The Impact of Pembrolizumab as a Salvage Therapy Based on HER2 Expression in Advanced Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. HER-2 (c-erbB-2) Immunohistochemistry Test
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Treatment Efficacy
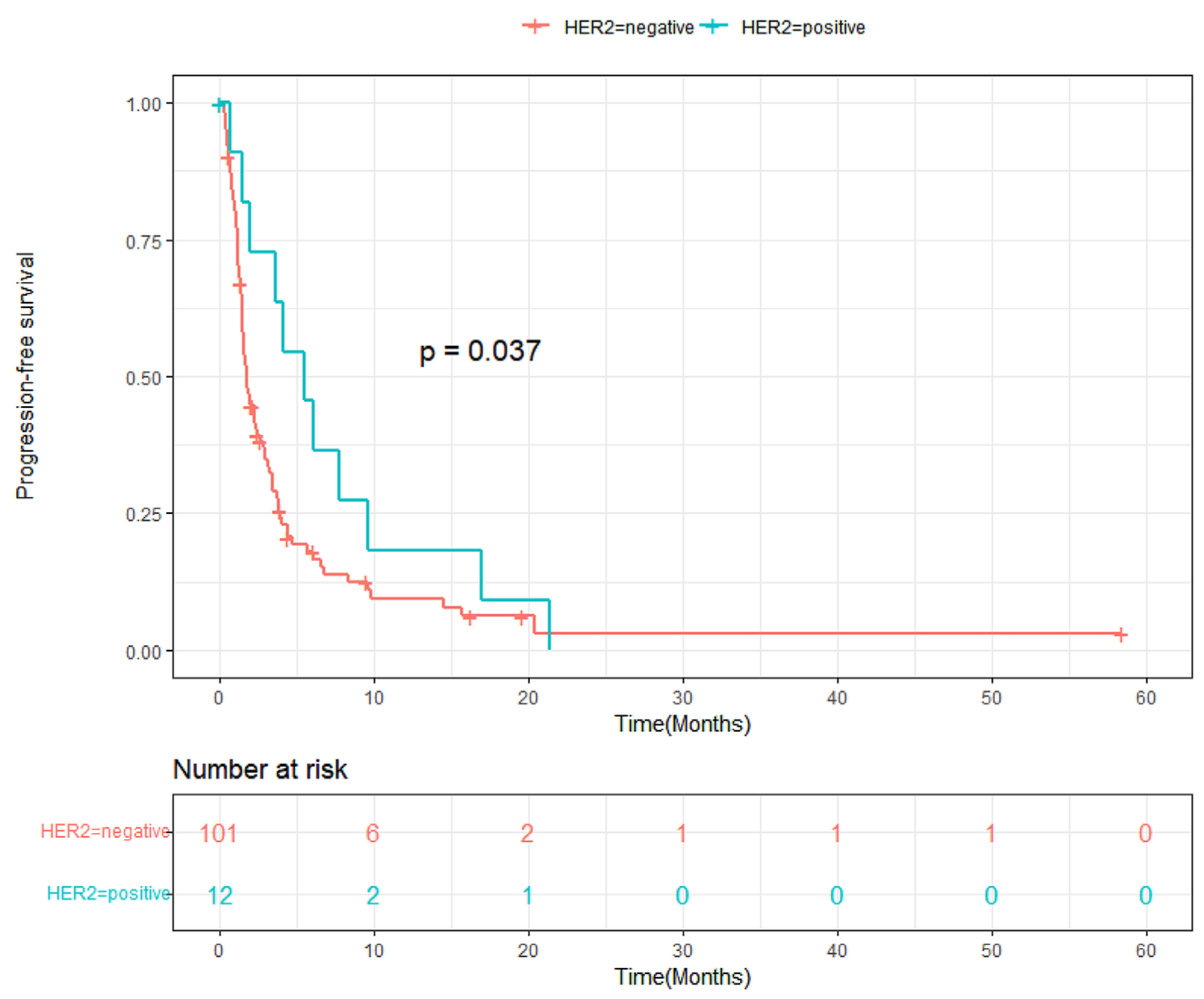
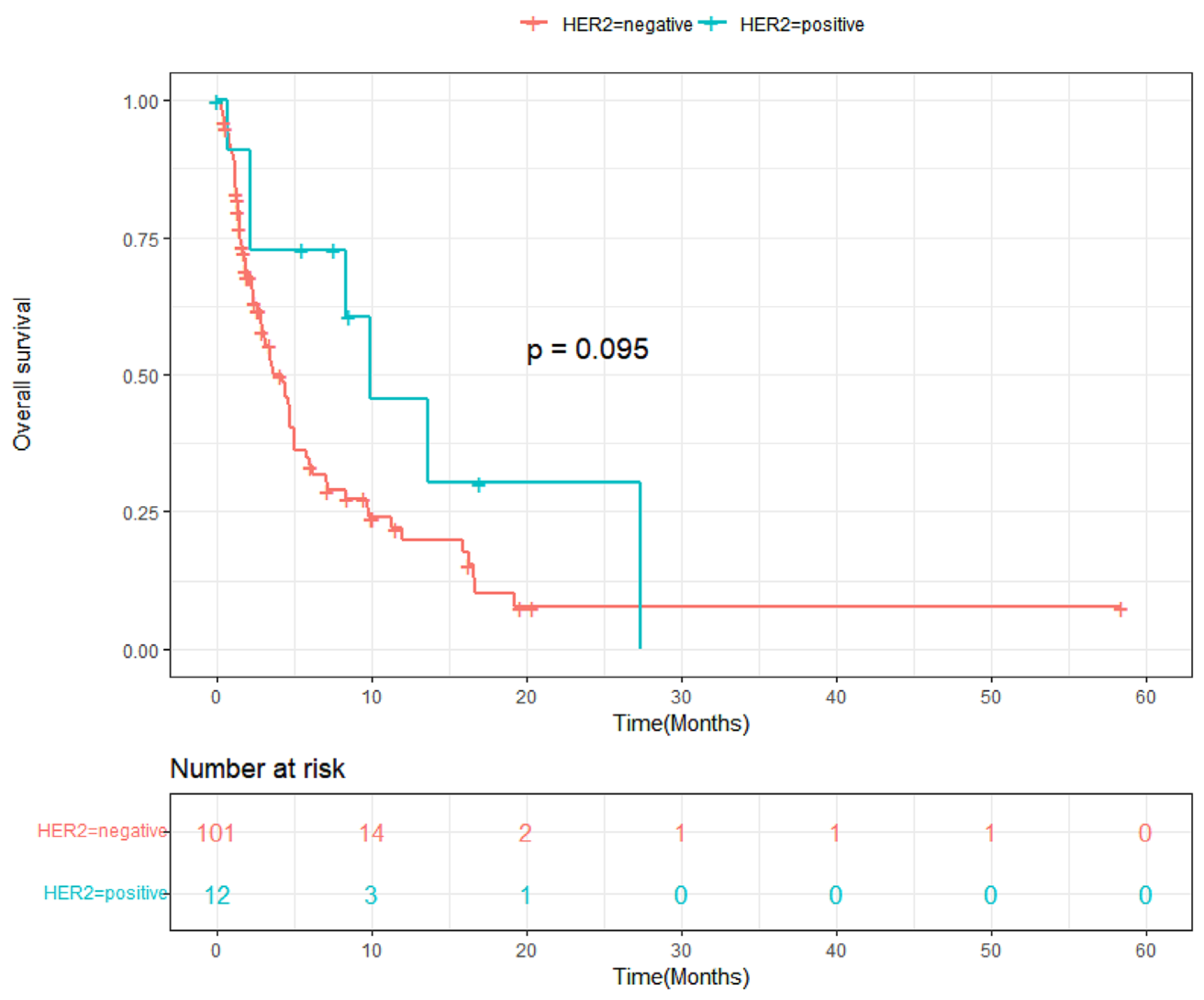
3.3. Progression-Free Survival and Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Jeung, H.-C.; Rha, S.Y.; Kim, H.S.; Jung, I.; Nam, B.H.; Lee, K.H.; Chung, H.C. A randomized phase II trial of S-1-oxaliplatin versus capecitabine–oxaliplatin in advanced gastric cancer. Eur. J. Cancer 2012, 48, 518–526. [Google Scholar] [CrossRef]
- Shitara, K.; Sawaki, A.; Matsuo, K.; Kondo, C.; Takahari, D.; Ura, T.; Tajika, M.; Niwa, Y.; Muro, K. A retrospective comparison of S-1 plus cisplatin and capecitabine plus cisplatin for patients with advanced or recurrent gastric cancer. Int. J. Clin. Oncol. 2012, 18, 539–546. [Google Scholar] [CrossRef]
- Yamada, Y.; Higuchi, K.; Nishikawa, K.; Gotoh, M.; Fuse, N.; Sugimoto, N.; Nishina, T.; Amagai, K.; Chin, K.; Niwa, Y.; et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann. Oncol. 2014, 26, 141–148. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.-J.; Feng-Yi, F.; Xu, J.M.; Lee, K.-W.; Jiao, S.-C.; Chong, J.L.; Lopez-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef]
- Tanner, M.; Hollmén, M.; Junttila, T.T.; Kapanen, A.I.; Tommola, S.; Soini, Y.; Helin, H.; Salo, J.; Joensuu, H.; Sihvo, E.; et al. Amplification of HER-2 in gastric carcinoma: Association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 2005, 16, 273–278. [Google Scholar] [CrossRef]
- Dang, H.Z.; Yu, Y.; Jiao, S.C. Prognosis of HER2 over-expressing gastric cancer patients with liver metastasis. World J. Gastroenterol. 2012, 18, 2402–2407. [Google Scholar] [CrossRef]
- Begnami, M.D.; Fukuda, E.; Fregnani, J.H.; Nonogaki, S.; Montagnini, A.L.; da Costa, W.L., Jr.; Soares, F.A. Prognostic Implications of Altered Human Epidermal Growth Factor Receptors (HERs) in Gastric Carcinomas: HER2 and HER3 Are Predictors of Poor Outcome. J. Clin. Oncol. 2011, 29, 3030–3036. [Google Scholar] [CrossRef]
