Clinical, Dermoscopic, and Molecular Features of Acantholytic Squamous Cell Carcinoma: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
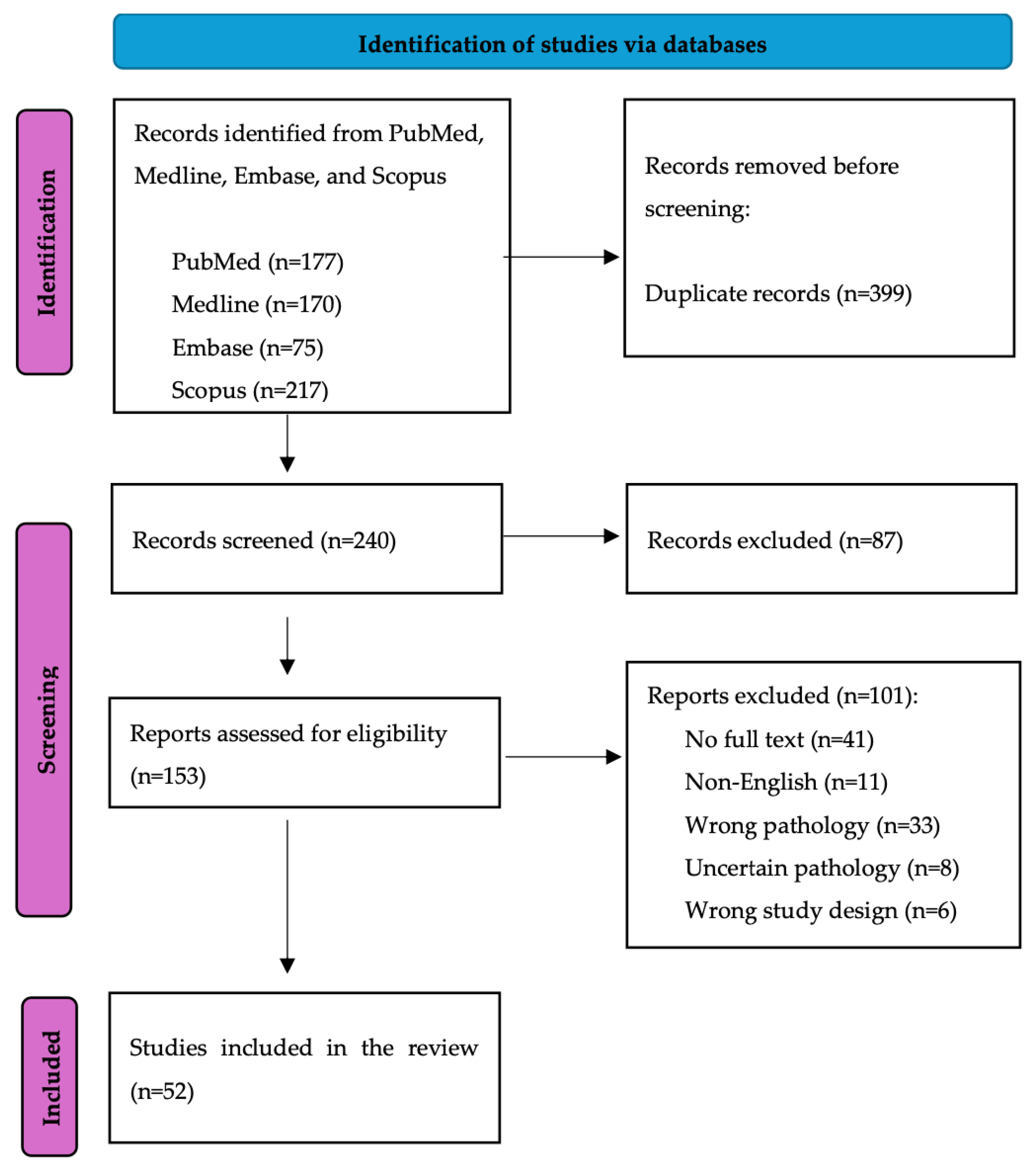
2. Methods
2.1. Search Strategy
2.2. Data Screening and Extraction
3. Results
3.1. Demographics
3.2. Clinical and Dermoscopic Features
3.3. Molecular Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cassarino, D.S.; DeRienzo, D.P.; Barr, R.J. Cutaneous squamous cell carcinoma: A comprehensive clinicopathologic classification. Part One. J. Cutan. Pathol. 2006, 33, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Conde-Ferreirós, A.; Moyano-Bueno, D.; Santos-Briz, A.; Revelles-Peñas, L.; Revilla-Nebreda, D.; Becerril-Andrés, S.; Román-Curto, C.; Cañueto, J. Clinical and histopathological evaluation of 50 acantholytic cutaneous squamous cell carcinomas: Analysis outcome in a retrospective case-control study. J. Cutan. Pathol. 2022, 49, 133–138. [Google Scholar] [CrossRef]
- Nappi, O.; Pettinato, G.; Wick, M.R. Adenoid (acantholytic) squamous cell carcinoma of the skin. J. Cutan. Pathol. 1989, 16, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sajin, M.; Prisăcaru, A.H.; Luchian, M.C.; Pătraşcu, O.M.; Dumitru, A.; Costache, D.; Dumitrescu, D.; Vrînceanu, D.; Voinea, L.M.; Simionescu, O.; et al. Acantholytic squamous cell carcinoma: Pathological study of nine cases with review of literature. Rom. J. Morphol. Embryol. 2014, 55, 279–283. [Google Scholar] [PubMed]
- Ogawa, T.; Kiuru, M.; Konia, T.H.; Fung, M.A. Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not “high risk”: Diagnosis, management, and clinical outcomes in a series of 115 cases. J. Am. Acad. Dermatol. 2017, 76, 327–333. [Google Scholar] [CrossRef]
- Cassarino, D.S.; DeRienzo, D.P.; Barr, R.J. Cutaneous squamous cell carcinoma: A comprehensive clinicopathologic classification-Part two. J. Cutan. Pathol. 2006, 33, 261–279. [Google Scholar] [CrossRef]
- Lim, J.Y.; Do, M.O.; Kim, S.H.; Hahm, J.H.; Whang, K.K. A Case of Acantholytic Squamous Cell Carcinoma. Ann. Dermatol. 2008, 20, 267–270. [Google Scholar] [CrossRef]
- Conte, S.; Ghezelbash, S.; Nallanathan, B.; Lefrançois, P. Clinical and Molecular Features of Morpheaform Basal Cell Carcinoma: A Systematic Review. Curr. Oncol. 2023, 30, 9906–9928. [Google Scholar] [CrossRef]
- Jacques, C.; De Aquino, A.M.; Ramos-E.-Silva, M. Cytokeratins and dermatology. Skinmed 2005, 4, 354–360. [Google Scholar] [CrossRef]
- Ellmark, P.; Woolfson, A.; Belov, L.; Christopherson, R.I. The applicability of a cluster of differentiation monoclonal antibody microarray to the diagnosis of human disease. Methods Mol. Biol. 2008, 439, 199–209. [Google Scholar] [CrossRef]
- Couchman, J.R. Syndecan-1 (CD138), Carcinomas and EMT. Int. J. Mol. Sci. 2021, 22, 4227. [Google Scholar] [CrossRef]
