Tumor-Infiltrating Lymphocyte Scoring in Neoadjuvant-Treated Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Histological Staining
2.3. Pathological Assessment
2.4. Digital Scoring
2.5. Statistical Analysis
3. Results
3.1. Inter-Pathologist Concordance for Scoring TIL
3.2. Comparison of Scoring TIL on H&E- versus CD3/CD20-Stained Tissues
3.3. Comparison of TIL Aggregate Scores
3.4. Scoring TIL in Heterogeneous Residual Tumor Tissue
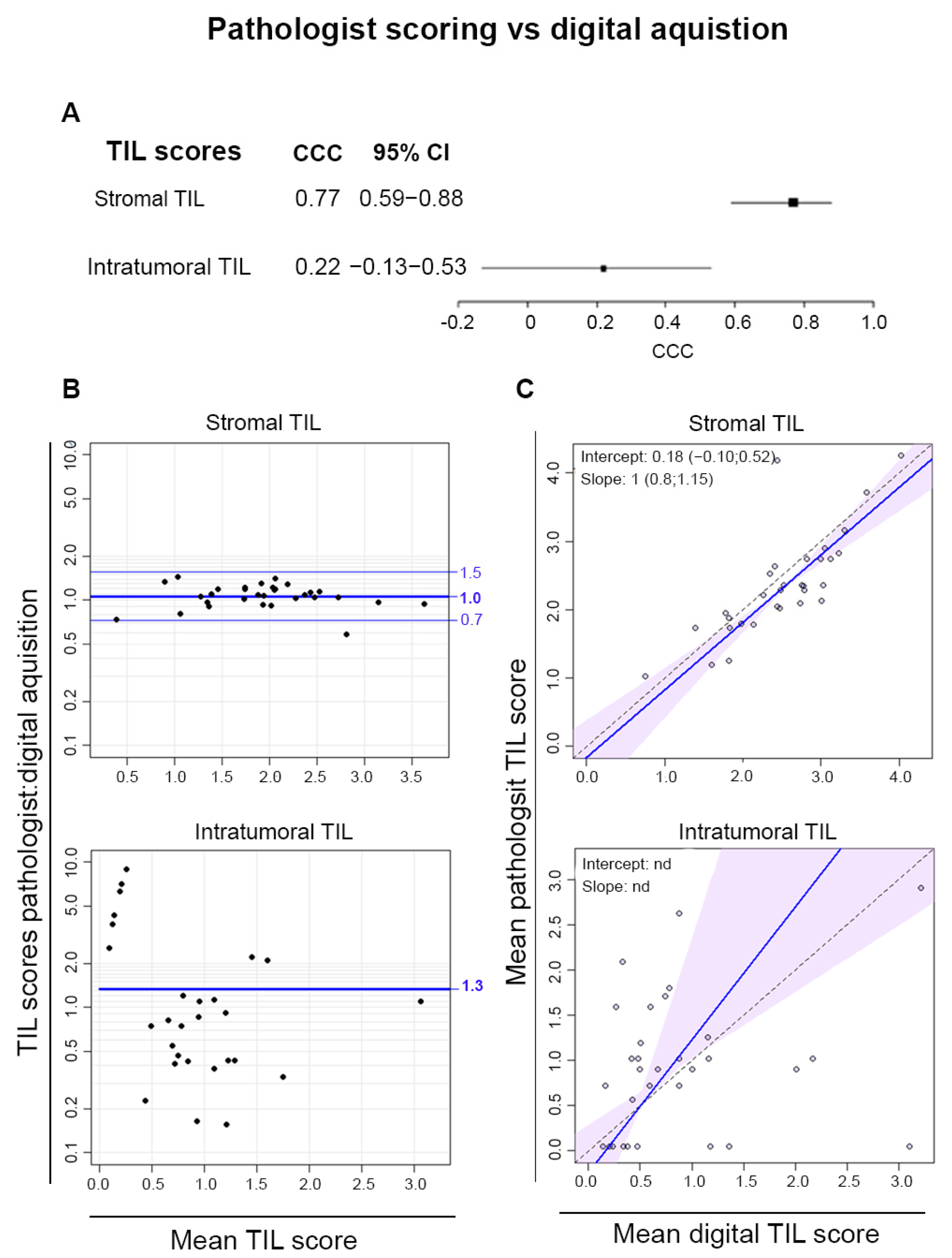
3.5. Digital Pathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teshome, M.; Hunt, K.K. Neoadjuvant Therapy in the Treatment of Breast Cancer. Surg. Oncol. Clin. N. Am. 2014, 23, 505–523. [Google Scholar] [CrossRef]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of Residual Breast Cancer Burden to Predict Survival after Neoadjuvant Chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef]
- Prowell, T.M.; Pazdur, R. Pathological Complete Response and Accelerated Drug Approval in Early Breast Cancer. N. Engl. J. Med. 2012, 366, 2438–2441. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Osdoit, M.; van der Noordaa, M.; Shad, S.; Wei, J.; de Croze, D.; Hamy, A.-S.; Laé, M.; Reyal, F.; Sonke, G.S.; et al. Residual Cancer Burden after Neoadjuvant Chemotherapy and Long-Term Survival Outcomes in Breast Cancer: A Multicentre Pooled Analysis of 5161 Patients. Lancet Oncol. 2022, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The Immune Contexture in Human Tumours: Impact on Clinical Outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- West, N.R.; Milne, K.; Truong, P.T.; Macpherson, N.; Nelson, B.H.; Watson, P.H. Tumor-Infiltrating Lymphocytes Predict Response to Anthracycline-Based Chemotherapy in Estrogen Receptor-Negative Breast Cancer. Breast Cancer Res. BCR 2011, 13, R126. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.A.; Hitre, E.; et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin with Doxorubicin-Based Chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The Evaluation of Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Denkert, C.; Wienert, S.; Poterie, A.; Loibl, S.; Budczies, J.; Badve, S.; Bago-Horvath, Z.; Bane, A.; Bedri, S.; Brock, J.; et al. Standardized Evaluation of Tumor-Infiltrating Lymphocytes in Breast Cancer: Results of the Ring Studies of the International Immuno-Oncology Biomarker Working Group. Mod. Pathol. 2016, 29, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Kos, Z.; Roblin, E.; Kim, R.S.; Michiels, S.; Gallas, B.D.; Chen, W.; van de Vijver, K.K.; Goel, S.; Adams, S.; Demaria, S.; et al. Pitfalls in Assessing Stromal Tumor Infiltrating Lymphocytes (sTILs) in Breast Cancer. Npj Breast Cancer 2020, 6, 17. [Google Scholar] [CrossRef]
