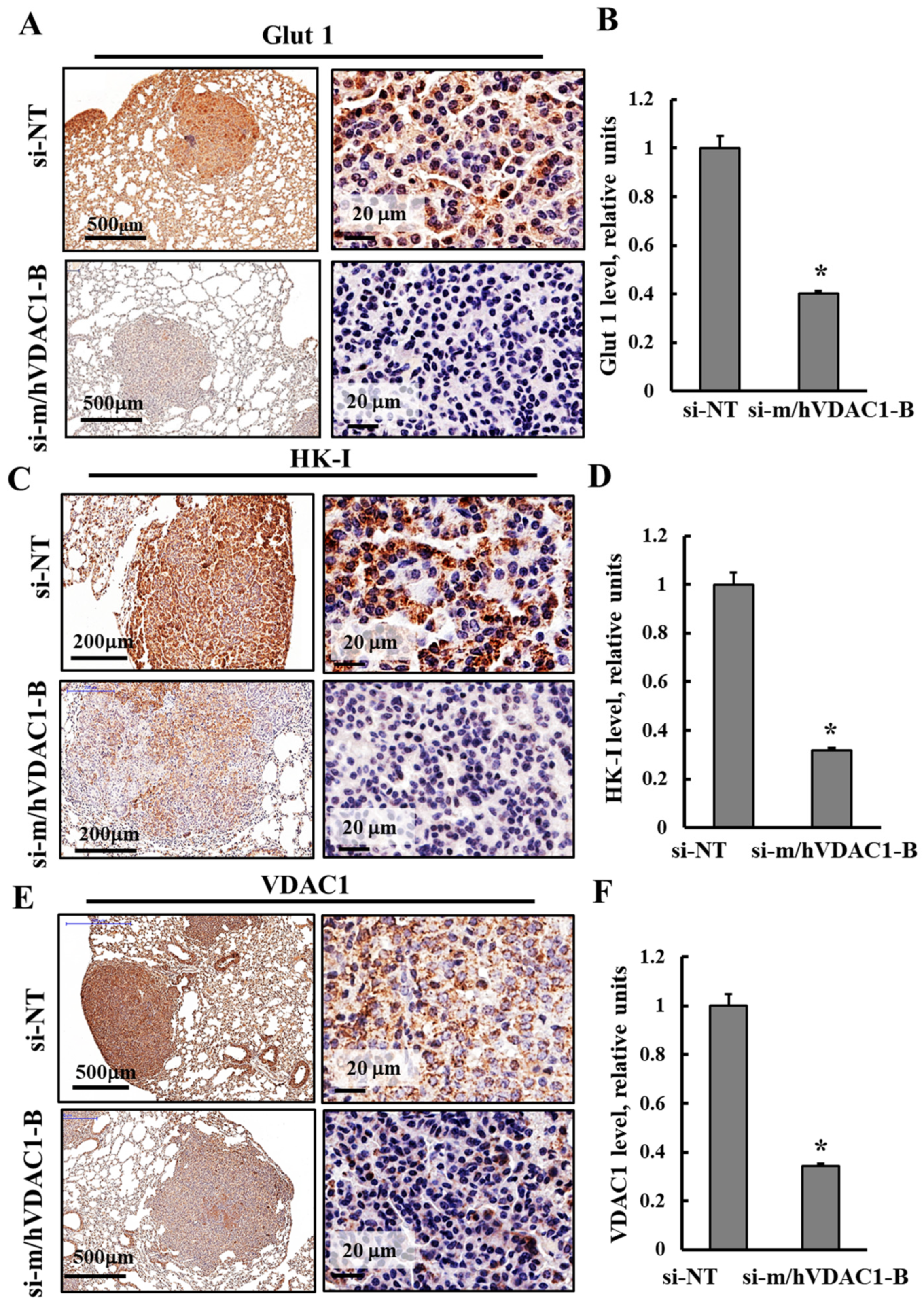
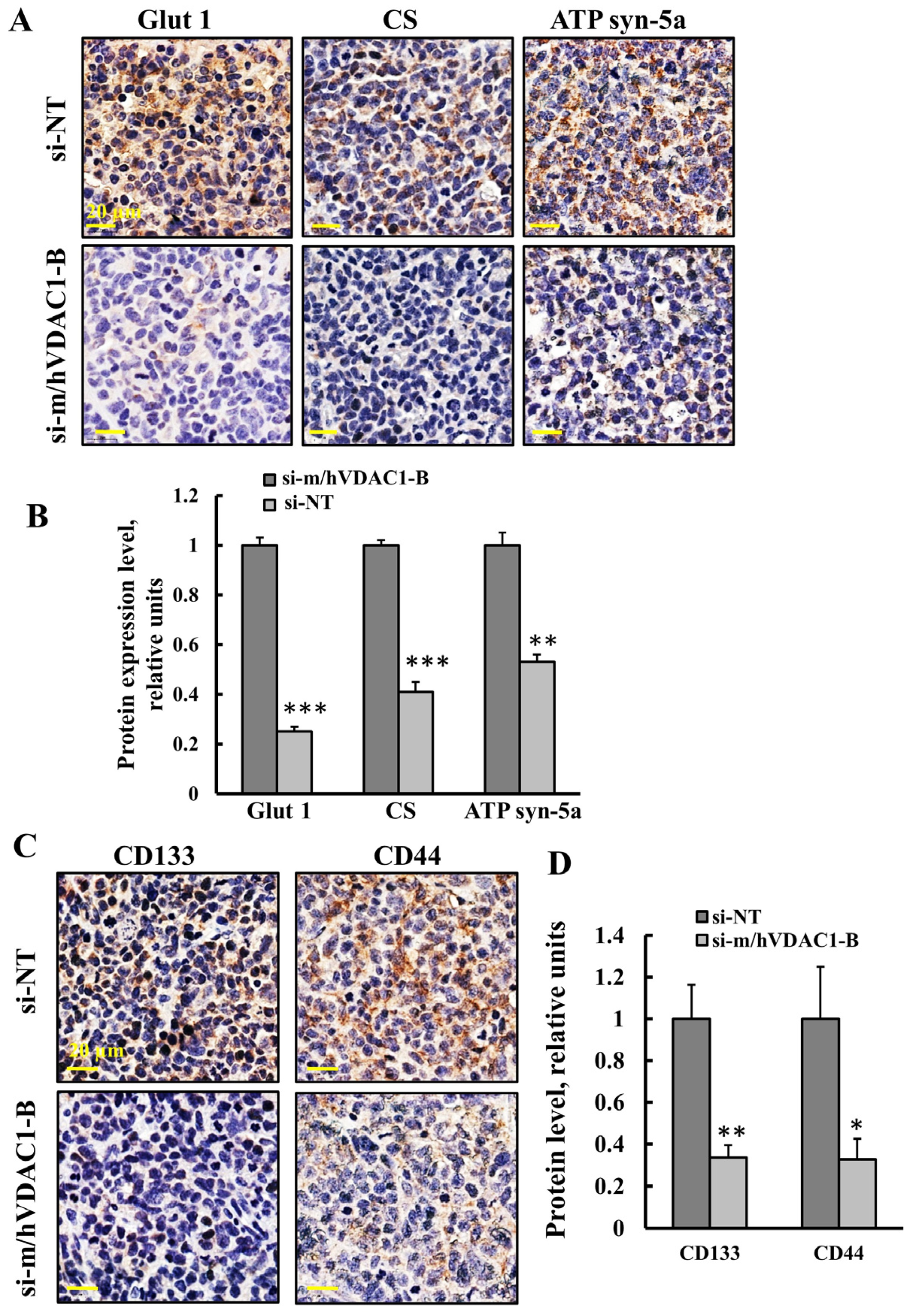
3.3. PLGA-PEI-si-m/hVDAC1-B Altered the Expression of Metabolism-Related Proteins in Tumors of Urethane-Induced Lung Cancer
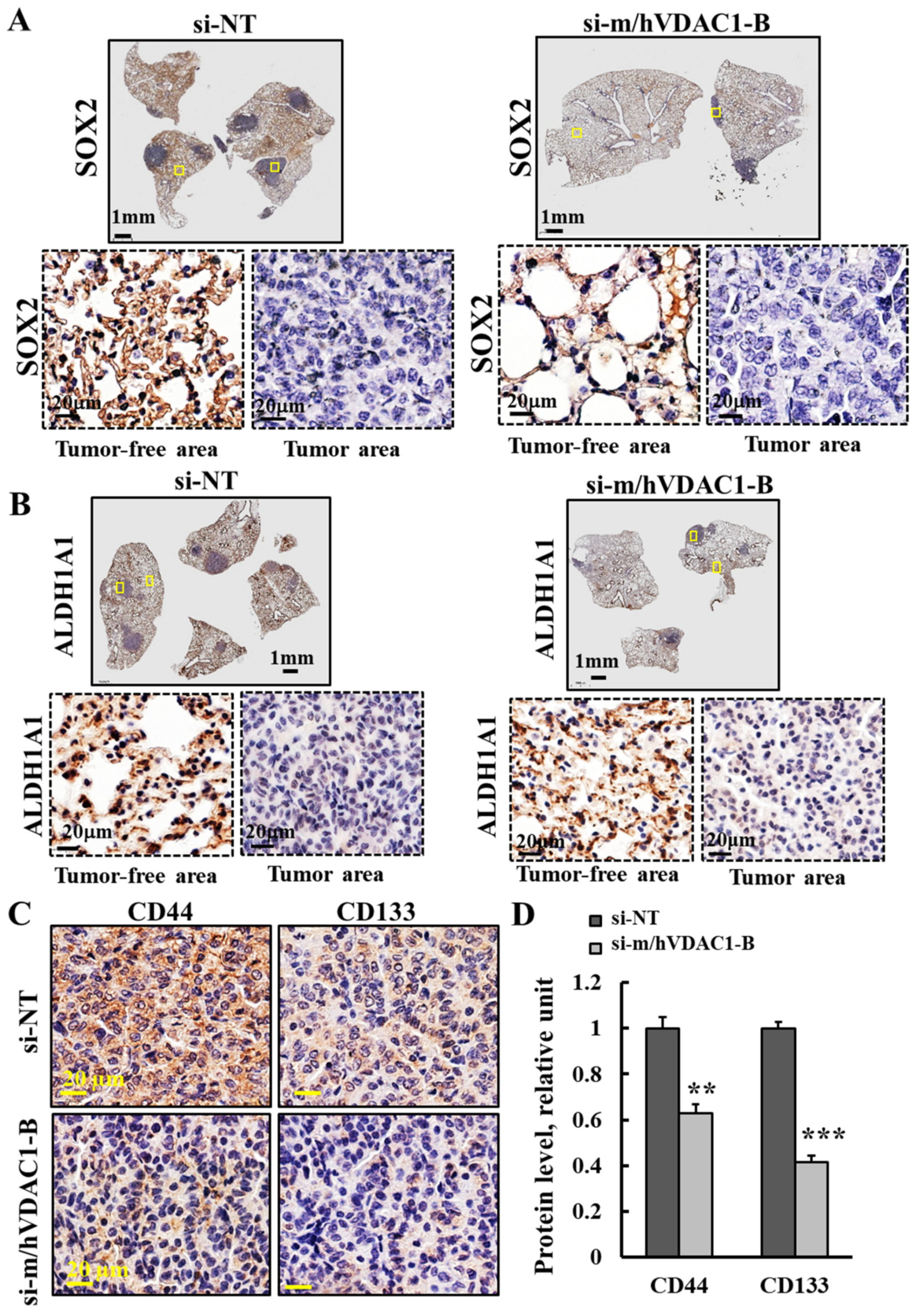
3.4. PLGA-PEI-si-m/hVDAC1-B Treatment of Urethane-Induced Lung Cancer Altered the Expression of CSC Markers
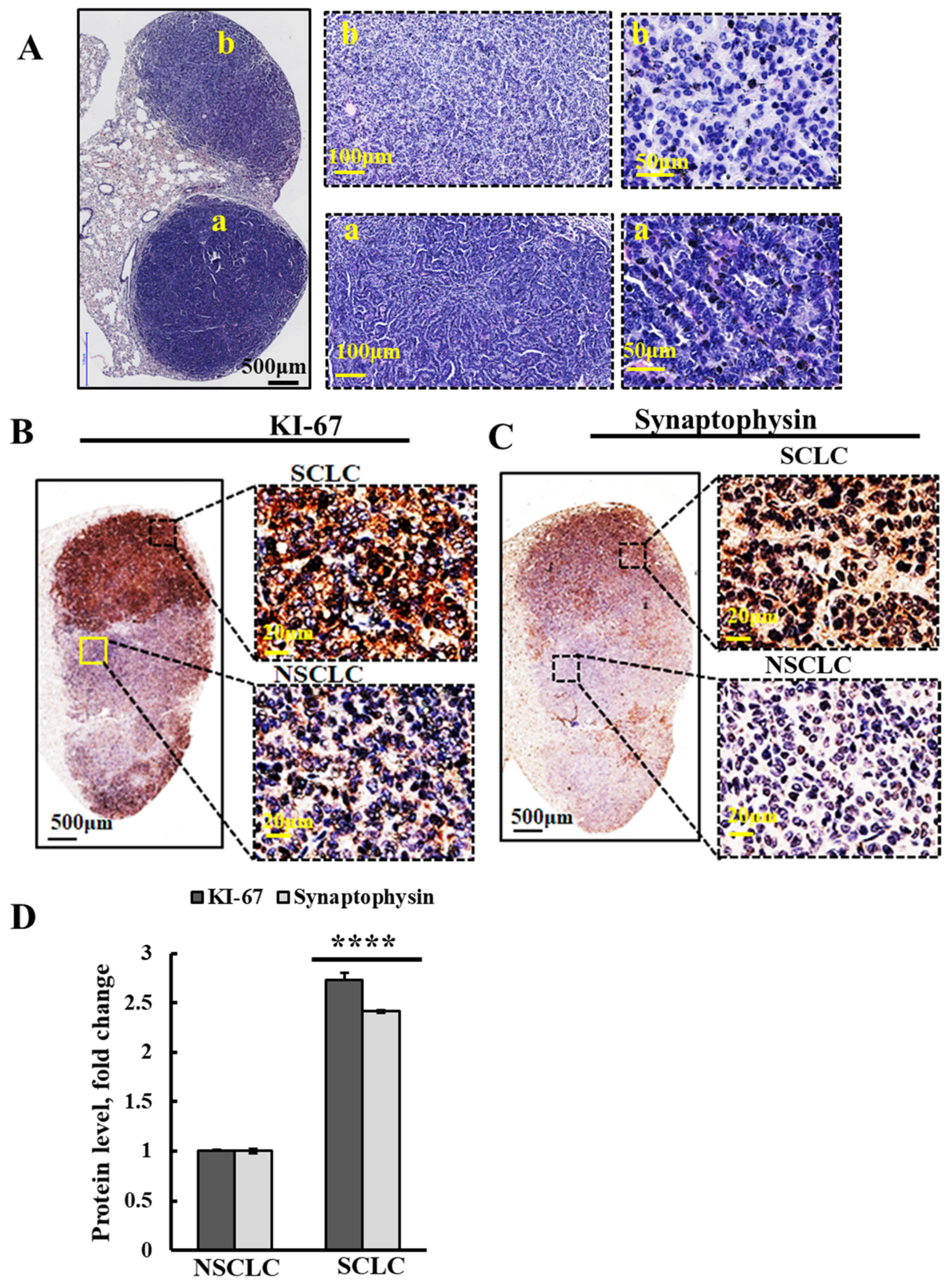
3.5. Urethane Treatment Induces NSCLC and also SCLC Cancers, and both Were Inhibited by PLGA-PEI-si-m/hVDAC1-B
3.6. si-m/hVDAC1-B Inhibited Tumor Growth in an SCLC Xenograft Mice Model
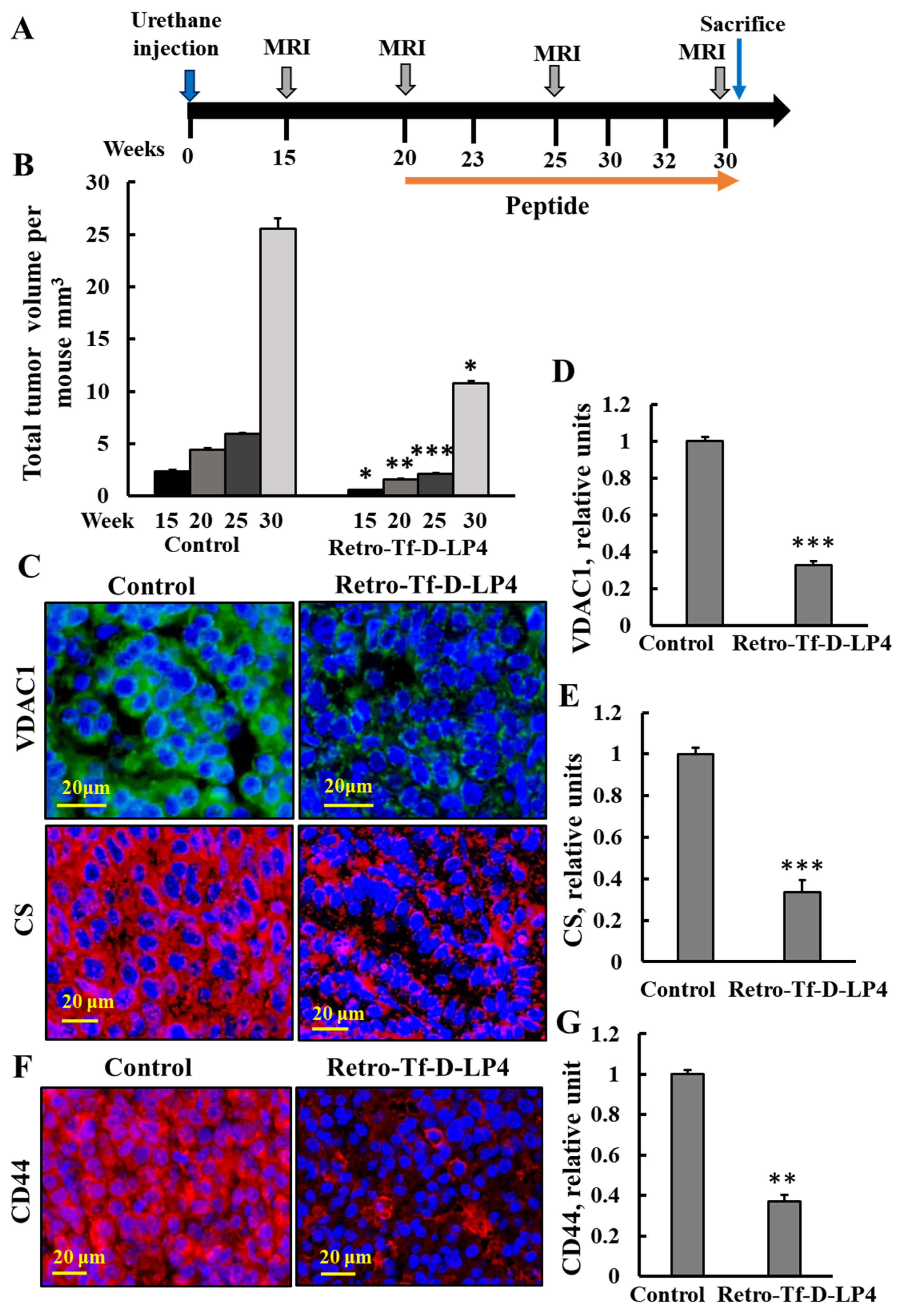
3.7. VDAC-1-Based Peptide, Retro-Tf-D-LP4, Inhibited Tumor Growth in Urethane-Exposed Mice
4. Discussion
4.1. PLGA-PEI-si-m/hVDAC1-B as a Potential Treatment for Lung Cancer
4.2. Tumor VDAC1 Depletion as a Possible Treatment of Small and Non-Small Lung Cancer
4.3. VDAC-1-Based Peptide, Retro-Tf-D-LP4 as a Potential Treatment for Lung Cancer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.M.; Baade, P.D. The International Epidemiology of Lung Cancer: Geographical distribution and secular trends. J. Thorac. Oncol. 2008, 3, 819–831. [Google Scholar] [CrossRef]
- Sasco, A.J.; Secretan, M.B.; Straif, K. Tobacco smoking and cancer: A brief review of recent epidemiological evidence. Lung Cancer 2004, 45 (Suppl. S2), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Cigarette smoking and lung cancer: Chemical mechanisms and approaches to prevention. Lancet Oncol. 2002, 3, 461–469. [Google Scholar] [CrossRef]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Galindo, I.; Gomez-Morales, M.; Diaz-Cano, I.; Andrades, A.; Caba-Molina, M.; Miranda-Leon, M.T.; Medina, P.P.; Martin-Padron, J.; Farez-Vidal, M.E. The value of desmosomal plaque-related markers to distinguish squamous cell carcinoma and adenocarcinoma of the lung. Ups. J. Med. Sci. 2020, 125, 19–29. [Google Scholar] [CrossRef]
- Iyoda, A.; Azuma, Y.; Sano, A. Neuroendocrine tumors of the lung: Clinicopathological and molecular features. Surg. Today 2020, 12, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D. 2015 WHO Classification of the Pathology and Genetics of Tumors of the Lung. J. Thorac. Oncol. 2015, 10, S68. [Google Scholar] [CrossRef]
- Menis, J.; Reck, M. Checkpoint Inhibitors in SCLC: How Much Can We Trust in Randomized Cohorts of Phase I/II Trials? J. Thorac. Oncol. 2020, 15, 308–310. [Google Scholar] [CrossRef]
- Zheng, M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 447–468. [Google Scholar] [CrossRef]
- Domine, M.; Moran, T.; Isla, D.; Marti, J.L.; Sullivan, I.; Provencio, M.; Olmedo, M.E.; Ponce, S.; Blasco, A.; Cobo, M. SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019). Clin. Transl. Oncol. 2020, 22, 245–255. [Google Scholar] [CrossRef]
