Sialylation Inhibition Can Partially Revert Acquired Resistance to Enzalutamide in Prostate Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Cell Culture
2.2. Inhibitors
2.3. Western Blotting
2.4. ELISA Assays
2.5. Immunocytochemistry
2.6. Lectin Immunofluorescence
2.7. CellTiter-Glo® Assays
2.8. Statistical Analyses
3. Results
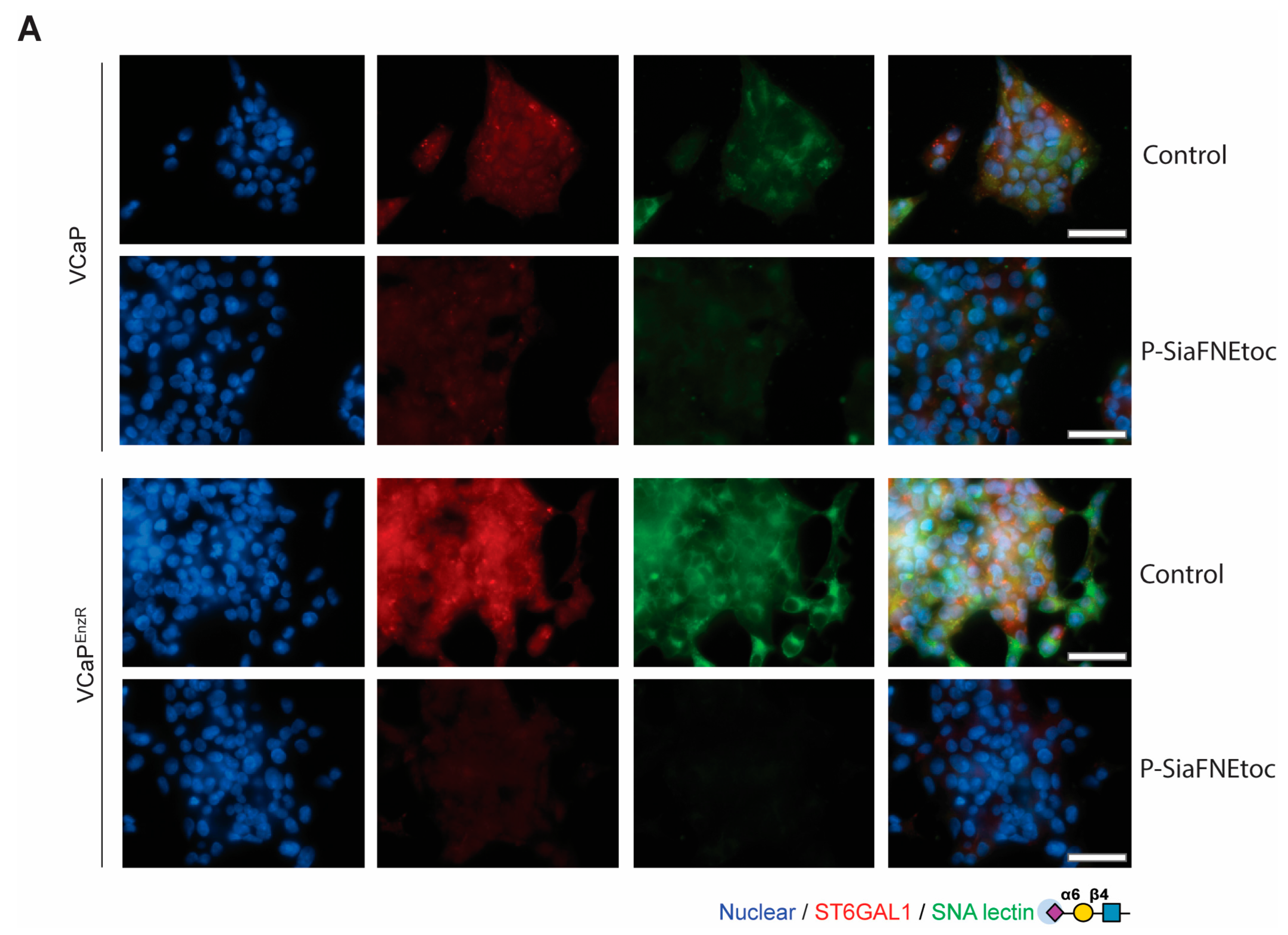
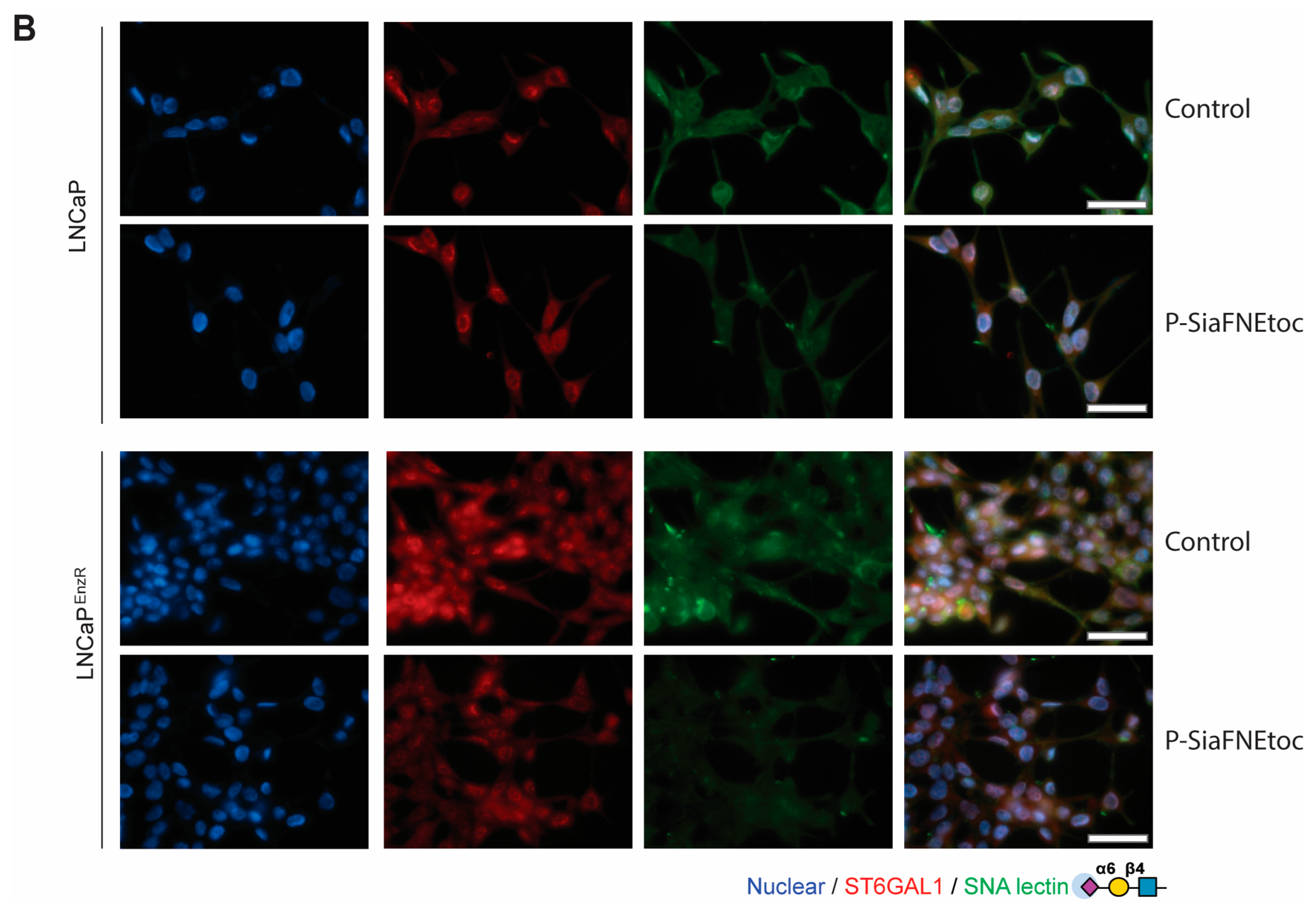
3.1. Prostate Cancer Cells with Acquired Enzalutamide Resistance Have Upregulated ST6GAL1
3.2. Enzalutamide-Resistant Prostate Cancer Cells Have Increased Levels of α2,6-Sialylated N-Glycans
3.3. The Sialyltransferase Inhibitor P-SiaFNEtoc Blocks α2,6 Sialylation in Enzalutamide-Resistant Prostate Cancer Cells
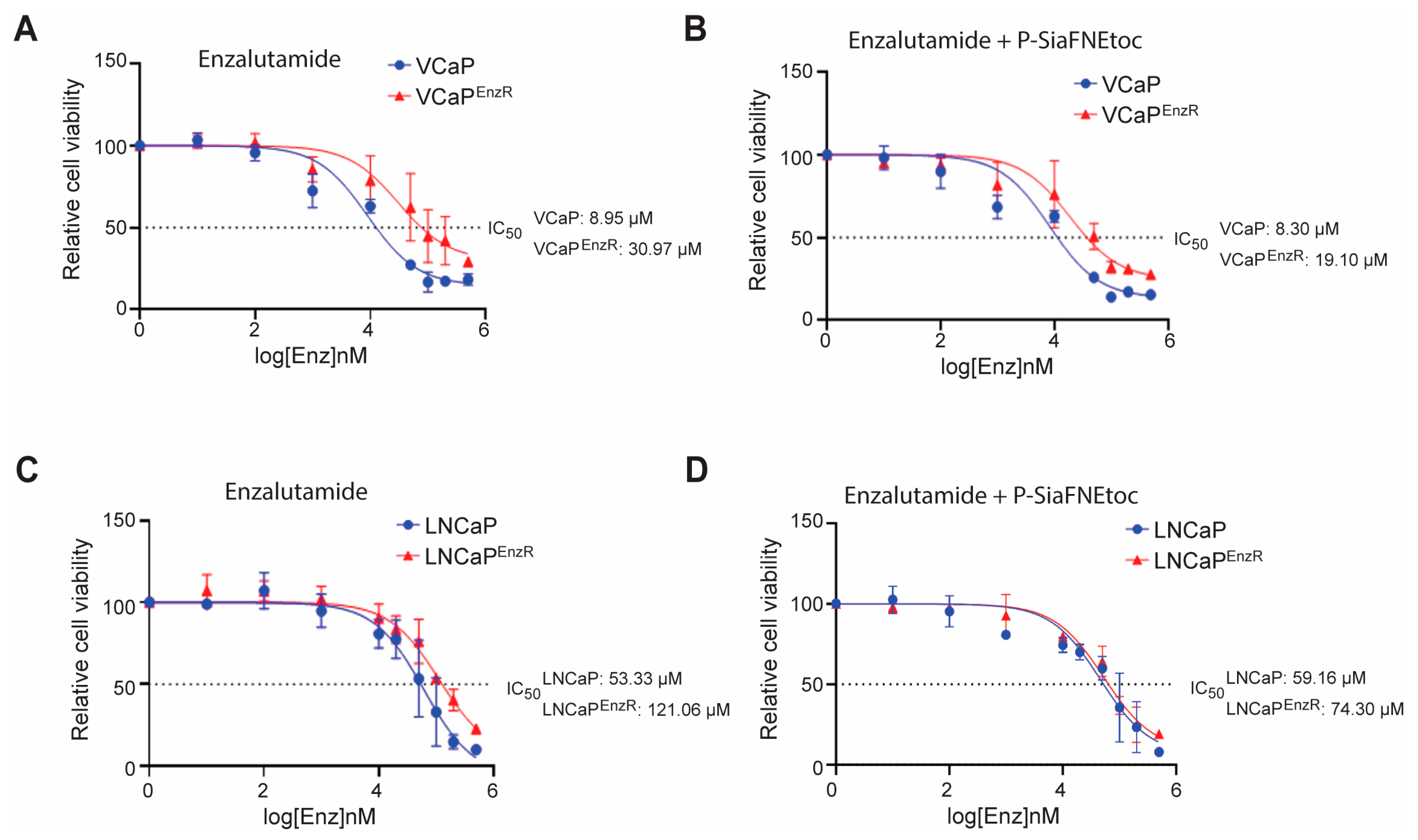
3.4. Sialic Acid Blockade Partially Reverts Acquired Resistance to Enzalutamide in Prostate Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Archer Goode, E.; Wang, N.; Munkley, J. Prostate cancer bone metastases biology and clinical management (Review). Oncol. Lett. 2023, 25, 163. [Google Scholar] [CrossRef]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Mens Health 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.; Ross, A.E.; Parikh, R.B.; Nohria, A.; Morgans, A.K. How to Treat Prostate Cancer With Androgen Deprivation and Minimize Cardiovascular Risk: A Therapeutic Tightrope. JACC CardioOncol. 2021, 3, 737–741. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef]
- Nanda, J.S.; Koganti, P.; Perri, G.; Ellis, L. Phenotypic Plasticity-Alternate Transcriptional Programs Driving Treatment Resistant Prostate Cancer. Crit. Rev. Oncog. 2022, 27, 45–60. [Google Scholar] [CrossRef]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef]
- Vellky, J.E.; Ricke, W.A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia 2020, 22, 566–575. [Google Scholar] [CrossRef]
- Adashek, J.J.; Jain, R.K.; Zhang, J. Clinical Development of PARP Inhibitors in Treating Metastatic Castration-Resistant Prostate Cancer. Cells 2019, 8, 860. [Google Scholar] [CrossRef]
- Aly, M.; Leval, A.; Schain, F.; Liwing, J.; Lawson, J.; Vágó, E.; Nordström, T.; Andersson, T.M.-L.; Sjöland, E.; Wang, C.; et al. Survival in patients diagnosed with castration-resistant prostate cancer: A population-based observational study in Sweden. Scand. J. Urol. 2020, 54, 115–121. [Google Scholar] [CrossRef]
- Amaral, T.M.; Macedo, D.; Fernandes, I.; Costa, L. Castration-resistant prostate cancer: Mechanisms, targets, and treatment. Prostate Cancer 2012, 2012, 327253. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Shore, N.D.; Chowdhury, S.; Villers, A.; Klotz, L.; Siemens, D.R.; Phung, D.; van Os, S.; Hasabou, N.; Wang, F.; Bhattacharya, S.; et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): A randomised, double-blind, phase 2 study. Lancet Oncol. 2016, 17, 153–163. [Google Scholar] [CrossRef]
- Dong, L.; Zieren, R.C.; Xue, W.; de Reijke, T.M.; Pienta, K.J. Metastatic prostate cancer remains incurable, why? Asian J. Urol. 2019, 6, 26–41. [Google Scholar] [CrossRef]