- O’Loughlin, M.; Andreu, X.; Bianchi, S.; Chemielik, E.; Cordoba, A.; Cserni, G.; Figueiredo, P.; Floris, G.; Foschini, M.P.; Heikkilä, P.; et al. Reproducibility and Predictive Value of Scoring Stromal Tumour Infiltrating Lymphocytes in Triple-Negative Breast Cancer: A Multi-Institutional Study. Breast Cancer Res. Treat. 2018, 171, 1–9. [Google Scholar] [CrossRef]
- Buisseret, L.; Desmedt, C.; Garaud, S.; Fornili, M.; Wang, X.; Van den Eyden, G.; de Wind, A.; Duquenne, S.; Boisson, A.; Naveaux, C.; et al. Reliability of Tumor-Infiltrating Lymphocyte and Tertiary Lymphoid Structure Assessment in Human Breast Cancer. Mod. Pathol. 2017, 30, 1204–1212. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-Infiltrating Lymphocytes and Prognosis in Different Subtypes of Breast Cancer: A Pooled Analysis of 3771 Patients Treated with Neoadjuvant Therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, J.; Zhang, T.; Xue, D. Tumor-Infiltrating Lymphocytes in Breast Cancer Predict the Response to Chemotherapy and Survival Outcome: A Meta-Analysis. Oncotarget 2016, 7, 44288–44298. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B Cells and Tertiary Lymphoid Structures Promote Immunotherapy Response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Noël, G.; Fontsa, M.L.; Garaud, S.; De Silva, P.; de Wind, A.; Van den Eynden, G.G.; Salgado, R.; Boisson, A.; Locy, H.; Thomas, N.; et al. Functional Th1-Oriented T Follicular Helper Cells That Infiltrate Human Breast Cancer Promote Effective Adaptive Immunity. J. Clin. Investig. 2021, 131, e139905. [Google Scholar] [CrossRef]
- Italiano, A.; Bessede, A.; Pulido, M.; Bompas, E.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Bertucci, F.; Toulmonde, M.; Bellera, C.; et al. Pembrolizumab in Soft-Tissue Sarcomas with Tertiary Lymphoid Structures: A Phase 2 PEMBROSARC Trial Cohort. Nat. Med. 2022, 28, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Song, I.H.; Heo, S.-H.; Bang, W.S.; Park, H.S.; Park, I.A.; Kim, Y.-A.; Park, S.Y.; Roh, J.; Gong, G.; Lee, H.J. Predictive Value of Tertiary Lymphoid Structures Assessed by High Endothelial Venule Counts in the Neoadjuvant Setting of Triple-Negative Breast Cancer. Cancer Res. Treat. 2017, 49, 399–407. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Han, Y.; Deng, Y.; Li, J.; Jiang, Y. The Presence of Tertiary Lymphoid Structures Provides New Insight Into the Clinicopathological Features and Prognosis of Patients With Breast Cancer. Front. Immunol. 2022, 13, 868155. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ji, J.; Li, S.; Yang, M.; Che, Y.; Xu, Z.; Zhang, Y.; Wang, M.; Fang, Z.; Luo, L.; et al. Analysis of the Correlation and Prognostic Significance of Tertiary Lymphoid Structures in Breast Cancer: A Radiomics-Clinical Integration Approach. J. Magn. Reson. Imaging JMRI 2024, 59, 1206–1217. [Google Scholar] [CrossRef]