- Rufini, V.; Calcagni, M.L.; Baum, R.P. Imaging of neuroendocrine tumors. Semin. Nucl. Med. 2006, 36, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; McCutcheon, J.N.; Kallakury, B.; Chahine, J.J.; Pratt, D.; Raffeld, M.; Chen, Y.; Wang, C.; Giaccone, G. Combined Small Cell Carcinoma of the Lung: Is It a Single Entity? J. Thorac. Oncol. 2018, 13, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Tokaz, M.C.; Baik, C.S.; Houghton, A.M.; Tseng, D. New Immuno-oncology Targets and Resistance Mechanisms. Curr. Treat. Options Oncol. 2022, 23, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, H. Immunotherapy resistance in non-small-cell lung cancer: From mechanism to clinical strategies. Front. Immunol. 2023, 14, 1129465. [Google Scholar] [CrossRef]
- Arif, T.; Krelin, Y.; Nakdimon, I.; Benharroch, D.; Paul, A.; Dadon-Klein, D.; Shoshan-Barmatz, V. VDAC1 is a molecular target in glioblastoma, with its depletion leading to reprogrammed metabolism and reversed oncogenic properties. Neuro-Oncol. 2017, 19, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Paul, A.; Krelin, Y.; Shteinfer-Kuzmine, A.; Shoshan-Barmatz, V. Mitochondrial VDAC1 Silencing Leads to Metabolic Rewiring and the Reprogramming of Tumour Cells into Advanced Differentiated States. Cancers 2018, 10, 499. [Google Scholar] [CrossRef]
- Arif, T.; Vasilkovsky, L.; Refaely, Y.; Konson, A.; Shoshan-Barmatz, V. Silencing VDAC1 Expression by siRNA Inhibits Cancer Cell Proliferation and Tumor Growth In Vivo. Mol. Ther. Nucleic Acids 2014, 3, e159. [Google Scholar] [CrossRef]
- Zerbib, E.; Arif, T.; Shteinfer-Kuzmine, A.; Chalifa-Caspi, V.; Shoshan-Barmatz, V. VDAC1 Silencing in Cancer Cells Leads to Metabolic Reprogramming That Modulates Tumor Microenvironment. Cancers 2021, 13, 2850. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Ben-Hail, D.; Admoni, L.; Krelin, Y.; Tripathi, S.S. The mitochondrial voltage-dependent anion channel 1 in tumor cells. Biochim. Biophys. Acta 2015, 1848, 2547–2575. [Google Scholar] [CrossRef]
- Alhozeel, B.; Pandey, S.K.; Shteinfer-Kuzmine, A.; Santhanam, M.; Shoshan-Barmatz, V. Silencing the Mitochondrial Gatekeeper VDAC1 as a Potential Treatment for Bladder Cancer. Cells 2024, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Machlof-Cohen, R.; Santhanam, M.; Shteinfer-Kuzmine, A.; Shoshan-Barmatz, V. Silencing VDAC1 to Treat Mesothelioma Cancer: Tumor Reprograming and Altering Tumor Hallmarks. Biomolecules 2022, 12, 895. [Google Scholar] [CrossRef]
- Prezma, T.; Shteinfer, A.; Admoni, L.; Raviv, Z.; Sela, I.; Levi, I.; Shoshan-Barmatz, V. VDAC1-based peptides: Novel pro-apoptotic agents and potential therapeutics for B-cell chronic lymphocytic leukemia. Cell Death Dis. 2013, 4, e809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shteinfer-Kuzmine, A.; Amsalem, Z.; Arif, T.; Zooravlov, A.; Shoshan-Barmatz, V. Selective induction of cancer cell death by VDAC1-based peptides and their potential use in cancer therapy. Mol. Oncol. 2018, 12, 1077–1103. [Google Scholar] [CrossRef] [PubMed]