- Cicero, G.D.E.L.; Dorangricchia, P.; Dieli, F. The Clinical Efficacy of Enzalutamide in Metastatic Prostate Cancer: Prospective Single-center Study. Anticancer. Res. 2017, 37, 1475–1480. [Google Scholar] [PubMed]
- Linder, S.; van der Poel, H.G.; Bergman, A.M.; Zwart, W.; Prekovic, S. Enzalutamide therapy for advanced prostate cancer: Efficacy, resistance and beyond. Endocr. Relat. Cancer 2018, 26, R31–R52. [Google Scholar] [CrossRef] [PubMed]
- Schalken, J.; Fitzpatrick, J.M. Enzalutamide: Targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2015, 117, 215–225. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.; Kelly, W.K. Enzalutamide: A novel antiandrogen for patients with castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; De Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Penson, D.F.; Armstrong, A.J.; Concepcion, R.; Agarwal, N.; Olsson, C.; Karsh, L.; Dunshee, C.; Wang, F.; Wu, K.; Krivoshik, A.; et al. Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J. Clin. Oncol. 2016, 34, 2098–2106. [Google Scholar] [CrossRef]
- Tucci, M.; Zichi, C.; Buttigliero, C.; Vignani, F.; Scagliotti, G.V.; Di Maio, M. Enzalutamide-resistant castration-resistant prostate cancer: Challenges and solutions. Onco Targets Ther. 2018, 11, 7353–7368. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wu, Z.; Ding, W.; Gao, S.; Gao, Y.; Xu, C. Mechanisms of enzalutamide resistance in castration-resistant prostate cancer and therapeutic strategies to overcome it. Br. J. Pharmacol. 2020, 178, 239–261. [Google Scholar] [CrossRef]
- Lin, H.-M.; Mak, B.; Yeung, N.; Huynh, K.; Meikle, T.G.; Mellett, N.A.; Kwan, E.M.; Fettke, H.; Tran, B.; Davis, I.D.; et al. Overcoming enzalutamide resistance in metastatic prostate cancer by targeting sphingosine kinase. EBioMedicine 2021, 72, 103625. [Google Scholar] [CrossRef] [PubMed]
- Claessens, F.; Helsen, C.; Prekovic, S.; Broeck, T.V.D.; Spans, L.; Van Poppel, H.; Joniau, S. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nat. Rev. Urol. 2014, 11, 712–716. [Google Scholar] [CrossRef]
- Hussain, A.; Dawson, N. Management of advanced/metastatic prostate cancer: 2000 update. Oncology 2000, 14, 1677–1688; discussion 1688, 1691–1694. [Google Scholar] [PubMed]
- Blatt, E.B.; Raj, G.V. Molecular mechanisms of enzalutamide resistance in prostate cancer. Cancer Drug Resist. 2019, 2, 189–197. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Eichler, J. Protein glycosylation. Curr. Biol. 2019, 29, R229–R231. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef] [PubMed]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2017, 34, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Rodrigues, J.G.; Duarte, H.O.; Reis, C.A.; Gomes, J. Aberrant protein glycosylation in cancer: Implications in targeted therapy. Biochem. Soc. Trans. 2021, 49, 843–854. [Google Scholar] [CrossRef]