- Bayer-Garner, I.B.; Sanderson, R.D.; Smoller, B.R. Syndecan-1 expression is diminished in acantholytic cutaneous squamous cell carcinoma. J. Cutan. Pathol. 1999, 26, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.R.; Wriston, C.C.; Peters, M.S.; Lehman, J.S. Decreased expression of intercellular adhesion molecules in acantholytic squamous cell carcinoma compared with invasive well-differentiated squamous cell carcinoma of the skin. Am. J. Clin. Pathol. 2013, 139, 442–447. [Google Scholar] [CrossRef]
- Ko, T.; Muramatsu, T.; Shirai, T. Distribution of lectin UEA-I in trichilemmal carcinoma, squamous cell carcinoma and other epithelial tumors of the skin. J. Dermatol. 1996, 23, 389–393. [Google Scholar] [CrossRef]
- Holthofer, H.; Virtanen, I.; Kariniemi, A.; Hormia, M.; Linder, E.; Miettinen, A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab. Investig. 1982, 4, 60–66. [Google Scholar]
- Walker, G.E.; Merlin, S.; Zanolini, D.; Vandoni, A.; Volpe, A.; Gaidano, G.; Valente, G.; Olivero, M.; Follenzi, A. Factor VIII as a potential player in cancer pathophysiology. J. Thromb. Haemost. 2022, 20, 648–660. [Google Scholar] [CrossRef]
- Hamasaki, H.; Koga, K.; Aoki, M.; Hamasaki, M.; Koshikawa, N.; Seiki, M.; Iwasaki, H.; Nakayama, J.; Nabeshima, K. Expression of laminin 5-γ2 chain in cutaneous squamous cell carcinoma and its role in tumour invasion. Br. J. Cancer 2011, 105, 824–832. [Google Scholar] [CrossRef]
- Green, H.; Easley, K.; Iuchi, S. Marker succession during the development of keratinocytes from cultured human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15625–15630. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Li, F.-Z.; Ye, Q.; Chen, K.-J.; Fang, S. Expression of Heat Shock Protein 105 in Cutaneous Squamous Cell Carcinoma: Correlation with Clinicopathological Characteristics. Clin. Cosmet. Investig. Dermatol. 2021, 14, 633–641. [Google Scholar] [CrossRef]
- Johnson, W.C.; Helwig, E.B. Adenoid squamous cell carcinoma (adenoacanthoma). A clinicopathologic study of 155 patients. Cancer 1966, 19, 1639–1650. [Google Scholar] [CrossRef]
- Barbosa, L.O.; Neto, J.O.B.; Teixeira-Júnior, A.A.L.; Nogueira, L.R.; Calixto, J.d.R.R.; Cunha, I.W.; Pinho, J.D.; Nascimento, F.S.M.S.D.; Melo, S.P.d.C.; Soares, F.A.; et al. Pseudoangiosarcomatous squamous cell carcinoma: First case report on penis. Transl. Androl. Urol. 2021, 10, 1803–1806. [Google Scholar] [CrossRef]
- Barron, C.R.; Paczos, T.A.; Varghese, S.M.; Smoller, B.R. Acantholytic Squamous Cell Carcinoma Arising From Lichen Sclerosus: A Rare Case Affecting Vulvar Skin. Int. J. Gynecol. Pathol. 2022, 41, 122–125. [Google Scholar] [CrossRef]
- Cockayne, S.; Shah, M.; Slater, D.N.; Harrington, C. Spindle and pseudoglandular squamous cell carcinoma arising in lichen sclerosus of the vulva. Br. J. Dermatol. 1998, 138, 695–697. [Google Scholar] [CrossRef]