- Jørgensen, J.T.; Hersom, M. HER2 as a Prognostic Marker in Gastric Cancer—A Systematic Analysis of Data from the Literature. J. Cancer 2012, 3, 137–144. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Werner, D.; Pauligk, C.; Steinmetz, K.; Kelsen, D.P.; Jäger, E.; Altmannsberger, H.-M.; Robinson, E.; Tafe, L.J.; Tang, L.H.; et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: A European and USA International collaborative analysis. Ann. Oncol. 2012, 23, 2656–2662. [Google Scholar] [CrossRef]
- Terashima, M.; Kitada, K.; Ochiai, A.; Ichikawa, W.; Kurahashi, I.; Sakuramoto, S.; Katai, H.; Sano, T.; Imamura, H.; Sasako, M.; et al. Impact of Expression of Human Epidermal Growth Factor Receptors EGFR and ERBB2 on Survival in Stage II/III Gastric Cancer. Clin. Cancer Res. 2012, 18, 5992–6000. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- Park, S.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.F.; Duret, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, E.D.; Park, S.; Jiang, Z.; Wang, S.; Fu, Y.-X. Effective Anti-Neu–Initiated Antitumor Responses Require the Complex Role of CD4+ T Cells. Clin. Cancer Res. 2013, 19, 1476–1486. [Google Scholar] [CrossRef]
- Patnaik, A.; Kang, S.P.; Rasco, D.; Papadopoulos, K.P.; Elassaiss-Schaap, J.; Beeram, M.; Drengler, R.; Chen, C.; Smith, L.; Espino, G.; et al. Phase I Study of Pembrolizumab (MK-3475; Anti–PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 4286–4293. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod. Pathol. 2015, 28, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Satoh, T.; Xu, R.H.; Chung, H.C.; Sun, G.P.; Doi, T.; Xu, J.M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.W.; et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—A randomized, phase III study. J. Clin. Oncol. 2014, 32, 2039–2049. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer 2018, 17, 129. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Khazanov, N.A.; Bulen, B.J.; Hovelson, D.H.; Shreve, M.J.; Lamb, L.E.; Matrana, M.R.; Burkard, M.E.; Yang, E.S.; Edenfield, W.J.; et al. Development and validation of an integrative pan-solid tumor predictor of PD-1/PD-L1 blockade benefit. Commun. Med. 2023, 3, 14. [Google Scholar] [CrossRef]