- Sinha, A.; Huang, V.; Livingstone, J.; Wang, J.; Fox, N.S.; Kurganovs, N.; Ignatchenko, V.; Fritsch, K.; Donmez, N.; Heisler, L.E.; et al. The Proteogenomic Landscape of Curable Prostate Cancer. Cancer Cell 2019, 35, 414–427 e6. [Google Scholar] [CrossRef]
- Cheng, Q.; Butler, W.; Zhou, Y.; Zhang, H.; Tang, L.; Perkinson, K.; Chen, X.; Jiang, X.; McCall, S.J.; Inman, B.A.; et al. Pre-existing Castration-resistant Prostate Cancer-like Cells in Primary Prostate Cancer Promote Resistance to Hormonal Therapy. Eur. Urol. 2022, 81, 446–455. [Google Scholar] [CrossRef]
- Ren, S.; Shao, Y.; Zhao, X.; Hong, C.S.; Wang, F.; Lu, X.; Li, J.; Ye, G.; Yan, M.; Zhuang, Z.; et al. Integration of Metabolomics and Transcriptomics Reveals Major Metabolic Pathways and Potential Biomarker Involved in Prostate Cancer. Mol. Cell Proteom. 2016, 15, 154–163. [Google Scholar] [CrossRef]
- Le, T.K.; Duong, Q.H.; Baylot, V.; Fargette, C.; Baboudjian, M.; Colleaux, L.; Taïeb, D.; Rocchi, P. Castration-Resistant Prostate Cancer: From Uncovered Resistance Mechanisms to Current Treatments. Cancers 2023, 15, 5047. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Powers, T.W.; Neely, B.A.; Shao, Y.; Tang, H.; Troyer, D.A.; Mehta, A.S.; Haab, B.B.; Drake, R.R. MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS ONE 2014, 9, e106255. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.R.; Powers, T.W.; Norris-Caneda, K.; Mehta, A.S.; Angel, P.M. In Situ Imaging of N-Glycans by MALDI Imaging Mass Spectrometry of Fresh or Formalin-Fixed Paraffin-Embedded Tissue. Curr. Protoc. Protein Sci. 2018, 94, e68. [Google Scholar] [CrossRef] [PubMed]
- West, C.A.; Liang, H.; Drake, R.R.; Mehta, A.S. New Enzymatic Approach to Distinguish Fucosylation Isomers of N-Linked Glycans in Tissues Using MALDI Imaging Mass Spectrometry. J. Proteome Res. 2020, 19, 2989–2996. [Google Scholar] [CrossRef]
- Wallace, E.N.; West, C.A.; McDowell, C.T.; Lu, X.; Bruner, E.; Mehta, A.S.; Aoki-Kinoshita, K.F.; Angel, P.M.; Drake, R.R. An N-glycome tissue atlas of 15 human normal and cancer tissue types determined by MALDI-imaging mass spectrometry. Sci. Rep. 2024, 14, 489. [Google Scholar] [CrossRef]
- Scott, E.; Munkley, J. Glycans as Biomarkers in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1389. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Mills, I.G.; Elliott, D.J. The role of glycans in the development and progression of prostate cancer. Nat. Rev. Urol. 2016, 13, 324–333. [Google Scholar] [CrossRef]
- Kałuża, A.; Szczykutowicz, J.; Ferens-Sieczkowska, M. Glycosylation: Rising Potential for Prostate Cancer Evaluation. Cancers 2021, 13, 3726. [Google Scholar] [CrossRef]
- Butler, W.; Huang, J. Glycosylation Changes in Prostate Cancer Progression. Front. Oncol. 2021, 11, 809170. [Google Scholar] [CrossRef]
- Costa, A.F.; Campos, D.; Reis, C.A.; Gomes, C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer 2020, 6, 757–766. [Google Scholar] [CrossRef]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef]