- Lam, K.Y.; Chan, K.W. Molecular Pathology and clinicopathologic features of penile tumors: With special reference to analyses of p21 and p53 expression and unusual histologic features. Arch. Pathol. Lab. Med. 1999, 123, 895–904. [Google Scholar] [CrossRef]
- Osama, A.; Gaur, K.; Chatterjee, P.; Agarwal, K.; Jyoti, D. Acantholytic Squamous Cell Carcinoma: A Diagnostic Pitfall on Cytology. Indian J. Surg. Oncol. 2023, 14, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Rush, P.; Shiau, J.; Hibler, B.; Longley, B.; Downs, T.; Bennett, D. Primary cutaneous adenosquamous carcinoma of the penis: The first characterization of HPV status in this rare and diagnostically challenging entity with review of glandular carcinomas of the penis. J. Cutan. Pathol. 2016, 43, 1226–1230. [Google Scholar] [CrossRef]
- Santos, L.D.; Krivanek, M.J.; Chan, F.; Killingsworth, M. Pseudoangiosarcomatous squamous cell carcinoma of the vulva. Pathology 2006, 38, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Mukawa, A.; Miyazaki, K.; Tsukahara, K. Adenoid squamous cell Carcinoma of the penis Report of a surgical case clinically manifested with rapid lung metastasis. Acta Pathol. Jpn. 1983, 33, 1243–1250. [Google Scholar] [CrossRef]
- Zamecnik, M.; Mukensnabl, P.; Chlumska, A. Pseudoglandular (adenoid, acantholytic) squamous cell carcinoma of the penis. A case report. Cesk. Patol. 2011, 47, 15–18. [Google Scholar] [PubMed]
- Hald, A.K.; Blaakaer, J. The possible role of human papillomavirus infection in the development of lichen sclerosus. Int. J. Dermatol. 2018, 57, 139–146. [Google Scholar] [CrossRef]
- Aidé, S.; Lattario, F.R.; Almeida, G.; do Val, I.C.; da Carvalho, M.C. Epstein-Barr virus and human papillomavirus infection in vulvar lichen sclerosus. J. Low. Genit. Tract Dis. 2010, 14, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Hayduk, K.; Missmahl, H.P.; Gerrads, J. Demonstration of amyloid substance in biopsy material from bronchi in generalized amyloidoses. Med. Klin. 1969, 64, 18–21. [Google Scholar]
- McDaniel, B.; Badri, T.; Steele, R.B. Basal Cell Carcinoma; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zito, P.M.; Scharf, R. Keratoacanthoma; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Combalia, A.; Carrera, C. Squamous Cell Carcinoma: An Update on Diagnosis and Treatment. Dermatol. Pract. Concept. 2020, 10, e2020066. [Google Scholar] [CrossRef]
- Kiyohara, T.; Miyamoto, M.; Shijimaya, T.; Nagano, N.; Nakamaru, S.; Makimura, K.; Tanimura, H. Pseudovascular squamous cell carcinoma: A review of the published work and reassessment of prognosis. J. Dermatol. 2018, 45, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Alegría-Landa, V.; Navarro-Triviño, F.J.; Aneiros-Fernandez, J.; Requena, L. Pseudoangiosarcomatous squamous cell carcinoma of the skin: A need for a more rigorous nomenclature for histopathological variants of squamous cell carcinoma. J. Dermatol. 2018, 45, 76–79. [Google Scholar] [CrossRef]