- Shteinfer-Kuzmine, A.; Arif, T.; Krelin, Y.; Tripathi, S.S.; Paul, A.; Shoshan-Barmatz, V. Mitochondrial VDAC1-based peptides: Attacking oncogenic properties in glioblastoma. Oncotarget 2017, 8, 31329–31346. [Google Scholar] [CrossRef][Green Version]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef]
- Abu-Hamad, S.; Sivan, S.; Shoshan-Barmatz, V. The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc. Natl. Acad. Sci. USA 2006, 103, 5787–5792. [Google Scholar] [CrossRef]
- Arif, T.; Stern, O.; Pittala, S.; Chalifa-Caspi, V.; Shoshan-Barmatz, V. Rewiring of Cancer Cell Metabolism by Mitochondrial VDAC1 Depletion Results in Time-Dependent Tumor Reprogramming: Glioblastoma as a Proof of Concept. Cells 2019, 8, 1330. [Google Scholar] [CrossRef]
- Amsalem, Z.; Arif, T.; Shteinfer-Kuzmine, A.; Chalifa-Caspi, V.; Shoshan-Barmatz, V. The Mitochondrial Protein VDAC1 at the Crossroads of Cancer Cell Metabolism: The Epigenetic Link. Cancers 2020, 12, 1031. [Google Scholar] [CrossRef]
- Alshamsan, A.; Haddadi, A.; Hamdy, S.; Samuel, J.; El-Kadi, A.O.; Uludag, H.; Lavasanifar, A. STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response. Mol. Pharm. 2010, 7, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Bernabeu, E.; Rodriguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta 2012, 1820, 291–317. [Google Scholar] [CrossRef]
- Gurley, K.E.; Moser, R.D.; Kemp, C.J. Induction of Lung Tumors in Mice with Urethane. Cold Spring Harb. Protoc. 2015, 2015, pdb-prot077446. [Google Scholar] [CrossRef] [PubMed]
- Redente, E.F.; Orlicky, D.J.; Bouchard, R.J.; Malkinson, A.M. Tumor signaling to the bone marrow changes the phenotype of monocytes and pulmonary macrophages during urethane-induced primary lung tumorigenesis in A/J mice. Am. J. Pathol. 2007, 170, 693–708. [Google Scholar] [CrossRef]
- Elsam, J. Histological and Histochemical Methods: Theory and Practice, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Patil, Y.; Panyam, J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int. J. Pharm. 2009, 367, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Monobe, Y.; Manabe, T. Morphological changes and proliferative activity of alveolar epithelium in mouse lungs treated with urethan. Virchows Arch. 1995, 425, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ravikumar, P.; Nguyen, K.T.; Hsia, C.C.; Hong, Y. Lung protection by inhalation of exogenous solubilized extracellular matrix. PLoS ONE 2017, 12, e0171165. [Google Scholar] [CrossRef]
