Vitamin D3 Upregulated Protein 1 Deficiency Promotes Azoxymethane/Dextran Sulfate Sodium-Induced Colorectal Carcinogenesis in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Animals
2.2. AOM/DSS-Induced Colitis
2.3. RNA Isolation and Quantification of mRNA Expression
2.4. Histology, Immunohistochemistry (IHC), and TdT-Mediated dUTP Nick-End Labeling (TUNEL) Assay
2.5. Western Immunoblotting Analysis
2.6. Statistical Analyses
3. Results
3.1. VDUP1 Expression Is Downregulated and Correlates with Poor Prognosis in CAC
3.2. VDUP1 Reduces Disease Severity in the AOM/DSS-Induced CAC Model Mice
3.3. VDUP1 Protects against AOM/DSS-Mediated Carcinogenesis
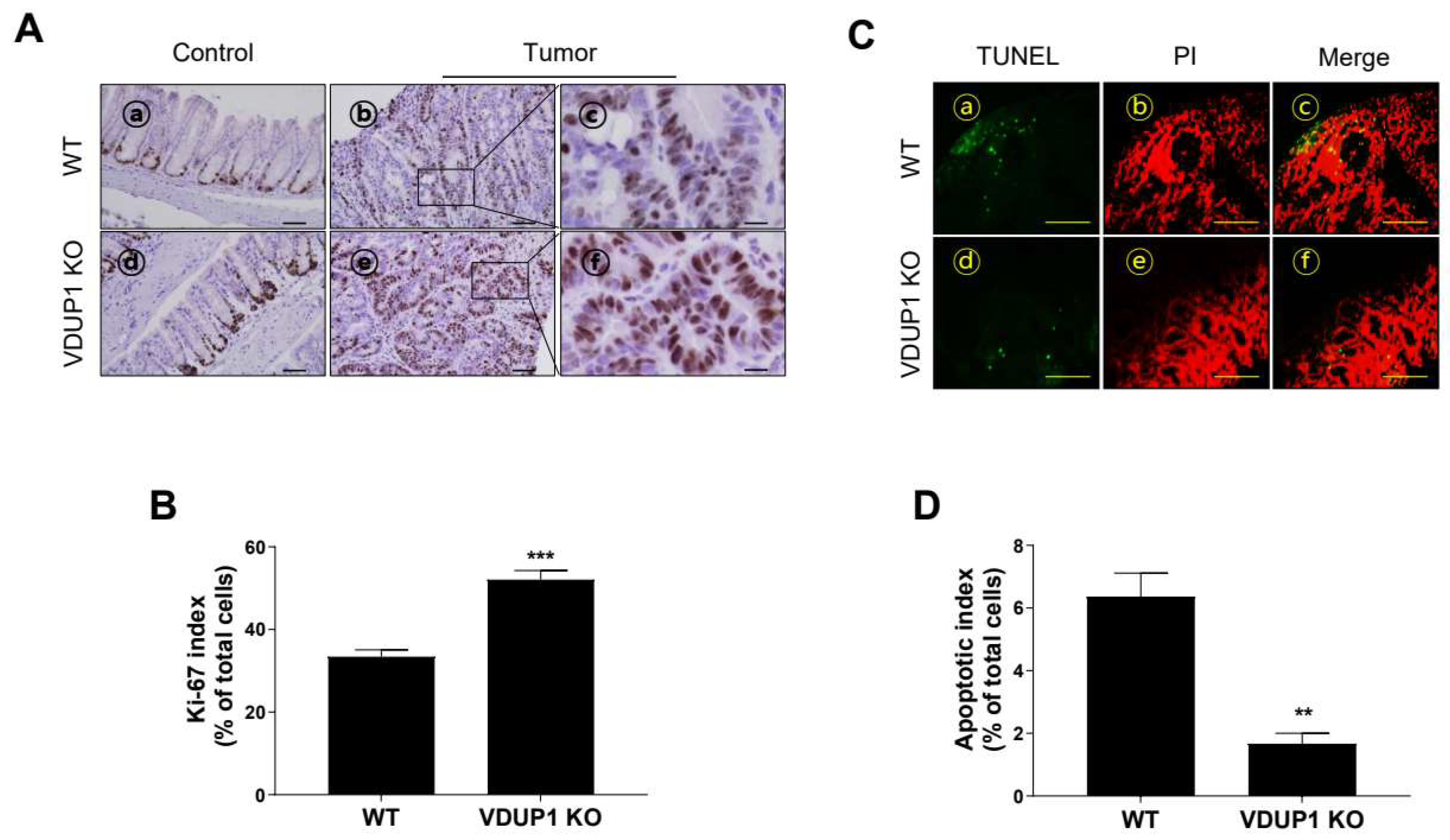
3.4. VDUP1 Deficiency Induces Cell Proliferation and Inhibits Apoptosis in AOM/DSS-Induced CAC Model Mice
3.5. VDUP1 Deficiency Induces the Molecular Patterns of Carcinogenesis in AOM/DSS-Induced CAC Model Mice
3.6. VDUP1 Deficiency Induces the Activation of STAT3 and NF-κB in AOM/DSS-Induced CAC Model Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diaz, S.; Preaudet, A.; Samson, A.L.; Nguyen, P.M.; Fung, K.Y.; Garnham, A.L.; Alexander, W.S.; Strasser, A.; Ernst, M.; Putoczki, T.L.; et al. Necroptosis is dispensable for the development of inflammation-associated or sporadic colon cancer in mice. Cell Death Differ. 2021, 28, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva-Posocco, O.; Dzutsev, A.; Posocco, D.F.; Hou, V.; Yuan, W.; Thovarai, V.; Mufazalov, I.A.; Gunzer, M.; Shilovskiy, I.P.; Khaitov, M.R.; et al. Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer. Immunity 2019, 50, 166–180.e167. [Google Scholar] [CrossRef]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef]
- Rajamaki, K.; Taira, A.; Katainen, R.; Valimaki, N.; Kuosmanen, A.; Plaketti, R.M.; Seppala, T.T.; Ahtiainen, M.; Wirta, E.V.; Vartiainen, E.; et al. Genetic and Epigenetic Characteristics of Inflammatory Bowel Disease-Associated Colorectal Cancer. Gastroenterology 2021, 161, 592–607. [Google Scholar] [CrossRef]
- Su, L.K.; Vogelstein, B.; Kinzler, K.W. Association of the APC tumor suppressor protein with catenins. Science 1993, 262, 1734–1737. [Google Scholar] [CrossRef]
- Robles, A.I.; Traverso, G.; Zhang, M.; Roberts, N.J.; Khan, M.A.; Joseph, C.; Lauwers, G.Y.; Selaru, F.M.; Popoli, M.; Pittman, M.E.; et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 2016, 150, 931–943. [Google Scholar] [CrossRef]
- Yaeger, R.; Shah, M.A.; Miller, V.A.; Kelsen, J.R.; Wang, K.; Heins, Z.J.; Ross, J.S.; He, Y.; Sanford, E.; Yantiss, R.K.; et al. Genomic Alterations Observed in Colitis-Associated Cancers Are Distinct From Those Found in Sporadic Colorectal Cancers and Vary by Type of Inflammatory Bowel Disease. Gastroenterology 2016, 151, 278–287.e276. [Google Scholar] [CrossRef]