- Banerjee, S.; Eyden, B.; Wells, S.; McWilliam, L.; Harris, M. Pseudoangiosarcomatous carcinoma: A clinicopathological study of seven cases. Histopathology 1992, 21, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O.; Yasuda, H. A Case of pseudovascular adenoid squamous cell carcinoma of the skin with spindle cell pattern. J. Dermatol. 1997, 24, 587–594. [Google Scholar] [CrossRef]
- Smirnov, A.; Anemona, L.; Novelli, F.; Piro, M.C.; Annicchiarico-Petruzzelli, M.; Melino, G.; Candi, E. p63 Is a Promising Marker in the Diagnosis of Unusual Skin Cancer. Int. J. Mol. Sci. 2019, 20, 5781. [Google Scholar] [CrossRef] [PubMed]
- Marco, V.S.; Rabasco, A.G.; Cebollada, M.M.; Estrada, R.B. Acantholytic squamous cell carcinoma arising in actinic keratoacanthoma: Two cases with E-cadherin expression supporting a potentially different prognosis. Rev. Española Patol. 2016, 49, 23–26. [Google Scholar] [CrossRef]
- Alferally, I.T.; Munir, D.; Putra, I.B.; Sembiring, R.J. Correlation of Ki-67 Expression as Tumor cell Proliferation Activity Marker with Cutaneous Squamous Cell Carcinoma Grading. Open Access Maced. J. Med. Sci. 2019, 7, 3384–3386. [Google Scholar] [CrossRef]
- Zhan, Y.; Wan, H.; Wu, L.; Ge, X.; Xie, X.; Wu, L.; Cai, Y. Pseudoangiosarcomatous squamous cell carcinoma: A rare subtype of squamous cell carcinoma that needs to be differentiated from angiosarcoma and has a poor prognosis. Cell Mol. Biol. 2022, 68, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, S.; Saida, T.; Takizawa, Y.; Tokuda, Y.; Ito, T.; Fujioka, F.; Sakaki, T.; Uchiha, N.; Arase, S.; Takeda, K. Vimentin-positive squamous cell carcinoma arising in a burn scar. A highly malignant neoplasm composed of acantholytic round keratinocytes. Arch. Dermatol. 1989, 125, 1672–1676. [Google Scholar] [PubMed]
- Bayer-Garner, I.B.; Smoller, B.R. The expression of syndecan-1 is preferentially reduced compared with that of E-cadherin in acantholytic squamous cell carcinoma. J. Cutan. Pathol. 2001, 28, 83–89. [Google Scholar] [CrossRef] [PubMed]
| Study Characteristics | Number of Studies N = 52, n (%) | ||
|---|---|---|---|
| Study type | Case reports | 30 (57.7%) | |
| Case series | 10 (19.2%) | ||
| Prospective observational studies | 3 (5.8%) | ||
| Retrospective observational studies | 9 (17.3%) | ||
| Randomized controlled studies | 0 (0%) | ||
| Quality of study | Level 1 | 0 (0%) | |
| Level 2 | 0 (0%) | ||
| Level 3 | 3a | 0 (0%) | |
| 3b | 0 (0%) | ||
| 3c | 0 (0%) | ||
| 3d | 4 (7.7%) | ||
| 3e | 6 (11.5%) | ||
| Level 4 | 4a | 0 (0%) | |
| 4b | 0 (0%) | ||
| 4c | 11 (21.2%) | ||
| 4d | 31 (59.6%) | ||
| 4e | 0 (0%) | ||
| Level 5 | 0 (0%) | ||
| Country of study | United States | 15 (28.8%) | |
| Japan | 9 (17.3%) | ||
| Spain | 6 (11.5%) | ||
| India | 4 (7.7%) | ||
| United Kingdom | 3 (5.8%) | ||
| China | 3 (5.8%) | ||
| Australia | 2 (3.8%) | ||
| South Korea | 2 (3.8%) | ||
