Inflammatory Bowel Disease and Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Pathogenesis and Epidemiology
4. Risk Factors
4.1. Patient-Related Factors
4.2. Disease-Related Factors
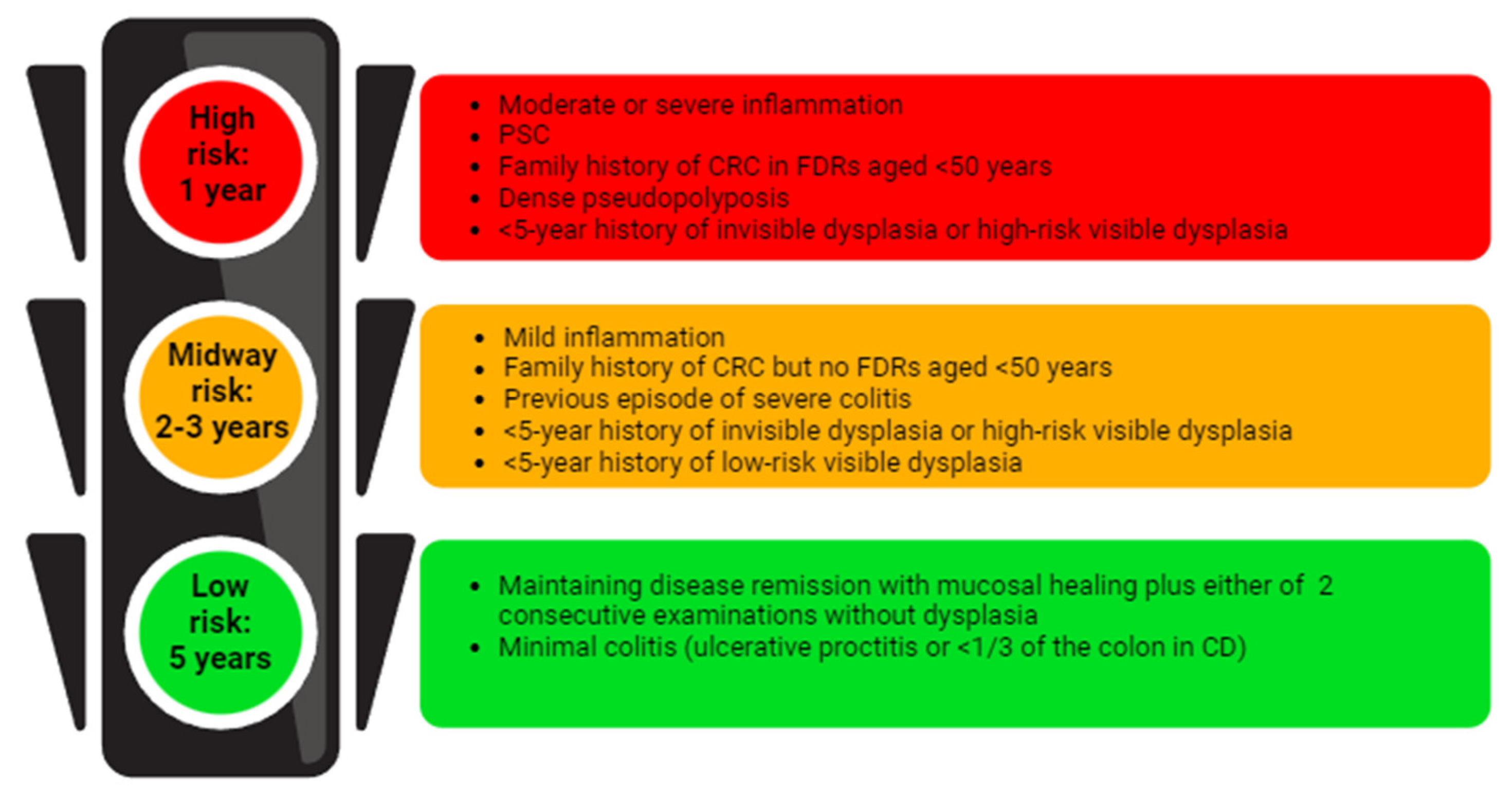
5. Endoscopic Surveillance
6. IBD Therapy and Cancer
6.1. Management of IBD Therapy in Patients with a History of Previous Cancer
6.2. Management of IBD Therapy in Patients with Current Cancer
7. Management of Chemotherapy, Immunotherapy, and Radiation Therapy in IBD
7.1. Chemotherapy
7.2. Immunotherapy
7.3. Radiotherapy
| Study, Authors, Year of Publication | Patients and IBD Subtype (IBD Remission or Active IBD, if Available) | Type of Cancer (Type of Cancer in IBD Remission, Type of Cancer in Active IBD) | Typer of Cancer Treatment (Type of Treatment in IBD Remission, Type of Treatment in Active IBD) | Main Results | ||
|---|---|---|---|---|---|---|
| Effects of Cancer Treatment on IBD Remission and Reactivation, Jordan E. Axelrad et al., 2012 [72] | 84 patients - UC 45 (40, 5) - CD 39 (29, 10) | Breast 37 (30, 7) Lung 12 (10, 2) GI 19 (16, 3) | Cytotoxic CT 46 (41, 5) Hormonal 22 (16, 6) Cytotoxic + hormonal 16 (12, 4) | Active IBD group 10 IBD remission: 5 cytotoxic CT 1 hormonal 4 combination therapy | Inactive IBD group 12 IBD flare-ups: 1 cytotoxic CT 6 hormonal 5 combination therapy | |
| Hormone Therapy for Cancer is a Risk Factor for Relapse of IBD, J. E. Axelrad et al., 2021 [73] | 447 patients - UC 238 (214, 24) - CD 197 (175, 22) - IBD-U 12 (11, 1) | Breast 346 (315, 31) Prostate 101 (85, 16) | Cytotoxic CT 34 (34, 0) Hormonal 187 (164, 23) Cytotoxic CT + hormonal 73 (65, 8) Other therapies or unknown 165 (148, 17) | Active IBD group, risk for IBD remission (95% CI) Cytotoxic CT: - Hormonal: HR 1.98 (0.42–9.34) Cytotoxic CT + hormonal: HR 2.09 (0.35–12.5) | Inactive IBD group, risk of flare-up (95% CI) Cytotoxic CT: HR 0.91 (0.34–2.42) Hormonal: HR 2.00 (1.21–3.29) Cytotoxic CT + hormonal: HR 1.86 (1.01–3.43) | |
| Acute and late toxicity of patients with IBD undergoing irradiation for abdominal and pelvic neoplasm, C. G. Willett et al., 1999 [87] | 28 patients - UC 18 - CD 10 | CRC 17 Prostate 7 Endometrial 2 Pancreatic 1 Small bowel 1 [no data regarding active or remission IBD] | Radiotherapy techniques Conventional 12 Specialized 16 [no data regarding active or remission IBD] | Frequency of toxicities (conventional, specialized) Total severe toxicity 46% (58%, 38%) - Severe acute toxicity 21% (17%, 25%) - Severe late toxicity 29% (50%, 13%) | ||
| Rates of Adverse IBD-Related Outcomes for Patients with IBD and Concomitant Prostate Cancer Treated With Radiation Therapy, L. A. Feagins et al., 2020 [89] | 100 patients - UC 66 - CD 29 - IBD-U 5 | Prostate 100 | XRT/brachytherapy 47 Nonradiation therapy 53 | Rates of IBD flare-up XRT/brachytherapy vs. nonradiation therapy - within 6 months: 10.6%, 5.7% - within 6–12 months: 4.3%, 1.9% - within 12–24 months: 8.5%, 9.4% | ||
| Implications of prostate cancer treatment in men with IBD, P. S. Kirk et al., 2018 [91] | 205 patients [no data regarding IBD type] | Prostate 205 | Surgery 85 Radiotherapy 56 ADT/observation 64 | Rate of IBD flare-up in years following treatment | ||
| Surgery 13% Radiotherapy 23% p = 0.28 |  | ADT/observation 19% | ||||
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Porter, R.J.; Arends, M.J.; Churchhouse, A.M.D.; Din, S. Inflammatory Bowel Disease-Associated Colorectal Cancer: Translational Risks from Mechanisms to Medicines. J. Crohns Colitis 2021, 15, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Sato, Y.; Tsujinaka, S.; Miura, T.; Kitamura, Y.; Suzuki, H.; Shibata, C. Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management. Cancers 2023, 15, 4154. [Google Scholar] [CrossRef]