- Cooks, T.; Pateras, I.S.; Tarcic, O.; Solomon, H.; Schetter, A.J.; Wilder, S.; Lozano, G.; Pikarsky, E.; Forshew, T.; Rosenfeld, N.; et al. Mutant p53 prolongs NF-kappaB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 2013, 23, 634–646. [Google Scholar] [CrossRef]
- Gowrikumar, S.; Ahmad, R.; Uppada, S.B.; Washington, M.K.; Shi, C.; Singh, A.B.; Dhawan, P. Upregulated claudin-1 expression promotes colitis-associated cancer by promoting beta-catenin phosphorylation and activation in Notch/p-AKT-dependent manner. Oncogene 2019, 38, 5321–5337. [Google Scholar] [CrossRef]
- Coskun, O.; Oztopuz, O.; Ozkan, O.F. Determination of IL-6, TNF-alpha and VEGF levels in the serums of patients with colorectal cancer. Cell Mol. Biol. 2017, 63, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.O.; Anatriello, E.; Azevedo, L.R.; Cordeiro, J.F.C.; Peria, F.M.; Floria-Santos, M.; Pereira-da-Silva, G. Elevated serum levels of proinflammatory cytokines potentially correlate with depression and anxiety in colorectal cancer patients in different stages of the antitumor therapy. Cytokine 2018, 104, 72–77. [Google Scholar] [CrossRef]
- De Simone, V.; Franze, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015, 34, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Investig. 2008, 118, 560–570. [Google Scholar] [CrossRef]
- Liu, L.; Gao, H.; Wen, T.; Gu, T.; Zhang, S.; Yuan, Z. Tanshinone IIA attenuates AOM/DSS-induced colorectal tumorigenesis in mice via inhibition of intestinal inflammation. Pharm. Biol. 2021, 59, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Zhang, H.X.; Li, W.W.; Ran, Y.; Liu, T.T.; Xiong, M.G.; Li, Q.L.; Wang, S.Y.; Wu, M.; Shu, H.B.; et al. FAM64A positively regulates STAT3 activity to promote Th17 differentiation and colitis-associated carcinogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 10447–10452. [Google Scholar] [CrossRef]
- Zhao, H.; Pan, W.M.; Zhang, H.H.; Song, Y.; Chen, J.; Xiang, Y.; Gu, B.; Li, S.Z.; Du, R.L.; Zhang, X.D. Cancer testis antigen 55 deficiency attenuates colitis-associated colorectal cancer by inhibiting NF-kappaB signaling. Cell Death Dis. 2019, 10, 304. [Google Scholar] [CrossRef]
- El-Daly, S.M.; Omara, E.A.; Hussein, J.; Youness, E.R.; El-Khayat, Z. Differential expression of miRNAs regulating NF-kappaB and STAT3 crosstalk during colitis-associated tumorigenesis. Mol. Cell Probes 2019, 47, 101442. [Google Scholar] [CrossRef]
- Kesselring, R.; Glaesner, J.; Hiergeist, A.; Naschberger, E.; Neumann, H.; Brunner, S.M.; Wege, A.K.; Seebauer, C.; Kohl, G.; Merkl, S.; et al. IRAK-M Expression in Tumor Cells Supports Colorectal Cancer Progression through Reduction of Antimicrobial Defense and Stabilization of STAT3. Cancer Cell 2016, 29, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Sun, B.; Gong, T.; Guo, S.; Zhang, J.; Wang, J.; Sugawara, A.; Jiang, M.; Yan, J.; Gurary, A.; et al. GNAI1 and GNAI3 Reduce Colitis-Associated Tumorigenesis in Mice by Blocking IL6 Signaling and Down-regulating Expression of GNAI2. Gastroenterology 2019, 156, 2297–2312. [Google Scholar] [CrossRef]