| Other | 8 (15.4%) | ||
| Study topic * | Clinical features | 48 (92.3%) | |
| Dermoscopy features | 6 (11.5%) | ||
| Molecular features | 34 (65.4%) | ||
| Demographics | N (%) or Mean ± SD (Range) | |
|---|---|---|
| Total patients | 482 | |
| Age at diagnosis | 68.9 ± 14.4 (20–102) | |
| Sex (male: female), unspecified | 367 (76.1%), 87 (18.0%), 28 (5.8%) | |
| Ethnicity | Caucasian | 181 (37.6%) |
| Arabic | 1 (0.2%) | |
| Asian | 22 (4.6%) | |
| Hispanic | 53 (11.0%) | |
| Black | 1 (0.2%) | |
| Unspecified | 224 (46.5%) | |
| Risk factors and comorbidities | Sunburn/intentional sun exposure | 2 (0.4%) |
| Other burn | 2 (0.4%) | |
| Smoking | 1 (0.2%) | |
| Immunosuppression | 5 (1.0%) | |
| History of skin cancer | 7 (1.5%) | |
| History of other malignancy | 4 (0.8%) | |
| Autoimmune condition | 2 (0.4%) | |
| Actinic keratosis | 4 (0.8%) | |
| Other * | 11 (2.3%) | |
| Final diagnosis | Acantholytic SCC | 445 (92.3%) |
| Pseudoangiosarcomatous SCC | 15 (3.1%) | |
| Adenoid SCC | 16 (3.3%) | |
| Pseudoglandular SCC | 3 (0.6%) | |
| Pseudovascular SCC | 3 (0.6%) | |
| Clinical Features | N (%) | |
|---|---|---|
| Number of cases | 430 | |
| Location | Head and neck | 329 (76.5%) |
| Trunk/back | 33 (7.7%) | |
| Upper limb | 36 (8.4%) | |
| Lower limb | 17 (4.0%) | |
| Genitalia | 11 (2.6%) | |
| Unspecified | 4 (0.9%) | |
| Morphology | Flat-like plaque | 1 (0.2%) |
| Raised nodules | 93 (21.6%) | |
| Erosion | 6 (1.4%) | |
| Ulceration | 54 (12.6%) | |
| Hyperkeratosis | 6 (1.4%) | |
| Other | Erythematous (7) vegetative (1) exophytic (4) sessile (1) crusting (3) papillomatous (1) friable (2) smooth (1) atrophic (2) pedunculated (1) indurated (2) well demarcated (1) irregular (2) semi-mobile/mobile (1) firm (2) soft (1) fixed (1) | |
| Symptoms | Pruritic | 3 (0.7%) |
| Painful | 7 (1.6%) | |
| Painless | 1 (0.2%) | |
| Purulent | 3 (0.7%) | |
| Hemorrhagic/bleeding | 3 (0.7%) | |
| Size/diameter, median (range), cm | 3.25 (0.2–10) | |
| Dermoscopy | Keratin clots (2) Erosions (2) Ulcerations (3) Background: white (1), red (3), pigmented (1) | |
| Confirmed acantholysis on histopathology | 292 (67.9%) | |
| Molecular Features | N (%) |
|---|---|
| Number of cases | 149 |
| Cytokeratins | AE1/AE3 positivity (20), negativity (2) CK1 positivity (6) CK5/6 positivity (3) CK7 positivity (1) CK10 positivity (1) CK8/18 negativity (1) CK19 negativity (1) CK20 negativity (1) CAM5.2 positivity (7), negativity (1) CK34βE12 positivity (6) KL-1 (pan-cytokeratin) positivity (4) High-molecular-weight CK positivity (3) Broad CK, not otherwise specified positivity (40) |
| Cluster of Differentiation Markers | CD15 (Leu-M1) positivity (14), negativity (23) CD31 positivity (1), negativity (15) CD34 positivity (2), negativity (16) CD56 negativity (1) CD68 positivity (1), negativity (1) CD117 negativity (2) CD138 (syndecan 1) diminished (46) |