- Narvaez, D.; Nadal, J.; Nervo, A.; Costanzo, M.V.; Paletta, C.; Petracci, F.E.; Rivero, S.; Ostinelli, A.; Freile, B.; Enrico, D.; et al. The Emerging Role of Tertiary Lymphoid Structures in Breast Cancer: A Narrative Review. Cancers 2024, 16, 396. [Google Scholar] [CrossRef]
- Dieci, M.V.; Criscitiello, C.; Goubar, A.; Viale, G.; Conte, P.; Guarneri, V.; Ficarra, G.; Mathieu, M.C.; Delaloge, S.; Curigliano, G.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes on Residual Disease after Primary Chemotherapy for Triple-Negative Breast Cancer: A Retrospective Multicenter Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Pelekanou, V.; Carvajal-Hausdorf, D.E.; Altan, M.; Wasserman, B.; Carvajal-Hausdorf, C.; Wimberly, H.; Brown, J.; Lannin, D.; Pusztai, L.; Rimm, D.L. Effect of Neoadjuvant Chemotherapy on Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Breast Cancer and Its Clinical Significance. Breast Cancer Res. BCR 2017, 19, 91. [Google Scholar] [CrossRef]
- Luen, S.J.; Salgado, R.; Dieci, M.V.; Vingiani, A.; Curigliano, G.; Gould, R.E.; Castaneda, C.; D’Alfonso, T.; Sanchez, J.; Cheng, E.; et al. Prognostic Implications of Residual Disease Tumor-Infiltrating Lymphocytes and Residual Cancer Burden in Triple-Negative Breast Cancer Patients after Neoadjuvant Chemotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 236–242. [Google Scholar] [CrossRef]
- Sethi, D.; Sen, R.; Parshad, S.; Khetarpal, S.; Garg, M.; Sen, J. Histopathologic Changes Following Neoadjuvant Chemotherapy in Various Malignancies. Int. J. Appl. Basic Med. Res. 2012, 2, 111–116. [Google Scholar] [CrossRef]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer, Including Recommendations to Assess TILs in Residual Disease after Neoadjuvant Therapy and in Carcinoma in Situ: A Report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Immuno-Oncol. Biomark. 2018, 52, 16–25. [Google Scholar] [CrossRef]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.-N.; Duvillier, H.; et al. Tumor-Infiltrating Lymphocyte Composition, Organization and PD-1/PD-L1 Expression Are Linked in Breast Cancer. OncoImmunology 2017, 6, e1257452. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman Analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Bilić-Zulle, L. Comparison of Methods: Passing and Bablok Regression. Biochem. Med. 2011, 21, 49–52. [Google Scholar] [CrossRef]
- Lawrence, I.; Lin, K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef]
- Carstensen, B.; Plummer, M.; Laara, E.; Hills, M. Epi: A Package for Statistical Analysis in Epidemiology. R Package Version 1.0-14. 2020. Available online: https://CRAN.R-project.org/package=Epi (accessed on 12 August 2024).