- Westwood, M.; Lang, S.; Armstrong, N.; Van Turenhout, S.; Cubiella, J.; Stirk, L.; Ramos, I.C.; Luyendijk, M.; Zaim, R.; Kleijnen, J.; et al. Faecal Immunochemical Tests (FIT) Can Help to Rule out Colorectal Cancer in Patients Presenting in Primary Care with Lower Abdominal Symptoms: A Systematic Review Conducted to Inform New NICE DG30 Diagnostic Guidance. BMC Med. 2017, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Yalchin, M.; Baker, A.-M.; Graham, T.A.; Hart, A. Predicting Colorectal Cancer Occurrence in IBD. Cancers 2021, 13, 2908. [Google Scholar] [CrossRef] [PubMed]
- Mattar, M.C.; Lough, D.; Pishvaian, M.J.; Charabaty, A. Current Management of Inflammatory Bowel Disease and Colorectal Cancer. Gastrointest. Cancer Res. GCR 2011, 4, 53–61. [Google Scholar]
- Ananthakrishnan, A.N.; Cagan, A.; Cai, T.; Gainer, V.S.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; Kohane, I.; Liao, K.P. Colonoscopy Is Associated with a Reduced Risk for Colon Cancer and Mortality in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2015, 13, 322–329.e1. [Google Scholar] [CrossRef]
- Beaugerie, L.; Itzkowitz, S.H. Cancers Complicating Inflammatory Bowel Disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and Cancer IV. Colorectal Cancer in Inflammatory Bowel Disease: The Role of Inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Wheat, C.L.; Clark-Snustad, K.; Devine, B.; Grembowski, D.; Thornton, T.A.; Ko, C.W. Worldwide Incidence of Colorectal Cancer, Leukemia, and Lymphoma in Inflammatory Bowel Disease: An Updated Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1632439. [Google Scholar] [CrossRef]
- Eaden, J.A. The Risk of Colorectal Cancer in Ulcerative Colitis: A Meta-Analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef]
- Zhang, Q.; Sha, S.; Xu, B.; Liang, S.; Wu, K. Prevalence of Colorectal Cancer in Patients with Ulcerative Colitis: A Retrospective, Monocenter Study in China. J. Cancer Res. Ther. 2015, 11, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Gamborg, M.; Matzen, P.; Munkholm, P.; Sorensen, T.I.A. Increased Risk of Intestinal Cancer in Crohn’s Disease: A Meta-Analysis of Population-Based Cohort Studies. Am. J. Gastroenterol. 2005, 100, 2724–2729. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin–Biroulet, L. Risk of Colorectal Cancer in Patients with Ulcerative Colitis: A Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, M.W.M.D.; Van Oijen, M.G.H.; Van Der Heijden, G.J.M.G.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining Risk of Colorectal Cancer in Inflammatory Bowel Disease: An Updated Meta-Analysis of Population-Based Cohort Studies. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Beaugerie, L.; Egan, L.; Biancone, L.; Bolling, C.; Brandts, C.; Dierickx, D.; Dummer, R.; Fiorino, G.; Gornet, J.M.; et al. European Evidence-Based Consensus: Inflammatory Bowel Disease and Malignancies. J. Crohns Colitis 2015, 9, 945–965. [Google Scholar] [CrossRef]
- Parigi, T.; Allocca, M.; Furfaro, F.; D’Amico, F.; Zilli, A.; Dal Buono, A.; Gabbiadini, R.; Bonovas, S.; Armuzzi, A.; Danese, S.; et al. Treat-to-Target and Regular Surveillance of Inflammatory Bowel Disease Are Associated with Low Incidence and Early-Stage Detection of Malignancies: A Retrospective Cohort Study. Cancers 2023, 15, 5754. [Google Scholar] [CrossRef]
- Selinger, C.P.; Andrews, J.M.; Titman, A.; Norton, I.; Jones, D.B.; McDonald, C.; Barr, G.; Selby, W.; Leong, R.W. Long-Term Follow-up Reveals Low Incidence of Colorectal Cancer, but Frequent Need for Resection, among Australian Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 644–650. [Google Scholar] [CrossRef]
- Qiu, X.; Ma, J.; Wang, K.; Zhang, H. Chemopreventive Effects of 5-Aminosalicylic Acid on Inflammatory Bowel Disease-Associated Colorectal Cancer and Dysplasia: A Systematic Review with Meta-Analysis. Oncotarget 2017, 8, 1031–1045. [Google Scholar] [CrossRef]