- Orozco-Moreno, M.; Visser, E.A.; Hodgson, K.; Hipgrave Ederveen, A.L.; Bastian, K.; Goode, E.A.; Öztürk, Ö.; Pijnenborg, J.F.A.; Eerden, N.; Moons, S.J.; et al. Targeting aberrant sialylation and fucosylation in prostate cancer cells using potent metabolic inhibitors. Glycobiology 2023, 33, 1155–1171. [Google Scholar] [CrossRef]
- Hodgson, K.; Orozco-Moreno, M.; Goode, E.A.; Fisher, M.; Garnham, R.; Beatson, R.; Turner, H.; Livermore, K.; Zhou, Y.; Wilson, L.; et al. Sialic acid blockade inhibits the metastatic spread of prostate cancer to bone. EBioMedicine 2024, 104, 105163. [Google Scholar] [CrossRef]
- Wen, R.; Stark, J.; Marti, G.E.; Riley, N.; Zhao, H.; Bertozzi, C.R.; Pitteri, S.; Brooks, J.D. 517 Blocking Siglec-7/9-sialic acid interactions induces immune cell-mediated suppression of prostate cancer. J. ImmunoTherapy Cancer 2023, 11 (Suppl. 1), A583. [Google Scholar]
- Wen, R.; Marti, G.E.; Stark, J.; Marques, F.J.G.; Riley, N.; Bermudez, A.; Zhao, H.; Bertozzi, C.; Pitteri, S.; Brooks, J. Abstract 7524: Targeting siglec-7/9 glyco-immune checkpoints in prostate cancer for enhanced immune responses and tumor suppression. Cancer Res. 2024, 84 (Suppl. 6), 7524. [Google Scholar] [CrossRef]
- Munkley, J. Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers 2022, 14, 4248. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2020, 124, 76–90. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Zhang, G. Insights into the Role of Sialylation in Cancer Metastasis, Immunity, and Therapeutic Opportunity. Cancers 2022, 23, 5840. [Google Scholar] [CrossRef]
- Jastrzab, P.; Narejko, K.; Car, H.; Wielgat, P. Cell Membrane Sialome: Sialic Acids as Therapeutic Targets and Regulators of Drug Resistance in Human Cancer Management. Cancers 2023, 15, 5103. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Ruan, Y.; Sun, L.; Xu, C.; Jiang, H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-beta1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018, 9, 1102. [Google Scholar] [CrossRef]
- Schultz, M.J.; Swindall, A.F.; Wright, J.W.; Sztul, E.S.; Landen, C.N.; Bellis, S.L. ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J. Ovarian Res. 2013, 6, 25. [Google Scholar] [CrossRef]
- Ou, L.; He, X.; Liu, N.; Song, Y.; Li, J.; Gao, L.; Huang, X.; Deng, Z.; Wang, X.; Lin, S. Sialylation of FGFR1 by ST6Gal-I overexpression contributes to ovarian cancer cell migration and chemoresistance. Mol. Med. Rep. 2020, 21, 1449–1460. [Google Scholar] [CrossRef]
- Santos, S.N.; Junqueira, M.S.; Francisco, G.; Vilanova, M.; Magalhães, A.; Baruffi, M.D.; Chammas, R.; Harris, A.L.; Reis, C.A.; Bernardes, E.S. O-glycan sialylation alters galectin-3 subcellular localization and decreases chemotherapy sensitivity in gastric cancer. Oncotarget 2016, 7, 83570–83587. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Zhao, Y.; Yuan, S.; Wu, Q.; Zhu, X.; Niang, B.; Wang, S.; Zhang, J. ST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathway. Oncotarget 2016, 7, 51955–51964. [Google Scholar] [CrossRef]