- Carstensen, B.; Gurrin, L.; Ekstrøm, C.T.; Figurski, M. MethComp: Analysis of Agreement in Method Comparison Studies. R Package Version 1.30.0. 2020. Available online: https://CRAN.R-project.org/package=MethComp (accessed on 12 August 2024).
- Watanabe, T.; Hida, A.I.; Inoue, N.; Imamura, M.; Fujimoto, Y.; Akazawa, K.; Hirota, S.; Miyoshi, Y. Abundant Tumor Infiltrating Lymphocytes after Primary Systemic Chemotherapy Predicts Poor Prognosis in Estrogen Receptor-Positive/HER2-Negative Breast Cancers. Breast Cancer Res. Treat. 2018, 168, 135–145. [Google Scholar] [CrossRef]
- García-Martínez, E.; Gil, G.L.; Benito, A.C.; González-Billalabeitia, E.; Conesa, M.A.V.; García, T.G.; García-Garre, E.; Vicente, V.; de la Peña, F.A. Tumor-Infiltrating Immune Cell Profiles and Their Change after Neoadjuvant Chemotherapy Predict Response and Prognosis of Breast Cancer. Breast Cancer Res. 2014, 16, 488. [Google Scholar] [CrossRef] [PubMed]
- Ochi, T.; Bianchini, G.; Ando, M.; Nozaki, F.; Kobayashi, D.; Criscitiello, C.; Curigliano, G.; Iwamoto, T.; Niikura, N.; Takei, H.; et al. Predictive and Prognostic Value of Stromal Tumour-Infiltrating Lymphocytes before and after Neoadjuvant Therapy in Triple Negative and HER2-Positive Breast Cancer. Eur. J. Cancer 2019, 118, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Pinard, C.; Debled, M.; Ben Rejeb, H.; Velasco, V.; Tunon de Lara, C.; Hoppe, S.; Richard, E.; Brouste, V.; Bonnefoi, H.; MacGrogan, G. Residual Cancer Burden Index and Tumor-Infiltrating Lymphocyte Subtypes in Triple-Negative Breast Cancer after Neoadjuvant Chemotherapy. Breast Cancer Res. Treat. 2020, 179, 11–23. [Google Scholar] [CrossRef]
- Kurozumi, S.; Inoue, K.; Matsumoto, H.; Fujii, T.; Horiguchi, J.; Oyama, T.; Kurosumi, M.; Shirabe, K. Prognostic Utility of Tumor-Infiltrating Lymphocytes in Residual Tumor after Neoadjuvant Chemotherapy with Trastuzumab for HER2-Positive Breast Cancer. Sci. Rep. 2019, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Takada, K.; Takahashi, K.; Hatano, T.; Noda, S.; Takashima, T.; Onoda, N.; Tomita, S.; et al. Prediction of Survival after Neoadjuvant Chemotherapy for Breast Cancer by Evaluation of Tumor-Infiltrating Lymphocytes and Residual Cancer Burden. BMC Cancer 2017, 17, 888. [Google Scholar] [CrossRef]
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The Tale of TILs in Breast Cancer: A Report from The International Immuno-Oncology Biomarker Working Group. Npj Breast Cancer 2021, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Laenkholm, A.-V.; Callagy, G.; Balancin, M.; Bartlett, J.M.S.; Sotiriou, C.; Marchio, C.; Kok, M.; Dos Anjos, C.H.; Salgado, R. Incorporation of TILs in Daily Breast Cancer Care: How Much Evidence Can We Bear? Virchows Arch. 2022, 480, 147–162. [Google Scholar] [CrossRef]