| Tumor Suppressor Genes, Cell Proliferation Markers | p16 positivity (1), negativity (1) p21 positivity (3) p40 positivity (6) p63 positivity (11) E6/E7 negativity (1) S100 negativity (7) Desmin negativity (1) Ki-67 positivity (9) Melan A negativity (1) |
| Epithelial Tissue Markers | EMA positivity (11), negativity (5) UEA-I positivity (35), negativity (7) Vimentin positivity (16), negativity (1) E-cadherin positivity (2), decreased (14) CEA positivity (2), negativity (7) |
| Markers for Inflammatory Environments | a-SMA positivity (5), negativity (2) ERG negativity (1) |
| Miscellaneous | Factor VIII-related antigen positivity (1), negativity (10) Laminin-5 positivity (3), negativity (3) MSA/HHF35 negativity (2) Involucrin positivity (2) HSP105 positivity (4), negativity (2) Chromogranin A negativity (1) Actin/myoglobin negativity (1) |
| Marker | Function |
|---|---|
| AE1/3 | Broad-spectrum cytokeratin marker for cytokeratins 1–8, 10, 14–16 and 19. Immunoreactivity observed in epithelia and most carcinomas (i.e., tumors of epithelial origin). |
| a-SMA | There are three isoforms of smooth muscle actin: alpha, beta and gamma. Alpha actins are found in muscle tissues and required for contraction. Usually negative in squamous cell carcinomas. |
| CAM5.2 | Usually positive in glandular epithelia and adenocarcinomas, whereas negative in squamous epithelium and squamous cell carcinomas. |
| CA15-3 (EMA, MUC1) | Epithelial membrane antigen highly expressed by most carcinomas (e.g., adenocarcinomas, squamous cell carcinomas) and hematologic cancers. |
| CD15 | Mostly used for diagnosis of Hodgkin’s lymphoma. May be a granulocyte marker. |
| CD31 | Most sensitive and specific endothelial marker in paraffin sections. Negative in adenomatoid tumor and pseudoangiomatous stromal hyperplasia of the breast. |
| CD34 | Distinguish Kaposi sarcoma, dermatofibrosarcoma protuberans, and epithelioid sarcoma from dermatofibroma; distinguish solitary fibrous tumor from desmoplastic mesothelioma; distinguish hemangiopericytoma from endometrial stromal sarcoma. |
| CD56 | Marker of natural killer (NK) cells and NK lymphomas. |
| CD68 | Lysosomal marker used to identify histiocytic and monocytic cells. Usually used to diagnose histiocytic sarcoma. |
| CD117 | Proto-oncogene activated in gastrointestinal stromal tumors (GISTs). Negativity is associated with leiomyoma, leiomyosarcoma, smooth muscle tumors and solitary fibrous tumors. |
| CD138 (syndecan 1) | Involved in cell proliferation, migration, adhesion and angiogenesis, with loss of CD138 expression leading to enhanced motility and invasion of neoplastic cells. Low or absent expression in testicular germ cell tumors, sarcomas and melanomas. |
| CEA | Also known as carcinoembryonic antigen. Usually considered epithelial marker with expression in many adenocarcinomas, lung squamous cell carcinoma and sweat gland carcinomas. Negative staining in melanomas, acantholytic squamous cell carcinoma (pseudoglandular). |