- Chen, K.S.; DeLuca, H.F. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim. Biophys. Acta 1994, 1219, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, Q.; Chng, W.J. TXNIP (VDUP-1, TBP-2): A major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2011, 43, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, M.; Zhang, H.; Chen, Q.; Hu, Y.; Meng, Y.; Wu, C.; Tu, C.; Liu, Y.; Li, A.; et al. A pan-cancer analysis of thioredoxin-interacting protein as an immunological and prognostic biomarker. Cancer. Cell Int. 2022, 22, 230. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Won, Y.S.; Nam, K.T.; Yoon, Y.D.; Jee, H.; Yoon, W.K.; Nam, K.H.; Kang, J.S.; Han, S.U.; Choi, I.P.; et al. Vitamin D(3) upregulated protein 1 deficiency promotes N-methyl-N-nitrosourea and Helicobacter pylori-induced gastric carcinogenesis in mice. Gut 2012, 61, 53–63. [Google Scholar] [CrossRef]
- Nishizawa, K.; Nishiyama, H.; Matsui, Y.; Kobayashi, T.; Saito, R.; Kotani, H.; Masutani, H.; Oishi, S.; Toda, Y.; Fujii, N.; et al. Thioredoxin-interacting protein suppresses bladder carcinogenesis. Carcinogenesis 2011, 32, 1459–1466. [Google Scholar] [CrossRef]
- Kwon, H.J.; Won, Y.S.; Suh, H.W.; Jeon, J.H.; Shao, Y.; Yoon, S.R.; Chung, J.W.; Kim, T.D.; Kim, H.M.; Nam, K.H.; et al. Vitamin D3 upregulated protein 1 suppresses TNF-alpha-induced NF-kappaB activation in hepatocarcinogenesis. J. Immunol. 2010, 185, 3980–3989. [Google Scholar] [CrossRef]
- Kato, T.; Shimono, Y.; Hasegawa, M.; Jijiwa, M.; Enomoto, A.; Asai, N.; Murakumo, Y.; Takahashi, M. Characterization of the HDAC1 complex that regulates the sensitivity of cancer cells to oxidative stress. Cancer Res. 2009, 69, 3597–3604. [Google Scholar] [CrossRef]
- Hu, J.; Feng, L.; Ren, M.; Zhao, Y.; Lu, G.; Lu, X.; Li, Y.; Wang, X.; Bu, X.; Wang, S.; et al. Colorectal Cancer Cell Differentiation Is Dependent on the Repression of Aerobic Glycolysis by NDRG2-TXNIP Axis. Dig. Dis. Sci. 2022, 67, 3763–3772. [Google Scholar] [CrossRef]
- Takahashi, Y.; Masuda, H.; Ishii, Y.; Nishida, Y.; Kobayashi, M.; Asai, S. Decreased expression of thioredoxin interacting protein mRNA in inflamed colonic mucosa in patients with ulcerative colitis. Oncol. Rep. 2007, 18, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Lee, H.; Kim, H.C.; Choi, I.; Han, S.B.; Kang, J.S. VDUP1 Deficiency Promotes the Severity of DSS-Induced Colitis in Mice by Inducing Macrophage Infiltration. Int. J. Mol. Sci. 2023, 24, 13584. [Google Scholar] [CrossRef]
- Lee, K.N.; Kang, H.S.; Jeon, J.H.; Kim, E.M.; Yoon, S.R.; Song, H.; Lyu, C.Y.; Piao, Z.H.; Kim, S.U.; Han, Y.H.; et al. VDUP1 is required for the development of natural killer cells. Immunity 2005, 22, 195–208. [Google Scholar] [CrossRef]