- Diaz-Ruiz, R.; Rigoulet, M.; Devin, A. The Warburg and Crabtree effects: On the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim. Biophys. Acta 2011, 1807, 568–576. [Google Scholar] [CrossRef]
- Majeed, R.; Hamid, A.; Qurishi, Y.; Qazi, A.K.; Hussain, A.; Ahmed, M.; Najar, R.A.; Bhat, J.A.; Singh, S.K.; Saxena, A.K. Therapeutic Targeting of Cancer Cell Metabolism: Role of Metabolic Enzymes, Oncogenes and Tumor Suppressor Genes. Cancer Sci. Ther. 2012, 4, 281–291. [Google Scholar] [CrossRef]
- Chou, Y.T.; Lee, C.C.; Hsiao, S.H.; Lin, S.E.; Lin, S.C.; Chung, C.H.; Chung, C.H.; Kao, Y.R.; Wang, Y.H.; Chen, C.T.; et al. The emerging role of SOX2 in cell proliferation and survival and its crosstalk with oncogenic signaling in lung cancer. Stem Cells 2013, 31, 2607–2619. [Google Scholar] [CrossRef]
- Huo, W.; Du, M.; Pan, X.; Zhu, X.; Li, Z. Prognostic value of ALDH1 expression in lung cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 2045–2051. [Google Scholar] [PubMed]
- Bertolini, G.; Roz, L.; Perego, P.; Tortoreto, M.; Fontanella, E.; Gatti, L.; Pratesi, G.; Fabbri, A.; Andriani, F.; Tinelli, S.; et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 16281–16286. [Google Scholar] [CrossRef]
- Leung, E.L.; Fiscus, R.R.; Tung, J.W.; Tin, V.P.; Cheng, L.C.; Sihoe, A.D.; Fink, L.M.; Ma, Y.; Wong, M.P. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS ONE 2010, 5, e14062. [Google Scholar] [CrossRef]
- Nicholson, S.A.; Beasley, M.B.; Brambilla, E.; Hasleton, P.S.; Colby, T.V.; Sheppard, M.N.; Falk, R.; Travis, W.D. Small cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases with surgical specimens. Am. J. Surg. Pathol. 2002, 26, 1184–1197. [Google Scholar] [CrossRef]
- Travis, W.D. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod. Pathol. 2012, 25 (Suppl. S1), S18–S30. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Amsalem, Z.; Shoshan-Barmatz, V. Metabolic Reprograming Via Silencing of Mitochondrial VDAC1 Expression Encourages Differentiation of Cancer Cells. Mol. Ther. Nucl. Acids 2019, 17, 24–37. [Google Scholar] [CrossRef]
- Prabavathy, D.; Ramadoss, N. Heterogeneity of Small Cell Lung Cancer Stem Cells. Adv. Exp. Med. Biol. 2019, 1139, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef]
- Colombini, M. VDAC: The channel at the interface between mitochondria and the cytosol. Mol. Cell Biochem. 2004, 256–257, 107–115. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Lemasters, J.J. Warburg revisited: Regulation of mitochondrial metabolism by voltage-dependent anion channels in cancer cells. J. Pharmacol. Exp. Ther. 2012, 342, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, G.T.; Sherrill, T.P.; Cheng, D.S.; Scoggins, R.M.; Han, W.; Polosukhin, V.V.; Connelly, L.; Yull, F.E.; Fingleton, B.; Blackwell, T.S. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18514–18519. [Google Scholar] [CrossRef] [PubMed]