- Peintinger, F.; Sinn, B.; Hatzis, C.; Albarracin, C.; Downs-Kelly, E.; Morkowski, J.; Gould, R.; Symmans, W.F. Reproducibility of Residual Cancer Burden for Prognostic Assessment of Breast Cancer after Neoadjuvant Chemotherapy. Mod. Pathol. 2015, 28, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Gupta, R.; Hou, L.; Abousamra, S.; Fassler, D.; Torre-Healy, L.; Moffitt, R.A.; Kurc, T.; Samaras, D.; Batiste, R.; et al. Utilizing Automated Breast Cancer Detection to Identify Spatial Distributions of Tumor-Infiltrating Lymphocytes in Invasive Breast Cancer. Am. J. Pathol. 2020, 190, 1491–1504. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, S.; Shao, W.; Wu, Y.; Zhang, J.; Han, Z.; Feng, Q.; Huang, K. Deep-Learning–Based Characterization of Tumor-Infiltrating Lymphocytes in Breast Cancers from Histopathology Images and Multiomics Data. JCO Clin. Cancer Inform. 2020, 4, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Verdicchio, M.; Brancato, V.; Cavaliere, C.; Isgrò, F.; Salvatore, M.; Aiello, M. A Pathomic Approach for Tumor-Infiltrating Lymphocytes Classification on Breast Cancer Digital Pathology Images. Heliyon 2023, 9, e14371. [Google Scholar] [CrossRef]
- Saednia, K.; Lagree, A.; Alera, M.A.; Fleshner, L.; Shiner, A.; Law, E.; Law, B.; Dodington, D.W.; Lu, F.-I.; Tran, W.T.; et al. Quantitative Digital Histopathology and Machine Learning to Predict Pathological Complete Response to Chemotherapy in Breast Cancer Patients Using Pre-Treatment Tumor Biopsies. Sci. Rep. 2022, 12, 9690. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, A.; Bifulco, C.; Baldin, P.; Galon, J. Digital Pathology for Better Clinical Practice. Cancers 2024, 16, 1686. [Google Scholar] [CrossRef]
- Amgad, M.; Stovgaard, E.S.; Balslev, E.; Thagaard, J.; Chen, W.; Dudgeon, S.; Sharma, A.; Kerner, J.K.; Denkert, C.; Yuan, Y.; et al. Report on Computational Assessment of Tumor Infiltrating Lymphocytes from the International Immuno-Oncology Biomarker Working Group. Npj Breast Cancer 2020, 6, 16. [Google Scholar] [CrossRef]
- Thagaard, J.; Broeckx, G.; Page, D.B.; Jahangir, C.A.; Verbandt, S.; Kos, Z.; Gupta, R.; Khiroya, R.; Abduljabbar, K.; Acosta Haab, G.; et al. Pitfalls in Machine Learning-Based Assessment of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. J. Pathol. 2023, 260, 498–513. [Google Scholar] [CrossRef]