| Chromogranin | Commonly used neuroendocrine marker. Specific for neuroendocrine cells, but not sensitive. |
| CK34βE12 | Positive staining in classic and basaloid squamous cell carcinoma, as well as amyloid deposits associated with squamous cell carcinoma and dysplasia in the head and neck. |
| CK1 | Highest-molecular-weight keratin. Positive staining associated with keratinizing squamous cell carcinoma. |
| CK5/6 | Together with p63, used to detect squamous cell origin in poorly differentiated carcinomas. |
| CK7 | Generally negative (with some variation) in colorectal carcinoma, Merkel cell carcinoma, hepatocellular carcinoma, prostatic adenocarcinoma, adrenocortical tumors and squamous cell carcinoma. |
| CK10 | Defects in CK10–CK1 protein network cause structural instability and weakness of keratinocytes, causing blistering, hyperproliferation and hyperkeratosis. |
| CK8/18 | Positive in bile duct, invasive ductal breast, hepatocellular, neuroendocrine, pancreatic, prostatic, renal cell, and squamous cell (cervical and oral) carcinomas. Usually negative in smooth muscle tumors. |
| CK19 | Present in simple and complex epithelium; positive staining in hair follicles; negative stain in trichilemmoma. |
| CK20 | Epithelial marker; positive staining in Merkel cell carcinoma and fibroepithelioma of Pinkus. Usually negative staining in squamous cell carcinomas. |
| High-molecular-weight CK | High-molecular-weight cytokeratins 1–6, 10, 14, 15 and 16. |
| Desmin | Good screening marker for neoplasms with myogenic differentiation such as rhabdomyosarcoma, rhabdomyoma, leiomyosarcoma, leiomyoma and smooth muscle. |
| E-cadherin | Transmembrane protein involved in cellular adhesion where loss is associated with tumor progression, chemoresistance and metastases. |
| ERG | Marker of endothelial differentiation including vascular neoplasms. Expression usually negative in all non-prostatic carcinomas. |
| Factor VIII-related antigen | Common endothelial marker with positive staining of endothelial cells, megakaryocytes, platelets and mast cells. |
| HSP105 1 | Heat shock proteins are a group of heat stress proteins that may be affected by malignant transformation. Expression is decreased in cutaneous squamous cell carcinomas, but poorly differentiated subtypes show higher expression. |
| Involucrin | Contributes to formation of the insoluble cell envelope with loricrin. |
| Ki-67 | Marker of cell proliferation. Commonly increased in most malignant and inflammatory conditions. |
| KL-1 (pan-cytokeratin) | Broad-spectrum keratin antibody for CK1–4, 10–11 or CK1, 2, 5–8, 11, 14, 16–18. Present in most carcinomas. |
| Laminin-5 2 | Associated with invading cancer cells such as cervical carcinomas and cutaneous squamous cell carcinoma invasion. |