- Horio, Y.; Chen, A.; Rice, P.; Roth, J.A.; Malkinson, A.M.; Schrump, D.S. Ki-ras and p53 mutations are early and late events, respectively, in urethane-induced pulmonary carcinogenesis in A/J mice. Mol. Carcinog. 1996, 17, 217–223. [Google Scholar] [CrossRef]
- Kelly-Spratt, K.S.; Philipp-Staheli, J.; Gurley, K.E.; Hoon-Kim, K.; Knoblaugh, S.; Kemp, C.J. Inhibition of PI-3K restores nuclear p27Kip1 expression in a mouse model of Kras-driven lung cancer. Oncogene 2009, 28, 3652–3662. [Google Scholar] [CrossRef]
- Alyaqoub, F.S.; Tao, L.; Kramer, P.M.; Steele, V.E.; Lubet, R.A.; Gunning, W.T.; Pereira, M.A. Prevention of mouse lung tumors and modulation of DNA methylation by combined treatment with budesonide and R115777 (Zarnestra MT). Carcinogenesis 2007, 28, 124–129. [Google Scholar] [CrossRef]
- Busch, S.E.; Moser, R.D.; Gurley, K.E.; Kelly-Spratt, K.S.; Liggitt, H.D.; Kemp, C.J. ARF inhibits the growth and malignant progression of non-small-cell lung carcinoma. Oncogene 2014, 33, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Getz, G.; Wheeler, D.A.; Mardis, E.R.; McLellan, M.D.; Cibulskis, K.; Sougnez, C.; Greulich, H.; Muzny, D.M.; Morgan, M.B.; et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008, 455, 1069–1075. [Google Scholar] [CrossRef]
- Westcott, P.M.; To, M.D. The genetics and biology of KRAS in lung cancer. Chin. J. Cancer 2013, 32, 63–70. [Google Scholar] [CrossRef]
- Riely, G.J.; Marks, J.; Pao, W. KRAS Mutations in Non–Small Cell Lung Cancer. Proc. Am. Thorac. Soc. 2009, 6, 201–205. [Google Scholar] [CrossRef]
- Kurokawa, K.; Matsui, T.; Ikeda, H.; Nishikawa, S.; Sone, T.; Kasahara, K. Significance of EGFR and KRAS gene mutation in small cell lung cancer. J. Clin. Oncol. 2013, 31, e18564. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Tarpin, C.; Diebel, M.; Esterni, B.; Houvenaeghel, G.; Extra, J.M.; Bertucci, F.; Jacquemier, J.; et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin. Cancer Res. 2010, 16, 45–55. [Google Scholar] [CrossRef]
- Yue, H.; Hu, Z.; Hu, R.; Guo, Z.; Zheng, Y.; Wang, Y.; Zhou, Y. ALDH1A1 in Cancers: Bidirectional Function, Drug Resistance, and Regulatory Mechanism. Front. Oncol. 2022, 12, 918778. [Google Scholar] [CrossRef]
- Pandey, M.; Sultana, S.; Gupta, K.P. Involvement of epigenetics and microRNA-29b in the urethane induced inception and establishment of mouse lung tumors. Exp. Mol. Pathol. 2014, 96, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Dragani, T.A.; Falvella, F.S.; Manenti, G.; Pierotti, M.A.; Gambetta, R.A. Downexpression of aldehyde dehydrogenase 1 in murine lung tumors. Mol. Carcinog. 1996, 16, 123–125. [Google Scholar] [CrossRef]