- Kong, J.; Ha, D.; Lee, J.; Kim, I.; Park, M.; Im, S.-H.; Shin, K.; Kim, S. Network-based machine learning approach to predict immunotherapy response in cancer patients. Nat. Commun. 2022, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, C.; Liu, Y.; Yan, X.; Huang, C.; Liu, Y.; Mei, J.; Wang, Z.; Liu, B.; Li, X.; et al. The age variation of HER2 immunohistochemistry positive rate in biopsy specimens of gastric cancer. Pathol. Res. Pract. 2020, 216, 152882. [Google Scholar] [CrossRef]
- Kataoka, Y.; Okabe, H.; Yoshizawa, A.; Minamiguchi, S.; Yoshimura, K.; Haga, H.; Sakai, Y. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer 2013, 16, 84–93. [Google Scholar] [CrossRef]
- Qiu, M.; Zhou, Y.; Zhang, X.; Wang, Z.; Wang, F.; Shao, J.; Lu, J.; Jin, Y.; Wei, X.; Zhang, D.; et al. Lauren classification combined with HER2 status is a better prognostic factor in Chinese gastric cancer patients. BMC Cancer 2014, 14, 823. [Google Scholar] [CrossRef]
- Kim, W.-H.; Gomez-Izquierdo, L.; Vilardell, F.; Chu, K.-M.; Soucy, G.; dos Santos, L.V.; Monges, G.; Viale, G.; Brito, M.J.; Osborne, S.; et al. HER2 Status in Gastric and Gastroesophageal Junction Cancer: Results of the Large, Multinational HER-EAGLE Study. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 239–245. [Google Scholar] [CrossRef]
- Leveque, D.; Gigou, L.; Bergerat, J.P. Clinical Pharmacology of Trastuzumab. Curr. Clin. Pharmacol. 2008, 3, 51–55. [Google Scholar] [CrossRef]
- Lian, J.; Zhang, G.; Zhang, Y.; Liu, H.; Zhang, J.; Nan, P.; Tian, W. PD-L1 and HER2 expression in gastric adenocarcinoma and their prognostic significance. Dig. Liver Dis. 2022, 54, 1419–1427. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Ni, S.; Tan, C.; Cai, X.; Huang, D.; Sheng, W. Programmed death-ligand 1 expression in gastric cancer: Correlation with mismatch repair deficiency and HER2-negative status. Cancer Med. 2018, 7, 2612–2620. [Google Scholar] [CrossRef]

| Study Characteristic | n = 113 | HER2-Positive (n = 12) | HER2-Negative (n = 101) | p-Value |
|---|---|---|---|---|
| Age–median (range), years | 61 (32–84) | 69.5 (50–82) | 60 (32–84) | 0.005 * |
| Sex–no. (%) | 0.999 | |||
| Male | 73 (64.6) | 65 | 8 | |
| Female | 40 (35.4) | 36 | 4 | |
| Performance status–no. (%) | 0.999 | |||
| ECOG 0–1 | 83 (73.4) | 9 | 74 | |
| ECOG 2–3 | 30 (26.5) | 3 | 27 | |
| Histologic classification–no. (%) | 0.001 | |||