- Patel, K.D.; De, M.; Jethva, D.D.; Rathod, B.S.; Patel, P.S. Alterations in Sialylation Patterns are Significantly Associated with Imatinib Mesylate Resistance in Chronic Myeloid Leukemia. Arch. Med. Res. 2022, 53, 51–58. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, H.; Song, X.; Shi, S.; Zhang, J.; Jia, L. Modification of sialylation is associated with multidrug resistance in human acute myeloid leukemia. Oncogene 2015, 34, 726–740. [Google Scholar] [CrossRef]
- Yen, H.-Y.; Liu, Y.-C.; Chen, N.-Y.; Tsai, C.-F.; Wang, Y.-T.; Chen, Y.-J.; Hsu, T.-L.; Yang, P.-C.; Wong, C.-H. Effect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibition. Proc. Natl. Acad. Sci. USA 2015, 112, 6955–6960. [Google Scholar] [CrossRef]
- Natoni, A.; Farrell, M.L.; Harris, S.; Falank, C.; Kirkham-McCarthy, L.; Macauley, M.S.; Reagan, M.R.; O’dwyer, M. Sialyltransferase inhibition leads to inhibition of tumor cell interactions with E-selectin, VCAM1, and MADCAM1, and improves survival in a human multiple myeloma mouse model. Haematologica 2020, 105, 457–467. [Google Scholar] [CrossRef]
- Lee, M.; Lee, H.J.; Bae, S.; Lee, Y.S. Protein sialylation by sialyltransferase involves radiation resistance. Mol. Cancer Res. 2008, 6, 1316–1325. [Google Scholar] [CrossRef]
- Lee, M.; Lee, H.J.; Seo, W.D.; Park, K.H.; Lee, Y.S. Sialylation of integrin beta1 is involved in radiation-induced adhesion and migration in human colon cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1528–1536. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, M. Increasing the alpha 2, 6 sialylation of glycoproteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver 2013, 7, 629–641. [Google Scholar] [CrossRef]
- Punch, P.R.; Irons, E.E.; Manhardt, C.T.; Marathe, H.; Lau, J.T.Y. The sialyltransferase ST6GAL1 protects against radiation-induced gastrointestinal damage. Glycobiology 2020, 30, 446–453. [Google Scholar] [CrossRef]
- Duarte, H.O.; Rodrigues, J.G.; Gomes, C.; Hensbergen, P.J.; Ederveen, A.L.H.; de Ru, A.H.; Mereiter, S.; Polónia, A.; Fernandes, E.; Ferreira, J.A.; et al. ST6Gal1 targets the ectodomain of ErbB2 in a site-specific manner and regulates gastric cancer cell sensitivity to trastuzumab. Oncogene 2021, 40, 3719–3733. [Google Scholar] [CrossRef]
- Smithson, M.; Irwin, R.; Williams, G.; Alexander, K.L.; Smythies, L.E.; Nearing, M.; McLeod, M.C.; Al Diffalha, S.; Bellis, S.L.; Hardiman, K.M. Sialyltransferase ST6GAL-1 mediates resistance to chemoradiation in rectal cancer. J. Biol. Chem. 2022, 298, 101594. [Google Scholar] [CrossRef]
- Butler, W.; McDowell, C.; Yang, Q.; He, Y.; Zhao, Y.; Hauck, J.S.; Zhou, Y.; Zhang, H.; Armstrong, A.J.; George, D.J.; et al. Rewiring of the N-Glycome with prostate cancer progression and therapy resistance. npj Precis. Oncol. 2023, 7, 22. [Google Scholar] [CrossRef]
- Wen, R.; Zhao, H.; Zhang, D.; Chiu, C.-L.; Brooks, J.D. Sialylated glycoproteins as biomarkers and drivers of progression in prostate cancer. Carbohydr. Res. 2022, 519, 108598. [Google Scholar] [CrossRef]