- Pan, Q.; Lou, X.; Zhang, J.; Zhu, Y.; Li, F.; Shan, Q.; Chen, X.; Xie, Y.; Su, S.; Wei, H.; et al. Genomic variants in mouse model induced by azoxymethane and dextran sodium sulfate improperly mimic human colorectal cancer. Sci. Rep. 2017, 7, 25. [Google Scholar] [CrossRef]
- Hu, B.; Elinav, E.; Huber, S.; Booth, C.J.; Strowig, T.; Jin, C.; Eisenbarth, S.C.; Flavell, R.A. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl. Acad. Sci. USA 2010, 107, 21635–21640. [Google Scholar] [CrossRef] [PubMed]
- Loo, D.T. TUNEL assay. An overview of techniques. Methods Mol. Biol. 2002, 203, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, K.; Sata, M.; Tanikawa, K. Significance of interleukin-6 in patients with inflammatory bowel disease. Gastroen. Jpn. 1991, 26, 20–28. [Google Scholar] [CrossRef]
- Huang, Z.G. Observation on therapeutic effect of warming needle therapy on chronic colitis. Zhongguo Zhen Jiu 2008, 28, 795–797. [Google Scholar]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel. Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hong, S.K.; Yoon, W.K.; Nam, K.H.; Choi, I.P.; Kim, D.Y.; Kim, H.C.; Won, Y.S. Vitamin D3 up-regulated protein 1 controls the priming phase of liver regeneration. J. Vet. Sci. 2013, 14, 257–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.; Wong, C.C.; Zhang, Y.; Huang, J.; Li, C.; Zhai, J.; Wang, G.; Wei, H.; Zhang, X.; He, H.H.; et al. ZNF545 loss promotes ribosome biogenesis and protein translation to initiate colorectal tumorigenesis in mice. Oncogene 2021, 40, 6590–6600. [Google Scholar] [CrossRef]
- Lou, Y.; Tian, X.; Sun, C.; Song, M.; Han, M.; Zhao, Y.; Song, Y.; Song, X.; Zhang, W.; Chen, Y.H.; et al. TNFAIP8 protein functions as a tumor suppressor in inflammation-associated colorectal tumorigenesis. Cell Death Dis. 2022, 13, 311. [Google Scholar] [CrossRef]
- Wang, K.; Ding, Y.; Xu, C.; Hao, M.; Li, H.; Ding, L. Cldn-7 deficiency promotes experimental colitis and associated carcinogenesis by regulating intestinal epithelial integrity. Oncoimmunology 2021, 10, 1923910. [Google Scholar] [CrossRef]
- Park, K.H.; Yang, J.W.; Kwon, J.H.; Lee, H.; Yoon, Y.D.; Choi, B.J.; Lee, M.Y.; Lee, C.W.; Han, S.B.; Kang, J.S. Targeted Induction of Endogenous VDUP1 by Small Activating RNA Inhibits the Growth of Lung Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7743. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Zhou, X.; Xu, W.S.; Scher, H.I.; Rifkind, R.A.; Marks, P.A.; Richon, V.M. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc. Natl. Acad. Sci. USA 2002, 99, 11700–11705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rong, Y.P.; Malone, M.H.; Davis, M.C.; Zhong, F.; Distelhorst, C.W. Thioredoxin-interacting protein (txnip) is a glucocorticoid-regulated primary response gene involved in mediating glucocorticoid-induced apoptosis. Oncogene 2006, 25, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Suh, H.W.; Chung, J.W.; Yoon, S.R.; Choi, I. Diverse functions of VDUP1 in cell proliferation, differentiation, and diseases. Cell Mol. Immunol. 2007, 4, 345–351. [Google Scholar]