- Avilion, A.A.; Nicolis, S.K.; Pevny, L.H.; Perez, L.; Vivian, N.; Lovell-Badge, R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes. Dev. 2003, 17, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Gontan, C.; de Munck, A.; Vermeij, M.; Grosveld, F.; Tibboel, D.; Rottier, R. Sox2 is important for two crucial processes in lung development: Branching morphogenesis and epithelial cell differentiation. Dev. Biol. 2008, 317, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Luo, X.; Schwartz, R.J.; Hogan, B.L. Multiple roles for Sox2 in the developing and adult mouse trachea. Development 2009, 136, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, D.H.; Besnard, V.; Lange, A.W.; Wert, S.E.; Keiser, A.R.; Smith, A.N.; Lang, R.; Whitsett, J.A. Sox2 Is Required for Maintenance and Differentiation of Bronchiolar Clara, Ciliated, and Goblet Cells. PLoS ONE 2009, 4, e8248. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.; Huser, L.; Elton, J.J.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2 in development and cancer biology. Semin. Cancer Biol. 2020, 67, 74–82. [Google Scholar] [CrossRef]
- Lu, Y.; Futtner, C.; Rock, J.R.; Xu, X.; Whitworth, W.; Hogan, B.L.M.; Onaitis, M.W. Evidence That SOX2 Overexpression Is Oncogenic in the Lung. PLoS ONE 2010, 5, e11022. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, N.; Zeng, Z.; Wu, Q.; Jiang, X.; Li, S.; Sun, W.; Zhang, J.; Li, Y.; Li, J.; et al. LncRNA PCAT1 activates SOX2 and suppresses radioimmune responses via regulating cGAS/STING signalling in non-small cell lung cancer. Clin. Transl. Med. 2022, 12, e792. [Google Scholar] [CrossRef]
- Mirzaei, S.; Paskeh, M.D.A.; Entezari, M.; Mirmazloomi, S.R.; Hassanpoor, A.; Aboutalebi, M.; Rezaei, S.; Hejazi, E.S.; Kakavand, A.; Heidari, H.; et al. SOX2 function in cancers: Association with growth, invasion, stemness and therapy response. Biomed. Pharmacother. 2022, 156, 113860. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Lee, K.Y.; Han, S.; Yun, C.W.; Park, C.H.; Jang, H. SOX2 Expression Does Not Guarantee Cancer Stem Cell-like Characteristics in Lung Adenocarcinoma. Cells 2024, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Hsieh, C.B.; Yan, M.D.; Tsao, C.M.; Hsieh, T.Y.; Liu, C.H.; Lin, Y.W. Frequent concomitant epigenetic silencing of SOX1 and secreted frizzled-related proteins (SFRPs) in human hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2013, 28, 551–559. [Google Scholar] [CrossRef]
- Devarakonda, S.; Morgensztern, D.; Govindan, R. Genomic alterations in lung adenocarcinoma. Lancet Oncol. 2015, 16, e342–e351. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretic, L.; Kong, G.; Leenders, F.; Lu, X.; Fernandez-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Gilad, S.; Lithwick-Yanai, G.; Barshack, I.; Benjamin, S.; Krivitsky, I.; Edmonston, T.B.; Bibbo, M.; Thurm, C.; Horowitz, L.; Huang, Y.; et al. Classification of the four main types of lung cancer using a microRNA-based diagnostic assay. J. Mol. Diagn. 2012, 14, 510–517. [Google Scholar] [CrossRef]