- Hartig, J.; Young, L.E.A.; Grimsley, G.; Mehta, A.S.; Ippolito, J.E.; Leach, R.J.; Angel, P.M.; Drake, R.R. The glycosylation landscape of prostate cancer tissues and biofluids. Adv Cancer Res 2024, 161, 1–30. [Google Scholar]
- Scott, E.; Goode, E.A.; Garnham, R.; Hodgson, K.; Orozco-Moreno, M.; Turner, H.; Livermore, K.; Nangkana, K.P.; Frame, F.M.; Bermudez, A.; et al. ST6GAL1-mediated aberrant sialylation promotes prostate cancer progression. J. Pathol. 2023, 261, 71–84. [Google Scholar] [CrossRef]
- Chakraborty, A.; Dorsett, K.A.; Trummell, H.Q.; Yang, E.S.; Oliver, P.G.; Bonner, J.A.; Buchsbaum, D.J.; Bellis, S.L. ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage. J. Biol. Chem. 2018, 293, 984–994. [Google Scholar] [CrossRef]
- Smithson, M.; Al Diffalha, S.; Irwin, R.K.; Williams, G.; McLeod, M.C.; Somasundaram, V.; Bellis, S.L.; Hardiman, K.M. ST6GAL1 is associated with poor response to chemoradiation in rectal cancer. Neoplasia 2024, 51, 100984. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, T.; Yang, L.; Kolarich, D.; Heisterkamp, N. Multi-Faceted Effects of ST6Gal1 Expression on Precursor B-Lineage Acute Lymphoblastic Leukemia. Front. Oncol. 2022, 12, 828041. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Sun, X.; Liu, B.; Xiao, Y.; Pan, S.; Zhou, H.; Dong, W.; Jia, L. The regulatory ZFAS1/miR-150/ST6GAL1 crosstalk modulates sialylation of EGFR via PI3K/Akt pathway in T-cell acute lymphoblastic leukemia. J. Exp. Clin. Cancer Res. 2019, 38, 199. [Google Scholar] [CrossRef]
- Heise, T.; Pijnenborg, J.F.A.; Büll, C.; van Hilten, N.; Kers-Rebel, E.D.; Balneger, N.; Elferink, H.; Adema, G.J.; Boltje, T.J. Potent Metabolic Sialylation Inhibitors Based on C-5-Modified Fluorinated Sialic Acids. J. Med. Chem. 2019, 62, 1014–1021. [Google Scholar] [CrossRef]
- Moons, S.J.; Rossing, E.; Janssen, M.A.C.H.; Heise, T.; Büll, C.; Adema, G.J.; Boltje, T.J. Structure-Activity Relationship of Metabolic Sialic Acid Inhibitors and Labeling Reagents. ACS Chem. Biol. 2022, 17, 590–597. [Google Scholar] [CrossRef]
- Munkley, J.; Li, L.; Krishnan, S.R.G.; Hysenaj, G.; Scott, E.; Dalgliesh, C.; Oo, H.Z.; Maia, T.M.; Cheung, K.; Ehrmann, I.; et al. Androgen-regulated transcription of ESRP2 drives alternative splicing patterns in prostate cancer. Elife 2019, 8, e47678. [Google Scholar] [CrossRef]
- Kregel, S.; Chen, J.L.; Tom, W.; Krishnan, V.; Kach, J.; Brechka, H.; Fessenden, T.B.; Isikbay, M.; Paner, G.P.; Szmulewitz, R.Z.; et al. Acquired resistance to the second-generation androgen receptor antagonist enzalutamide in castration-resistant prostate cancer. Oncotarget 2016, 7, 26259–26274. [Google Scholar] [CrossRef]
- Scott, E.; Hodgson, K.; Calle, B.; Turner, H.; Cheung, K.; Bermudez, A.; Marques, F.J.G.; Pye, H.; Yo, E.C.; Islam, K.; et al. Upregulation of GALNT7 in prostate cancer modifies O-glycosylation and promotes tumour growth. Oncogene 2023, 42, 926–937. [Google Scholar] [CrossRef]