- Marabotto, E.; Kayali, S.; Buccilli, S.; Levo, F.; Bodini, G.; Giannini, E.G.; Savarino, V.; Savarino, E.V. Colorectal Cancer in Inflammatory Bowel Diseases: Epidemiology and Prevention: A Review. Cancers 2022, 14, 4254. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Valentine, J.F.; Guthery, S.; Singh, H.; Bernstein, C.N.; Leighton, J.A.; Wan, Y.; Wong, J.; Boucher, K.; Pappas, L.; et al. Family History Associates with Increased Risk of Colorectal Cancer in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 1807–1813.e1. [Google Scholar] [CrossRef]
- Nuako, K.; Ahlquist, D.; Mahoney, D.; Schaid, D.; Siems, D.; Lindor, N. Familial Predisposition for Colorectal Cancer in Chronic Ulcerative Colitis: A Case-Control Study. Gastroenterology 1998, 115, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, A.M.; De Jong, M.E.; Lutgens, M.W.M.D.; Hoentjen, F.; Elias, S.G.; Oldenburg, B. Prognostic Factors for Advanced Colorectal Neoplasia in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Gastroenterology 2021, 160, 1584–1598. [Google Scholar] [CrossRef]
- Söderlund, S.; Granath, F.; Broström, O.; Karlén, P.; Löfberg, R.; Ekbom, A.; Askling, J. Inflammatory Bowel Disease Confers a Lower Risk of Colorectal Cancer to Females Than to Males. Gastroenterology 2010, 138, 1697–1703.e2. [Google Scholar] [CrossRef]
- Soetikno, R.M.; Lin, O.S.; Heidenreich, P.A.; Young, H.S.; Blackstone, M.O. Increased Risk of Colorectal Neoplasia in Patients with Primary Sclerosing Cholangitis and Ulcerative Colitis: A Meta-Analysis. Gastrointest. Endosc. 2002, 56, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-Intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-Anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Ekbom, A.; Helmick, C.; Zack, M.; Adami, H.-O. Ulcerative Colitis and Colorectal Cancer: A Population-Based Study. N. Engl. J. Med. 1990, 323, 1228–1233. [Google Scholar] [CrossRef]
- Rutter, M.; Saunders, B.; Wilkinson, K.; Rumbles, S.; Schofield, G.; Kamm, M.; Williams, C.; Price, A.; Talbot, I.; Forbes, A. Severity of Inflammation Is a Risk Factor for Colorectal Neoplasia in Ulcerative Colitis. Gastroenterology 2004, 126, 451–459. [Google Scholar] [CrossRef]
- Flores, B.M.; O’Connor, A.; Moss, A.C. Impact of Mucosal Inflammation on Risk of Colorectal Neoplasia in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2017, 86, 1006–1011.e8. [Google Scholar] [CrossRef]
- Gupta, R.B.; Harpaz, N.; Itzkowitz, S.; Hossain, S.; Matula, S.; Kornbluth, A.; Bodian, C.; Ullman, T. Histologic Inflammation Is a Risk Factor for Progression to Colorectal Neoplasia in Ulcerative Colitis: A Cohort Study. Gastroenterology 2007, 133, 1099–1105. [Google Scholar] [CrossRef]
- Rubin, D.T.; Huo, D.; Kinnucan, J.A.; Sedrak, M.S.; McCullom, N.E.; Bunnag, A.P.; Raun–Royer, E.P.; Cohen, R.D.; Hanauer, S.B.; Hart, J.; et al. Inflammation Is an Independent Risk Factor for Colonic Neoplasia in Patients with Ulcerative Colitis: A Case–Control Study. Clin. Gastroenterol. Hepatol. 2013, 11, 1601–1608.e4. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Svrcek, M.; Le Gall, G.; Landman, C.; Dray, X.; Bourrier, A.; Nion-Larmurier, I.; Hoyeau, N.; Sokol, H.; Seksik, P.; et al. Nancy Index Scores of Chronic Inflammatory Bowel Disease Activity Associate with Development of Colorectal Neoplasia. Clin. Gastroenterol. Hepatol. 2020, 18, 150–157.e1. [Google Scholar] [CrossRef]
- Coelho-Prabhu, N.; Lewis, J.D. Update on Endoscopic Dysplasia Surveillance in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2023, 118, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Huguet, J.M.; Ferrer-Barceló, L.; Suárez, P.; Sanchez, E.; Prieto, J.D.; Garcia, V.; Sempere, J. Colorectal Cancer Screening and Surveillance in Patients with Inflammatory Bowel Disease in 2021. World J. Gastroenterol. 2022, 28, 502–516. [Google Scholar] [CrossRef]
- Vento, P.; Lepistö, A.; Kärkkäinen, P.; Ristimäki, A.; Haglund, C.; Järvinen, H.J. Risk of Cancer in Patients with Chronic Pouchitis after Restorative Proctocolectomy for Ulcerative Colitis. Colorectal Dis. 2011, 13, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Derikx, L.A.A.P.; Nissen, L.H.C.; Smits, L.J.T.; Shen, B.; Hoentjen, F. Risk of Neoplasia After Colectomy in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 798–806.e20. [Google Scholar] [CrossRef]