- Zarogoulidis, K.; Zarogoulidis, P.; Darwiche, K.; Boutsikou, E.; Machairiotis, N.; Tsakiridis, K.; Katsikogiannis, N.; Kougioumtzi, I.; Karapantzos, I.; Huang, H.; et al. Treatment of non-small cell lung cancer (NSCLC). J. Thorac. Dis. 2013, 5 (Suppl. S4), S389–S396. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Aapro, M.; Ardizzoni, A.; Balducci, L.; De Marinis, F.; Kelly, K.; Le Chevalier, T.; Manegold, C.; Perrone, F.; Rosell, R.; et al. Treatment of advanced non-small-cell lung cancer in the elderly: Results of an international expert panel. J. Clin. Oncol. 2005, 23, 3125–3137. [Google Scholar] [CrossRef] [PubMed]
- Imakita, T.; Fujita, K.; Kanai, O.; Terashima, T.; Mio, T. Small cell lung cancer transformation during immunotherapy with nivolumab: A case report. Respir. Med. Case Rep. 2017, 21, 52–55. [Google Scholar] [CrossRef]
- Shiroyama, T.; Nasu, S.; Tanaka, A.; Takata, S.; Masuhiro, K.; Takada, H.; Morita, S.; Morishita, N.; Suzuki, H.; Okamoto, N.; et al. Transformation to small cell lung cancer after first-line afatinib treatment. Respir. Med. Case Rep. 2018, 23, 188–190. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 2017, 17, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Sabari, J.K.; Lok, B.H.; Laird, J.H.; Poirier, J.T.; Rudin, C.M. Unravelling the biology of SCLC: Implications for therapy. Nat. Rev. Clin. Oncol. 2017, 14, 549–561. [Google Scholar] [CrossRef]
- Zhong, L.; Suo, J.; Wang, Y.; Han, J.; Zhou, H.; Wei, H.; Zhu, J. Prognosis of limited-stage small cell lung cancer with comprehensive treatment including radical resection. World J. Surg. Oncol. 2020, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Otoshi, R.; Sekine, A.; Okudela, K.; Asaoka, M.; Sato, Y.; Ikeda, S.; Baba, T.; Komatsu, S.; Hagiwara, E.; Ogura, T. Small-cell lung carcinoma transformation of lung adenocarcinoma diagnosed by pericardial effusion: A case report. Mol. Clin. Oncol. 2020, 13, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; You, Y.; Zhang, X.; Wang, H.; Wang, M.; Zhang, L. Histologic transformation of lung cancer during pembrolizumab therapy: A case report. Thorac. Cancer 2020, 11, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Gill, D.M.; Pal, S.K.; Agarwal, N. The future of immune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy 2017, 9, 681–692. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, N.; Gonzalez-Cao, M.; Sosa, A.; Berenguer, J.; Bracht, J.W.P.; Ito, M.; Rosell, R. The combination of checkpoint immunotherapy and targeted therapy in cancer. Ann. Transl. Med. 2017, 5, 388. [Google Scholar] [CrossRef]