- Munkley, J.; Vodak, D.; Livermore, K.E.; James, K.; Wilson, B.T.; Knight, B.; Mccullagh, P.; Mcgrath, J.; Crundwell, M.; Harries, L.W.; et al. Glycosylation is an Androgen-Regulated Process Essential for Prostate Cancer Cell Viability. EBioMedicine 2016, 8, 103–116. [Google Scholar] [CrossRef]
- Wei, A.; Fan, B.; Zhao, Y.; Zhang, H.; Wang, L.; Yu, X.; Yuan, Q.; Yang, D.; Wang, S. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3beta/beta-catenin signaling pathway. Oncotarget 2016, 7, 65374–65388. [Google Scholar] [CrossRef]
- Garnham, R.; Scott, E.; Livermore, K.E.; Munkley, J. ST6GAL1: A key player in cancer. Oncol. Lett. 2019, 18, 983–989. [Google Scholar]
- Gc, S.; Bellis, S.L.; Hjelmeland, A.B. ST6Gal1: Oncogenic signaling pathways and targets. Front. Mol. Biosci. 2022, 9, 962908. [Google Scholar] [CrossRef]
- Bojar, D.; Meche, L.; Meng, G.; Eng, W.; Smith, D.F.; Cummings, R.D.; Mahal, L.K. A Useful Guide to Lectin Binding: Machine-Learning Directed Annotation of 57 Unique Lectin Specificities. ACS Chem. Biol. 2022, 17, 2993–3012. [Google Scholar] [CrossRef] [PubMed]
- Korenchuk, S.; Lehr, J.E.; McLean, L.; Lee, Y.G.; Whitney, S.; Vessella, R.; Lin, D.L.; Pienta, K.J. VCaP, a cell-based model system of human prostate cancer. In Vivo 2001, 15, 163–168. [Google Scholar]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z. Molecular Mechanisms of Enzalutamide Resistance in Prostate Cancer. Curr. Mol. Biol. Rep. 2017, 3, 230–235. [Google Scholar] [CrossRef]
- Prekovic, S.; van den Broeck, T.; Linder, S.; van Royen, M.E.; Houtsmuller, A.B.; Handle, F.; Joniau, S.; Zwart, W.; Claessens, F. Molecular underpinnings of enzalutamide resistance. Endocr. Relat. Cancer 2018, 25, R545–R557. [Google Scholar] [CrossRef] [PubMed]
- Dorsett, K.A.; Marciel, M.P.; Hwang, J.; E Ankenbauer, K.; Bhalerao, N.; Bellis, S.L. Regulation of ST6GAL1 sialyltransferase expression in cancer cells. Glycobiology 2021, 31, 530–539. [Google Scholar] [CrossRef]
- Gc, S.; Tuy, K.; Rickenbacker, L.; Jones, R.; Chakraborty, A.; Miller, C.R.; Beierle, E.A.; Hanumanthu, V.S.; Tran, A.N.; Mobley, J.A.; et al. α2,6 Sialylation mediated by ST6GAL1 promotes glioblastoma growth. J. Clin. Investig. 2022, 7, 158799. [Google Scholar] [CrossRef]
- Pearce, O.M.; Laubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2016, 26, 111–128. [Google Scholar] [CrossRef]
- Britain, C.M.; Holdbrooks, A.T.; Anderson, J.C.; Willey, C.D.; Bellis, S.L. Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J. Ovarian Res. 2018, 11, 12. [Google Scholar] [CrossRef]
- Cerasuolo, M.; Maccarinelli, F.; Coltrini, D.; Mahmoud, A.M.; Marolda, V.; Ghedini, G.C.; Rezzola, S.; Giacomini, A.; Triggiani, L.; Kostrzewa, M.; et al. Modeling Acquired Resistance to the Second-Generation Androgen Receptor Antagonist Enzalutamide in the TRAMP Model of Prostate Cancer. Cancer Res. 2020, 80, 1564–1577. [Google Scholar] [CrossRef]
- Al Saoud, R.; Hamrouni, A.; Idris, A.; Mousa, W.K.; Izneid, T.A. Recent advances in the development of sialyltransferase inhibitors to control cancer metastasis: A comprehensive review. Biomed Pharmacother 2023, 165, 115091. [Google Scholar] [CrossRef]