- Murthy, S.K.; Feuerstein, J.D.; Nguyen, G.C.; Velayos, F.S. AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology 2021, 161, 1043–1051.e4. [Google Scholar] [CrossRef]
- Al Bakir, I.; Kabir, M.; Yalchin, M.; Hart, A. Optimising Inflammatory Bowel Disease Surveillance and Dysplasia Management—Where Do We Stand? United Eur. Gastroenterol. J. 2022, 10, 1054–1062. [Google Scholar] [CrossRef]
- Laine, L.; Kaltenbach, T.; Barkun, A.; McQuaid, K.R.; Subramanian, V.; Soetikno, R.; East, J.E.; Farraye, F.A.; Feagan, B.; Ioannidis, J.; et al. SCENIC International Consensus Statement on Surveillance and Management of Dysplasia in Inflammatory Bowel Disease. Gastroenterology 2015, 148, 639–651.e28. [Google Scholar] [CrossRef]
- Rabinowitz, L.G.; Kumta, N.A.; Marion, J.F. Beyond the SCENIC Route: Updates in Chromoendoscopy and Dysplasia Screening in Patients with Inflammatory Bowel Disease. Gastrointest. Endosc. 2022, 95, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; De Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohns Colitis 2023, 17, 827–854. [Google Scholar] [CrossRef]
- Jang, J.-Y. The Past, Present, and Future of Image-Enhanced Endoscopy. Clin. Endosc. 2015, 48, 466–475. [Google Scholar] [CrossRef]
- Marion, J.F.; Waye, J.D.; Present, D.H.; Israel, Y.; Bodian, C.; Harpaz, N.; Chapman, M.; Itzkowitz, S.; Steinlauf, A.F.; Abreu, M.T.; et al. Chromoendoscopy-Targeted Biopsies Are Superior to Standard Colonoscopic Surveillance for Detecting Dysplasia in Inflammatory Bowel Disease Patients: A Prospective Endoscopic Trial. Am. J. Gastroenterol. 2008, 103, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, R.; Bessissow, T.; Joseph, J.A.; Baert, F.; Ferrante, M.; Ballet, V.; Willekens, H.; Demedts, I.; Geboes, K.; De Hertogh, G.; et al. Chromoendoscopy versus Narrow Band Imaging in UC: A Prospective Randomised Controlled Trial. Gut 2018, 67, 1087–1094. [Google Scholar] [CrossRef]
- Moussata, D.; Allez, M.; Cazals-Hatem, D.; Treton, X.; Laharie, D.; Reimund, J.-M.; Bertheau, P.; Bourreille, A.; Lavergne-Slove, A.; Brixi, H.; et al. Are Random Biopsies Still Useful for the Detection of Neoplasia in Patients with IBD Undergoing Surveillance Colonoscopy with Chromoendoscopy? Gut 2018, 67, 616–624. [Google Scholar] [CrossRef]
- Kandiah, K.; Subramaniam, S.; Thayalasekaran, S.; Chedgy, F.J.; Longcroft-Wheaton, G.; Fogg, C.; Brown, J.F.; Smith, S.C.; Iacucci, M.; Bhandari, P. Multicentre Randomised Controlled Trial on Virtual Chromoendoscopy in the Detection of Neoplasia during Colitis Surveillance High-Definition Colonoscopy (the VIRTUOSO Trial). Gut 2021, 70, 1684–1690. [Google Scholar] [CrossRef]
- Halligan, S.; Altman, D.G.; Taylor, S.A.; Mallett, S.; Deeks, J.J.; Bartram, C.I.; Atkin, W. CT Colonography in the Detection of Colorectal Polyps and Cancer: Systematic Review, Meta-Analysis, and Proposed Minimum Data Set for Study Level Reporting. Radiology 2005, 237, 893–904. [Google Scholar] [CrossRef]
- Kadari, M.; Subhan, M.; Saji Parel, N.; Krishna, P.V.; Gupta, A.; Uthayaseelan, K.; Uthayaseelan, K.; Sunkara, N.A.B.S. CT Colonography and Colorectal Carcinoma: Current Trends and Emerging Developments. Cureus 2022, 14, e24916. [Google Scholar] [CrossRef] [PubMed]
- Acuna, S.A.; Huang, J.W.; Dossa, F.; Shah, P.S.; Kim, S.J.; Baxter, N.N. Cancer Recurrence after Solid Organ Transplantation: A Systematic Review and Meta-Analysis. Transplant. Rev. 2017, 31, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, O.; Colombel, J.-F.; Ullman, T.A.; Laharie, D.; Beaugerie, L.; Itzkowitz, S.H. The Management of Immunosuppression in Patients with Inflammatory Bowel Disease and Cancer. Gut 2013, 62, 1523–1528. [Google Scholar] [CrossRef]