- Yoneyama, M.; Zormpas-Petridis, K.; Robinson, R.; Sobhani, F.; Provenzano, E.; Steel, H.; Lightowlers, S.; Towns, C.; Castillo, S.P.; Anbalagan, S.; et al. Longitudinal Assessment of Tumor-Infiltrating Lymphocytes in Primary Breast Cancer Following Neoadjuvant Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Berben, L.; Wildiers, H.; Marcelis, L.; Antoranz, A.; Bosisio, F.; Hatse, S.; Floris, G. Computerised Scoring Protocol for Identification and Quantification of Different Immune Cell Populations in Breast Tumour Regions by the Use of QuPath Software. Histopathology 2020, 77, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Vanhersecke, L.; Bougouin, A.; Crombé, A.; Brunet, M.; Sofeu, C.; Parrens, M.; Pierron, H.; Bonhomme, B.; Lembege, N.; Rey, C.; et al. Standardized Pathology Screening of Mature Tertiary Lymphoid Structures in Cancers. Lab. Investig. 2023, 103, 100063. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, I.S.; Mahmutovic, A.; Young, S.J.; Slingluff, C.L.J. Multiplex Immunofluorescence Histology for Immune Cell Infiltrates in Melanoma-Associated Tertiary Lymphoid Structures. In Melanoma; Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2265, pp. 573–587. [Google Scholar] [CrossRef]
- Serna, G.; Simonetti, S.; Fasani, R.; Pagliuca, F.; Guardia, X.; Gallego, P.; Jimenez, J.; Peg, V.; Saura, C.; Eppenberger-Castori, S.; et al. Sequential Immunohistochemistry and Virtual Image Reconstruction Using a Single Slide for Quantitative KI67 Measurement in Breast Cancer. Breast 2020, 53, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Qu, F.-J.; Liu, H.; Li, Z.-J.; Zhang, Y.-C.; Han, X.; Zhu, Z.-Y.; Lv, Y. Prognostic Impact of Tertiary Lymphoid Structures in Breast Cancer Prognosis: A Systematic Review and Meta-Analysis. Cancer Cell Int. 2021, 21, 536. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, N.; Garaud, S.; Langouo, M.; Sofronii, D.; Boisson, A.; De Wind, A.; Duwel, V.; Craciun, L.; Larsimont, D.; Awada, A.; et al. Tumor-Infiltrating Lymphocyte Scoring in Neoadjuvant-Treated Breast Cancer. Cancers 2024, 16, 2895. https://doi.org/10.3390/cancers16162895
Thomas N, Garaud S, Langouo M, Sofronii D, Boisson A, De Wind A, Duwel V, Craciun L, Larsimont D, Awada A, et al. Tumor-Infiltrating Lymphocyte Scoring in Neoadjuvant-Treated Breast Cancer. Cancers. 2024; 16(16):2895. https://doi.org/10.3390/cancers16162895
Chicago/Turabian StyleThomas, Noémie, Soizic Garaud, Mireille Langouo, Doïna Sofronii, Anaïs Boisson, Alexandre De Wind, Valérie Duwel, Ligia Craciun, Dennis Larsimont, Ahmad Awada, and et al. 2024. "Tumor-Infiltrating Lymphocyte Scoring in Neoadjuvant-Treated Breast Cancer" Cancers 16, no. 16: 2895. https://doi.org/10.3390/cancers16162895
APA StyleThomas, N., Garaud, S., Langouo, M., Sofronii, D., Boisson, A., De Wind, A., Duwel, V., Craciun, L., Larsimont, D., Awada, A., & Willard-Gallo, K. (2024). Tumor-Infiltrating Lymphocyte Scoring in Neoadjuvant-Treated Breast Cancer. Cancers, 16(16), 2895. https://doi.org/10.3390/cancers16162895