- Büll, C.; Brok, M.H.D.; Adema, G.J. Sweet escape: Sialic acids in tumor immune evasion. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2014, 1846, 238–246. [Google Scholar] [CrossRef]
- Zhou, X.; Chi, K.; Zhang, C.; Liu, Q.; Yang, G. Sialylation: A Cloak for Tumors to Trick the Immune System in the Microenvironment. Biology 2023, 12, 832. [Google Scholar] [CrossRef]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef]
- Lim, J.; Sari-Ak, D.; Bagga, T. Siglecs as Therapeutic Targets in Cancer. Biology 2021, 10, 1178. [Google Scholar] [CrossRef]
- Long, X.; Hou, H.; Wang, X.; Liu, S.; Diao, T.; Lai, S.; Hu, M.; Zhang, S.; Liu, M.; Zhang, H. Immune signature driven by ADT-induced immune microenvironment remodeling in prostate cancer is correlated with recurrence-free survival and immune infiltration. Cell Death Dis. 2020, 11, 779. [Google Scholar] [CrossRef]
- Gamat-Huber, M.; McNeel, D.G. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr.-Relat. Cancer 2017, 24, T297–T310. [Google Scholar] [CrossRef]
- Garnham, R.; Geh, D.; Nelson, R.; Ramon-Gil, E.; Wilson, L.; Schmidt, E.N.; Walker, L.; Adamson, B.; Buskin, A.; Hepburn, A.C.; et al. ST3 beta-galactoside alpha-2,3-sialyltransferase 1 (ST3Gal1) synthesis of Siglec ligands mediates anti-tumour immunity in prostate cancer. Commun Biol. 2024, 7, 276. [Google Scholar] [CrossRef]
- Berois, N.; Pittini, A.; Osinaga, E. Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives. Cancers 2022, 14, 645. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goode, E.A.; Orozco-Moreno, M.; Hodgson, K.; Nabilah, A.; Murali, M.; Peng, Z.; Merx, J.; Rossing, E.; Pijnenborg, J.F.A.; Boltje, T.J.; et al. Sialylation Inhibition Can Partially Revert Acquired Resistance to Enzalutamide in Prostate Cancer Cells. Cancers 2024, 16, 2953. https://doi.org/10.3390/cancers16172953
Goode EA, Orozco-Moreno M, Hodgson K, Nabilah A, Murali M, Peng Z, Merx J, Rossing E, Pijnenborg JFA, Boltje TJ, et al. Sialylation Inhibition Can Partially Revert Acquired Resistance to Enzalutamide in Prostate Cancer Cells. Cancers. 2024; 16(17):2953. https://doi.org/10.3390/cancers16172953
Chicago/Turabian StyleGoode, Emily Archer, Margarita Orozco-Moreno, Kirsty Hodgson, Amirah Nabilah, Meera Murali, Ziqian Peng, Jona Merx, Emiel Rossing, Johan F. A. Pijnenborg, Thomas J. Boltje, and et al. 2024. "Sialylation Inhibition Can Partially Revert Acquired Resistance to Enzalutamide in Prostate Cancer Cells" Cancers 16, no. 17: 2953. https://doi.org/10.3390/cancers16172953
APA StyleGoode, E. A., Orozco-Moreno, M., Hodgson, K., Nabilah, A., Murali, M., Peng, Z., Merx, J., Rossing, E., Pijnenborg, J. F. A., Boltje, T. J., Wang, N., Elliott, D. J., & Munkley, J. (2024). Sialylation Inhibition Can Partially Revert Acquired Resistance to Enzalutamide in Prostate Cancer Cells. Cancers, 16(17), 2953. https://doi.org/10.3390/cancers16172953