- Beaugerie, L.; Carrat, F.; Colombel, J.-F.; Bouvier, A.-M.; Sokol, H.; Babouri, A.; Carbonnel, F.; Laharie, D.; Faucheron, J.-L.; Simon, T.; et al. Risk of New or Recurrent Cancer under Immunosuppressive Therapy in Patients with IBD and Previous Cancer. Gut 2014, 63, 1416–1423. [Google Scholar] [CrossRef]
- Shelton, E.; Laharie, D.; Scott, F.I.; Mamtani, R.; Lewis, J.D.; Colombel, J.-F.; Ananthakrishnan, A.N. Cancer Recurrence Following Immune-Suppressive Therapies in Patients with Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2016, 151, 97–109.e4. [Google Scholar] [CrossRef]
- Poullenot, F.; Amiot, A.; Nachury, M.; Viennot, S.; Altwegg, R.; Bouhnik, Y.; Abitbol, V.; Nancey, S.; Vuitton, L.; Peyrin-Biroulet, L.; et al. Comparative Risk of Incident Cancer in Patients with Inflammatory Bowel Disease with Prior Non-Digestive Malignancy According to Immunomodulator: A Multicentre Cohort Study. J. Crohns Colitis 2022, 16, 1523–1530. [Google Scholar] [CrossRef]
- Poullenot, F.; Laharie, D. Management of Inflammatory Bowel Disease in Patients with Current or Past Malignancy. Cancers 2023, 15, 1083. [Google Scholar] [CrossRef] [PubMed]
- Mañosa, M.; Chaparro, M.; Juan, A.; Aràjol, C.; Alfaro, I.; Mínguez, M.; Velayos, B.; Benítez, J.M.; Mesonero, F.; Sicilia, B.; et al. Immunomodulatory Therapy Does Not Increase the Risk of Cancer in Persons with Inflammatory Bowel Disease and a History of Extracolonic Cancers. Am. J. Gastroenterol. 2019, 114, 771–776. [Google Scholar] [CrossRef]
- Laredo, V.; García-Mateo, S.; Martínez-Domínguez, S.J.; López De La Cruz, J.; Gargallo-Puyuelo, C.J.; Gomollón, F. Risk of Cancer in Patients with Inflammatory Bowel Diseases and Keys for Patient Management. Cancers 2023, 15, 871. [Google Scholar] [CrossRef]
- Waljee, A.K.; Higgins, P.D.R.; Jensen, C.B.; Villumsen, M.; Cohen-Mekelburg, S.A.; Wallace, B.I.; Berinstein, J.A.; Allin, K.H.; Jess, T. Anti-Tumour Necrosis Factor-α Therapy and Recurrent or New Primary Cancers in Patients with Inflammatory Bowel Disease, Rheumatoid Arthritis, or Psoriasis and Previous Cancer in Denmark: A Nationwide, Population-Based Cohort Study. Lancet Gastroenterol. Hepatol. 2020, 5, 276–284. [Google Scholar] [CrossRef]
- Cohen, R.D.; Bhayat, F.; Blake, A.; Travis, S. The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn’s Disease: 4 Years of Global Post-Marketing Data. J. Crohns Colitis 2020, 14, 192–204. [Google Scholar] [CrossRef]
- Card, T.; Ungaro, R.; Bhayat, F.; Blake, A.; Hantsbarger, G.; Travis, S. Vedolizumab Use Is Not Associated with Increased Malignancy Incidence: GEMINI LTS Study Results and Post-marketing Data. Aliment. Pharmacol. Ther. 2020, 51, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Zenger, C.; Pecoriello, J.; Pang, A.; Vallely, M.; Hudesman, D.P.; Chang, S.; Axelrad, J.E. Ustekinumab and Vedolizumab Are Not Associated with Subsequent Cancer in IBD Patients with Prior Malignancy. Inflamm. Bowel Dis. 2022, 28, 1826–1832. [Google Scholar] [CrossRef]
- Ghosh, S.; Feagan, B.G.; Ott, E.; Gasink, C.; Godwin, B.; Marano, C.; Miao, Y.; Ma, T.; Loftus, E.V.; Sandborn, W.J.; et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis through 5 Years in Crohn’s Disease and 4 Years in Ulcerative Colitis. J. Crohns Colitis 2024, 18, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Panés, J.; D’Haens, G.R.; Sands, B.E.; Ng, S.C.; Lawendy, N.; Kulisek, N.; Guo, X.; Wu, J.; Vranic, I.; Panaccione, R.; et al. Analysis of Tofacitinib Safety in Ulcerative Colitis from the Completed Global Clinical Developmental Program up to 9.2 Years of Drug Exposure. United Eur. Gastroenterol. J. 2024, 12, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Feagan, B.G.; Panés, J.; Baert, F.; Louis, E.; Dewit, O.; Kaser, A.; Duan, W.R.; Pang, Y.; Lee, W.-J.; et al. Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J. Crohns Colitis 2021, 15, 2001–2010. [Google Scholar] [CrossRef]