| Melan A | Melanocyte lineage-specific marker in the diagnosis of metastatic melanoma. Also positive in nonmelanocytic tumors with melanosomes (e.g., angiomyolipoma, PEComa, lymphangioleiomyomatosis). Negative in most carcinomas, lymphomas. |
| MSA (HHF35) | Identifies skeletal muscle, smooth muscle cells. Known for negative staining in angiomyofibroblastoma and epithelioid mesothelioma. |
| p16 | Tumor suppressor protein that prevents progression into S phase of cell cycle. Protein function is silenced in many HPV and non-HPV (e.g., breast, colon, pancreatic, head and neck, melanoma)-related tumors. |
| p21 | Negative cell cycle regular in G2–M phase and G1–S phase. |
| p40 | Stimulates cell proliferation and favors unrestrained tumor growth. Nuclear marker with expression in squamous, urothelial, myoepithelial cell carcinomas. |
| P63 | Important regulator of epidermal keratinocyte proliferation and embryonic epidermal growth. Usually positive in squamous and basal cell carcinomas and helps differentiate from melanomas. |
| S100 | Marker of melanocytes and useful for evaluating nerve sheath tumors and melanoma. |
| UEA-1 3,4 | Ulex europaeus agglutinin I is a plant lectin with an affinity for L-fucosyl residues associated with dorsal root ganglion neurons and their axonal processes. In the context of skin carcinomas, UEA-1 stains positive in endothelial cells and negative/weakly positive in the epidermis. |
| Vimentin | Intermediate filament for mesenchymal tissues. Usually positive in melanomas, negative for carcinomas (but many exceptions) and epithelial tumors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.K.; Mija, L.A.; Conte, S.; Ghezelbash, S.; Nallanathan, B.; Fortier-Riberdy, G.; Redpath, M.; Lefrançois, P. Clinical, Dermoscopic, and Molecular Features of Acantholytic Squamous Cell Carcinoma: A Systematic Review. Cancers 2024, 16, 2905. https://doi.org/10.3390/cancers16162905
Zhu CK, Mija LA, Conte S, Ghezelbash S, Nallanathan B, Fortier-Riberdy G, Redpath M, Lefrançois P. Clinical, Dermoscopic, and Molecular Features of Acantholytic Squamous Cell Carcinoma: A Systematic Review. Cancers. 2024; 16(16):2905. https://doi.org/10.3390/cancers16162905
Chicago/Turabian StyleZhu, Catherine Keying, Lorena Alexandra Mija, Santina Conte, Sarah Ghezelbash, Bonika Nallanathan, Geneviève Fortier-Riberdy, Margaret Redpath, and Philippe Lefrançois. 2024. "Clinical, Dermoscopic, and Molecular Features of Acantholytic Squamous Cell Carcinoma: A Systematic Review" Cancers 16, no. 16: 2905. https://doi.org/10.3390/cancers16162905
APA StyleZhu, C. K., Mija, L. A., Conte, S., Ghezelbash, S., Nallanathan, B., Fortier-Riberdy, G., Redpath, M., & Lefrançois, P. (2024). Clinical, Dermoscopic, and Molecular Features of Acantholytic Squamous Cell Carcinoma: A Systematic Review. Cancers, 16(16), 2905. https://doi.org/10.3390/cancers16162905