| Intestinal or mixed | 44 (38.9) | 11 | 33 | |
| Diffuse or indeterminate | 69 (61.1) | 1 | 68 | |
| PD-L1 status–no. (%) | 0.286 | |||
| CPS ≥ 1 | 33 (29.2) | 2 | 31 | |
| CPS < 1 | 12 (10.6) | 2 | 10 | |
| Unavailable | 68 (60.2) | 8 | 60 | |
| EBV status–no. (%) | 0.999 | |||
| Positive | 3 (2.6) | 0 | 3 | |
| Negative | 83 (73.5) | 8 | 75 | |
| Unknown | 27 (23.9) | 4 | 33 | |
| MSI status–no. (%) | 0.999 | |||
| Unstable (MSI high) | 2 (1.8) | 0 | 2 | |
| Stable | 99 (87.6) | 9 | 90 | |
| Unknown | 12 (10.6) | 3 | 9 | |
| Prior palliative chemotherapy | 0.140 | |||
| Median (range) | 3 (2–5) | 3 (2–5) | 3 (2–5) | |
| 2–no (%) | 3 (25) | 49 (48.5) | ||
| ≥3–no (%) | 9 (75) | 52 (51.5) | ||
| Prior anti-HER2 therapy–no. (%) | 12 (100%) |
| Overall (n = 92) | HER2-Positive (n = 10) | HER2-Negative (n = 82) | Odds Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|---|---|---|
| Complete response | 0 | 0 | 0 | ||
| Partial response | 9 | 5 | 4 | ||
| Stable disease | 29 | 2 | 27 | ||
| Progressive disease | 54 | 3 | 51 | ||
| <0.001 * | |||||
| Overall response rate (%) | 9.8 | 50 | 4.9 | 18.1 (2.9–127.0) | <0.001 * |
| Disease control rate (%) | 41.3 | 70 | 37.8 | 3.8 (0.8–24.3) | 0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.H.; Kim, M.J.; Lee, J.; Lim, H.Y.; Kang, W.K.; Kim, S.T. The Impact of Pembrolizumab as a Salvage Therapy Based on HER2 Expression in Advanced Gastric Cancer. Cancers 2024, 16, 2969. https://doi.org/10.3390/cancers16172969
Lim SH, Kim MJ, Lee J, Lim HY, Kang WK, Kim ST. The Impact of Pembrolizumab as a Salvage Therapy Based on HER2 Expression in Advanced Gastric Cancer. Cancers. 2024; 16(17):2969. https://doi.org/10.3390/cancers16172969
Chicago/Turabian StyleLim, Sung Hee, Min Jung Kim, Jeeyun Lee, Ho Yeong Lim, Won Ki Kang, and Seung Tae Kim. 2024. "The Impact of Pembrolizumab as a Salvage Therapy Based on HER2 Expression in Advanced Gastric Cancer" Cancers 16, no. 17: 2969. https://doi.org/10.3390/cancers16172969
APA StyleLim, S. H., Kim, M. J., Lee, J., Lim, H. Y., Kang, W. K., & Kim, S. T. (2024). The Impact of Pembrolizumab as a Salvage Therapy Based on HER2 Expression in Advanced Gastric Cancer. Cancers, 16(17), 2969. https://doi.org/10.3390/cancers16172969