- Blauvelt, A.; Lebwohl, M.; Langley, R.G.; Rowland, K.; Yang, Y.-W.; Chan, D.; Miller, M.; You, Y.; Yu, J.; Thaҫi, D.; et al. Malignancy Rates through 5 Years of Follow-up in Patients with Moderate-to-Severe Psoriasis Treated with Guselkumab: Pooled Results from the VOYAGE 1 and VOYAGE 2 Trials. J. Am. Acad. Dermatol. 2023, 89, 274–282. [Google Scholar] [CrossRef]
- Wetwittayakhlang, P.; Tselekouni, P.; Al-Jabri, R.; Bessissow, T.; Lakatos, P.L. The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer. J. Clin. Med. 2023, 12, 2432. [Google Scholar] [CrossRef]
- Sebastian, S.; Neilaj, S. Practical Guidance for the Management of Inflammatory Bowel Disease in Patients with Cancer. Which Treatment? Ther. Adv. Gastroenterol. 2019, 12, 175628481881729. [Google Scholar] [CrossRef]
- Khoury, W.; Lavery, I.C.; Kiran, R.P. Effects of Chronic Immunosuppression on Long-Term Oncologic Outcomes for Colorectal Cancer Patients Undergoing Surgery. Ann. Surg. 2011, 253, 323–327. [Google Scholar] [CrossRef]
- Grimsdottir, S.; Attauabi, M.; Kristine Dahl, E.; Burisch, J.; Seidelin, J.B. Systematic Review with Meta-Analysis: The Impact of Cancer Treatments on the Disease Activity of Inflammatory Bowel Diseases. J. Crohns Colitis 2023, 17, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Fowler, S.A.; Friedman, S.; Ananthakrishnan, A.N.; Yajnik, V. Effects of Cancer Treatment on Inflammatory Bowel Disease Remission and Reactivation. Clin. Gastroenterol. Hepatol. 2012, 10, 1021–1027.e1. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Bazarbashi, A.; Zhou, J.; Castañeda, D.; Gujral, A.; Sperling, D.; Glass, J.; Agrawal, M.; Hong, S.; Lawlor, G.; et al. Hormone Therapy for Cancer Is a Risk Factor for Relapse of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 872–880.e1. [Google Scholar] [CrossRef]
- Conceição, D.; Saraiva, M.R.; Rosa, I.; Claro, I. Inflammatory Bowel Disease Treatment in Cancer Patients—A Comprehensive Review. Cancers 2023, 15, 3130. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Mellotte, G.; Ryan, B.; O’Connor, A. Gastrointestinal Side Effects of Cancer Treatments. Ther. Adv. Chronic Dis. 2020, 11, 204062232097035. [Google Scholar] [CrossRef]
- Stein, A.; Voigt, W.; Jordan, K. Review: Chemotherapy-Induced Diarrhea: Pathophysiology, Frequency and Guideline-Based Management. Ther. Adv. Med. Oncol. 2010, 2, 51–63. [Google Scholar] [CrossRef]
- Soularue, E.; Lepage, P.; Colombel, J.F.; Coutzac, C.; Faleck, D.; Marthey, L.; Collins, M.; Chaput, N.; Robert, C.; Carbonnel, F. Enterocolitis Due to Immune Checkpoint Inhibitors: A Systematic Review. Gut 2018, 67, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, V.; Jauregui-Amezaga, A.; Fierens, L.; Aspeslagh, S.; Dekervel, J.; Wauters, E.; Peeters, M.; Sabino, J.; Crapé, L.; Somers, M.; et al. Position Statement on the Management of the Immune Checkpoint Inhibitor-Induced Colitis via Multidisciplinary Modified Delphi Consensus. Eur. J. Cancer 2023, 187, 36–57. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Marthey, L.; Mateus, C.; Mussini, C.; Nachury, M.; Nancey, S.; Grange, F.; Zallot, C.; Peyrin-Biroulet, L.; Rahier, J.F.; Bourdier De Beauregard, M.; et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 395–401. [Google Scholar] [CrossRef]
- Bergqvist, V.; Hertervig, E.; Gedeon, P.; Kopljar, M.; Griph, H.; Kinhult, S.; Carneiro, A.; Marsal, J. Vedolizumab Treatment for Immune Checkpoint Inhibitor-Induced Enterocolitis. Cancer Immunol. Immunother. 2017, 66, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Bishu, S.; Melia, J.; Sharfman, W.; Lao, C.D.; Fecher, L.A.; Higgins, P.D.R. Efficacy and Outcome of Tofacitinib in Immune Checkpoint Inhibitor Colitis. Gastroenterology 2021, 160, 932–934.e3. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, R.; Feehan, J.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Cancer Immunotherapy: The Checkpoint between Chronic Colitis and Colorectal Cancer. Cancers 2022, 14, 6131. [Google Scholar] [CrossRef] [PubMed]