- Ventola, C.L. Cancer Immunotherapy, Part 1: Current Strategies and Agents. Pharm. Ther. 2017, 42, 375–383. [Google Scholar]
- Clevers, H. The cancer stem cell: Premises, promises and challenges. Nat. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef]


| Antibody | Source and Cat. No. | Dilution | ||
|---|---|---|---|---|
| IHC | WB | IF | ||
| Rabbit polyclonal anti-VDAC1 | ab15895, Abcam, Cambridge, UK | 1:400 | 1:15,000 | 1:500 |
| Rabbit polyclonal anti-KI-67 | ab15580, Abcam, Cambridge, UK | 1:500 | ||
| Rabbit polyclonal anti-Glut 1 | ab652, Abcam, Cambridge, UK, | 1:500 | ||
| Rabbit polyclonal anti-ATP5A | ab151229, Abcam, Cambridge, UK | 1:500 | ||
| Mouse monoclonal anti-SOX2 | ab171380, Abcam, Cambridge, UK | 1:200 | ||
| Rabbit polyclonal anti-CD44 | ab157107, Abcam, Cambridge, UK | 1:800 | 1:1000 | |
| Rabbit monoclonal anti-HK-I | ab150423, Abcam, Cambridge, UK | 1:100 | ||
| Rabbit polyclonal anti-CD133 | ab19898, Abcam, Cambridge, UK | 1:200 | ||
| Rabbit monoclonal anti-synaptophysin | ab32127, Abcam, Cambridge UK | 1:500 | ||
| Rabbit polyclonal anti-citrate synthetase | ab96600, Abcam, Cambridge, UK | 1:500 | 1:500 | |
| Rabbit monoclonal anti-ALDH1 | ab52492, Abcam, Cambridge, UK | 1:50 | ||
| Mouse monoclonal anti-β-actin | MAB1501, Millipore, Billerica, MA | 1:40,000 | ||
| Donkey anti-Mouse (Alexa Fluor 488) | ab150109, Abcam, Cambridge, UK | 1:750 | ||
| Goat anti-Rabbit (Alexa Fluor 555) | ab150086, Abcam, Cambridge, UK | 1:850 | ||
| Goat anti-Rabbit HRP | W4018, Promega, Wisconsin | 1:1000 | 1:15,000 | |
| Donkey anti-Mouse HRP | ab98799, Abcam, Cambridge, UK | 1:1000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikov, N.; Pittala, S.; Shteinfer-Kuzmine, A.; Shoshan-Barmatz, V. Mitochondrial VDAC1 Silencing in Urethane-Induced Lung Cancer Inhibits Tumor Growth and Alters Cancer Oncogenic Properties. Cancers 2024, 16, 2970. https://doi.org/10.3390/cancers16172970
Melnikov N, Pittala S, Shteinfer-Kuzmine A, Shoshan-Barmatz V. Mitochondrial VDAC1 Silencing in Urethane-Induced Lung Cancer Inhibits Tumor Growth and Alters Cancer Oncogenic Properties. Cancers. 2024; 16(17):2970. https://doi.org/10.3390/cancers16172970
Chicago/Turabian StyleMelnikov, Nataly, Srinivas Pittala, Anna Shteinfer-Kuzmine, and Varda Shoshan-Barmatz. 2024. "Mitochondrial VDAC1 Silencing in Urethane-Induced Lung Cancer Inhibits Tumor Growth and Alters Cancer Oncogenic Properties" Cancers 16, no. 17: 2970. https://doi.org/10.3390/cancers16172970
APA StyleMelnikov, N., Pittala, S., Shteinfer-Kuzmine, A., & Shoshan-Barmatz, V. (2024). Mitochondrial VDAC1 Silencing in Urethane-Induced Lung Cancer Inhibits Tumor Growth and Alters Cancer Oncogenic Properties. Cancers, 16(17), 2970. https://doi.org/10.3390/cancers16172970