- Bienz, M.; Clevers, H. Linking colorectal cancer to Wnt signaling. Cell 2000, 103, 311–320. [Google Scholar] [CrossRef]
- Schulz-Heddergott, R.; Stark, N.; Edmunds, S.J.; Li, J.; Conradi, L.C.; Bohnenberger, H.; Ceteci, F.; Greten, F.R.; Dobbelstein, M.; Moll, U.M. Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion. Cancer Cell 2018, 34, 298–314.e297. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, H. The Role of Proinflammatory Pathways in the Pathogenesis of Colitis-Associated Colorectal Cancer. Mediat. Inflamm. 2017, 2017, 5126048. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.J.; Neurath, M.F. Mechanisms of Immune Signaling in Colitis-Associated Cancer. Cell Mol. Gastroen. Hepatol. 2015, 1, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Fantini, M.C.; Wirtz, S.; Nikolaev, A.; Lehr, H.A.; Galle, P.R.; Rose-John, S.; Neurath, M.F. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle 2005, 4, 217–220. [Google Scholar] [CrossRef]
- Burkitt, M.D.; Hanedi, A.F.; Duckworth, C.A.; Williams, J.M.; Tang, J.M.; O’Reilly, L.A.; Putoczki, T.L.; Gerondakis, S.; Dimaline, R.; Caamano, J.H.; et al. NF-kappaB1, NF-kappaB2 and c-Rel differentially regulate susceptibility to colitis-associated adenoma development in C57BL/6 mice. J. Pathol. 2015, 236, 326–336. [Google Scholar] [CrossRef]
- Lee, H.; Herrmann, A.; Deng, J.H.; Kujawssski, M.; Niu, G.; Li, Z.; Forman, S.; Jove, R.; Pardoll, D.M.; Yu, H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 2009, 15, 283–293. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.H.; Kim, H.-C.; Won, Y.-S.; Yoon, W.K.; Choi, I.; Han, S.-B.; Kang, J.S. Vitamin D3 Upregulated Protein 1 Deficiency Promotes Azoxymethane/Dextran Sulfate Sodium-Induced Colorectal Carcinogenesis in Mice. Cancers 2024, 16, 2934. https://doi.org/10.3390/cancers16172934
Park KH, Kim H-C, Won Y-S, Yoon WK, Choi I, Han S-B, Kang JS. Vitamin D3 Upregulated Protein 1 Deficiency Promotes Azoxymethane/Dextran Sulfate Sodium-Induced Colorectal Carcinogenesis in Mice. Cancers. 2024; 16(17):2934. https://doi.org/10.3390/cancers16172934
Chicago/Turabian StylePark, Ki Hwan, Hyoung-Chin Kim, Young-Suk Won, Won Kee Yoon, Inpyo Choi, Sang-Bae Han, and Jong Soon Kang. 2024. "Vitamin D3 Upregulated Protein 1 Deficiency Promotes Azoxymethane/Dextran Sulfate Sodium-Induced Colorectal Carcinogenesis in Mice" Cancers 16, no. 17: 2934. https://doi.org/10.3390/cancers16172934
APA StylePark, K. H., Kim, H.-C., Won, Y.-S., Yoon, W. K., Choi, I., Han, S.-B., & Kang, J. S. (2024). Vitamin D3 Upregulated Protein 1 Deficiency Promotes Azoxymethane/Dextran Sulfate Sodium-Induced Colorectal Carcinogenesis in Mice. Cancers, 16(17), 2934. https://doi.org/10.3390/cancers16172934