- Meserve, J.; Facciorusso, A.; Holmer, A.K.; Annese, V.; Sandborn, W.J.; Singh, S. Systematic Review with Meta-analysis: Safety and Tolerability of Immune Checkpoint Inhibitors in Patients with Pre-existing Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2021, 53, 374–382. [Google Scholar] [CrossRef]
- Sleiman, J.; Wei, W.; Shah, R.; Faisal, M.S.; Philpott, J.; Funchain, P. Incidence of Immune Checkpoint Inhibitor–Mediated Diarrhea and Colitis (imDC) in Patients with Cancer and Preexisting Inflammatory Bowel Disease: A Propensity Score–Matched Retrospective Study. J. Immunother. Cancer 2021, 9, e002567. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Willett, C.G.; Ooi, C.-J.; Zietman, A.L.; Menon, V.; Goldberg, S.; Sands, B.E.; Podolsky, D.K. Acute and Late Toxicity of Patients with Inflammatory Bowel Disease Undergoing Irradiation for Abdominal and Pelvic Neoplasms. Int. J. Radiat. Oncol. 2000, 46, 995–998. [Google Scholar] [CrossRef]
- Bodofsky, S.; Freeman, R.H.; Hong, S.S.; Chundury, A.; Hathout, L.; Deek, M.P.; Jabbour, S.K. Inflammatory Bowel Disease-Associated Malignancies and Considerations for Radiation Impacting Bowel: A Scoping Review. J. Gastrointest. Oncol. 2022, 13, 2565–2582. [Google Scholar] [CrossRef]
- Feagins, L.A.; Kim, J.; Chandrakumaran, A.; Gandle, C.; Naik, K.H.; Cipher, D.J.; Hou, J.K.; Yao, M.D.; Gaidos, J.K.J. Rates of Adverse IBD-Related Outcomes for Patients with IBD and Concomitant Prostate Cancer Treated with Radiation Therapy. Inflamm. Bowel Dis. 2020, 26, 728–733. [Google Scholar] [CrossRef]
- Green, S.; Stock, R.G.; Greenstein, A.J. Rectal Cancer and Inflammatory Bowel Disease: Natural History and Implications for Radiation Therapy. Int. J. Radiat. Oncol. 1999, 44, 835–840. [Google Scholar] [CrossRef]
- Kirk, P.S.; Govani, S.; Borza, T.; Hollenbeck, B.K.; Davis, J.; Shumway, D.; Waljee, A.K.; Skolarus, T.A. Implications of Prostate Cancer Treatment in Men with Inflammatory Bowel Disease. Urology 2017, 104, 131–136. [Google Scholar] [CrossRef]
- Brodersen, J.B.; Knudsen, T.; Kjeldsen, J.; Juel, M.A.; Rafaelsen, S.R.; Jensen, M.D. Diagnostic Accuracy of Pan-enteric Capsule Endoscopy and Magnetic Resonance Enterocolonography in Suspected Crohn’s Disease. United Eur. Gastroenterol. J. 2022, 10, 973–982. [Google Scholar] [CrossRef]
- Solitano, V.; Zilli, A.; Franchellucci, G.; Allocca, M.; Fiorino, G.; Furfaro, F.; D’Amico, F.; Danese, S.; Al Awadhi, S. Artificial Endoscopy and Inflammatory Bowel Disease: Welcome to the Future. J. Clin. Med. 2022, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Da Rio, L.; Spadaccini, M.; Parigi, T.L.; Gabbiadini, R.; Dal Buono, A.; Busacca, A.; Maselli, R.; Fugazza, A.; Colombo, M.; Carrara, S.; et al. Artificial Intelligence and Inflammatory Bowel Disease: Where Are We Going? World J. Gastroenterol. 2023, 29, 508–520. [Google Scholar] [CrossRef]
- Onuora, S. Cancer Recurrence Risk Not Increased by DMARDs. Nat. Rev. Rheumatol. 2021, 17, 707. [Google Scholar] [CrossRef] [PubMed]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; Van Der Heijde, D.; Dougados, M.; Van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; De Wit, M.; et al. Safety of Synthetic and Biological DMARDs: A Systematic Literature Review Informing the 2019 Update of the EULAR Recommendations for the Management of Rheumatoid Arthritis. Ann. Rheum. Dis. 2020, 79, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Melmed, G.Y. Multidisciplinary Team-Based Approaches to IBD Management: How Might “One-Stop Shopping” Work for Complex IBD Care? Am. J. Gastroenterol. 2017, 112, 825–827. [Google Scholar] [CrossRef]
- Helwig, U.; Fischer, I.; Hammer, L.; Kolterer, S.; Rath, S.; Maaser, C.; Kucharzik, T. Transmural Response and Transmural Healing Defined by Intestinal Ultrasound: New Potential Therapeutic Targets? J. Crohns Colitis 2022, 16, 57–67. [Google Scholar] [CrossRef]
- D’Amico, F.; Rubin, D.T.; Kotze, P.G.; Magro, F.; Siegmund, B.; Kobayashi, T.; Olivera, P.A.; Bossuyt, P.; Pouillon, L.; Louis, E.; et al. International Consensus on Methodological Issues in Standardization of Fecal Calprotectin Measurement in Inflammatory Bowel Diseases. United Eur. Gastroenterol. J. 2021, 9, 451–460. [Google Scholar] [CrossRef]
| Guideline (Year of Publication) | Type of Endoscopic Surveillance | Random or Targeted Biopsies |
|---|---|---|
| SCENIC Consensus (2015) [40] | HD recommended If SD, dye-CE recommended If HD, dye-CE suggested | No consensus |
| SCENIC commentary (2022) [41] | HD-WLE, dye-CE, or VCE | Random limited to highest-risk groups only (PSC, prior dysplasia, atrophic scarred colon, ongoing active inflammation) |
| ECCO Guideline (2017) [27] | HD recommended | Random if WL Targeted only if dye-CE |
| ECCO Guideline (2023) [44] | HD-WLE, dye-CE, or VCE | Targeted biopsies Random in high-risk (PSC or history of dysplasia) |
| ACG Clinical Guideline (2019) [43] | If SD, dye-CE recommended If HD, dye-CE or VCE recommended | No recommendation |
| AGA Clinical Practice update (2021) [38] | HD recommended Dye-CE should be considered VCE acceptable alternative if HD | Random if WL only and all patients with high risk (PSC or history of dysplasia) Targeted if dye-CE or VCE |
| BSG Guideline (2019) [42] | HD recommended If SD, dye-CE recommended If HD, dye-CE suggested NBI not suggested | Targeted recommended |
| Study Title | Aim of the Study | Patients | NCT Number |
|---|---|---|---|
| IBD neoplasia surveillance pilot RCT (IBD Dysplasia) | Random and targeted biopsies vs. targeted biopsies alone for CRC screening in adult persons with colonic IBD | 600 with long-standing CD or UC or any disease duration in case of PSC | NCT04067778 |
| Back-to-back endoscopy versus single-pass endoscopy and CE in IBD surveillance (HELIOS) | Back-to-back HD-WLE vs. single-pass HD-WLE vs. CE | 563 with long-standing CD or UC or any disease duration in case of PSC | NCT04291976 |
| HD colonoscopy vs. dye-spraying chromo-colonoscopy in screening patients with long-standing IBD | HD colonoscopy vs. dye-spraying chromo-colonoscopy | 500 (estimated) with long-standing CD, UC, or IBD-U or any disease duration in case of PSC | NCT04191655 |
| LCI vs. WLE for colorectal dysplasia in UC | LCI vs. conventional colonoscopy using WL for detection of colorectal dysplasia in UC | 60 (estimated) with UC | NCT02772406 |
| Dysplasia in inflammatory chronic idiopathic colitis long-standing | Assess the incidence of dysplasia and CRC in patients with chronic idiopathic inflammatory colitis treated with biologics, mesalamine, and immunosuppressive drug combinations | 300 (estimated) with long-standing CD or UC | NCT03096717 |
| Testing atorvastatin to lower colon cancer risk in long-standing UC | To determine the effect of atorvastatin treatment on reducing the fraction of colonic epithelial cells expressing mutant p53 protein | 70 (estimated) with long-standing UC | NCT04767984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanizza, J.; Bencardino, S.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 2943. https://doi.org/10.3390/cancers16172943
Fanizza J, Bencardino S, Allocca M, Furfaro F, Zilli A, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S, D’Amico F. Inflammatory Bowel Disease and Colorectal Cancer. Cancers. 2024; 16(17):2943. https://doi.org/10.3390/cancers16172943
Chicago/Turabian StyleFanizza, Jacopo, Sarah Bencardino, Mariangela Allocca, Federica Furfaro, Alessandra Zilli, Tommaso Lorenzo Parigi, Gionata Fiorino, Laurent Peyrin-Biroulet, Silvio Danese, and Ferdinando D’Amico. 2024. "Inflammatory Bowel Disease and Colorectal Cancer" Cancers 16, no. 17: 2943. https://doi.org/10.3390/cancers16172943
APA StyleFanizza, J., Bencardino, S., Allocca, M., Furfaro, F., Zilli, A., Parigi, T. L., Fiorino, G., Peyrin-Biroulet, L., Danese, S., & D’Amico, F. (2024). Inflammatory Bowel Disease and Colorectal Cancer. Cancers, 16(17), 2943. https://doi.org/10.3390/cancers16172943







