Outcome of Endoprosthetic Hip Reconstruction Following Resection of Malignant Bone Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Follow-Up Examination
2.3. Surgical and Histopathological Data
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
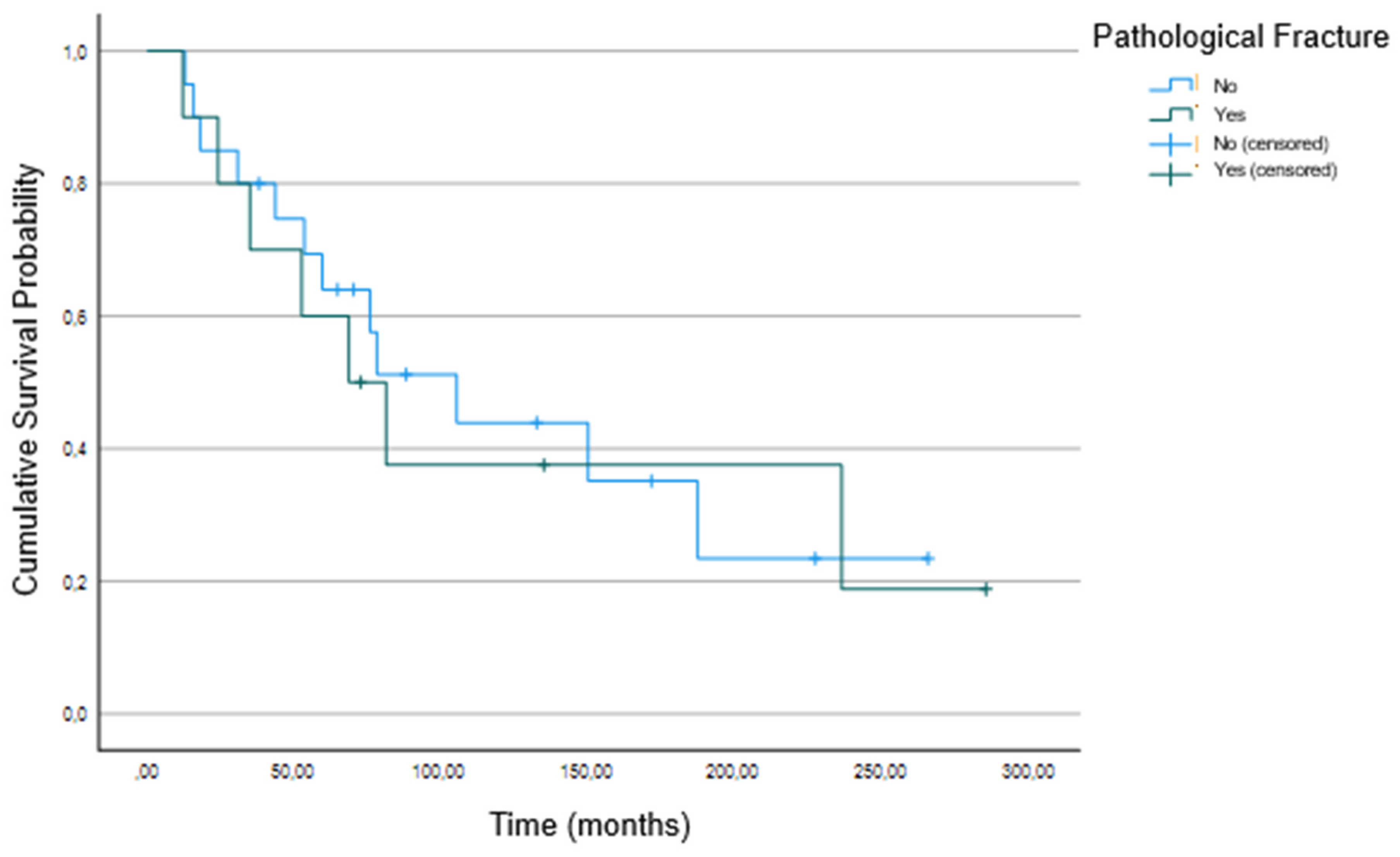
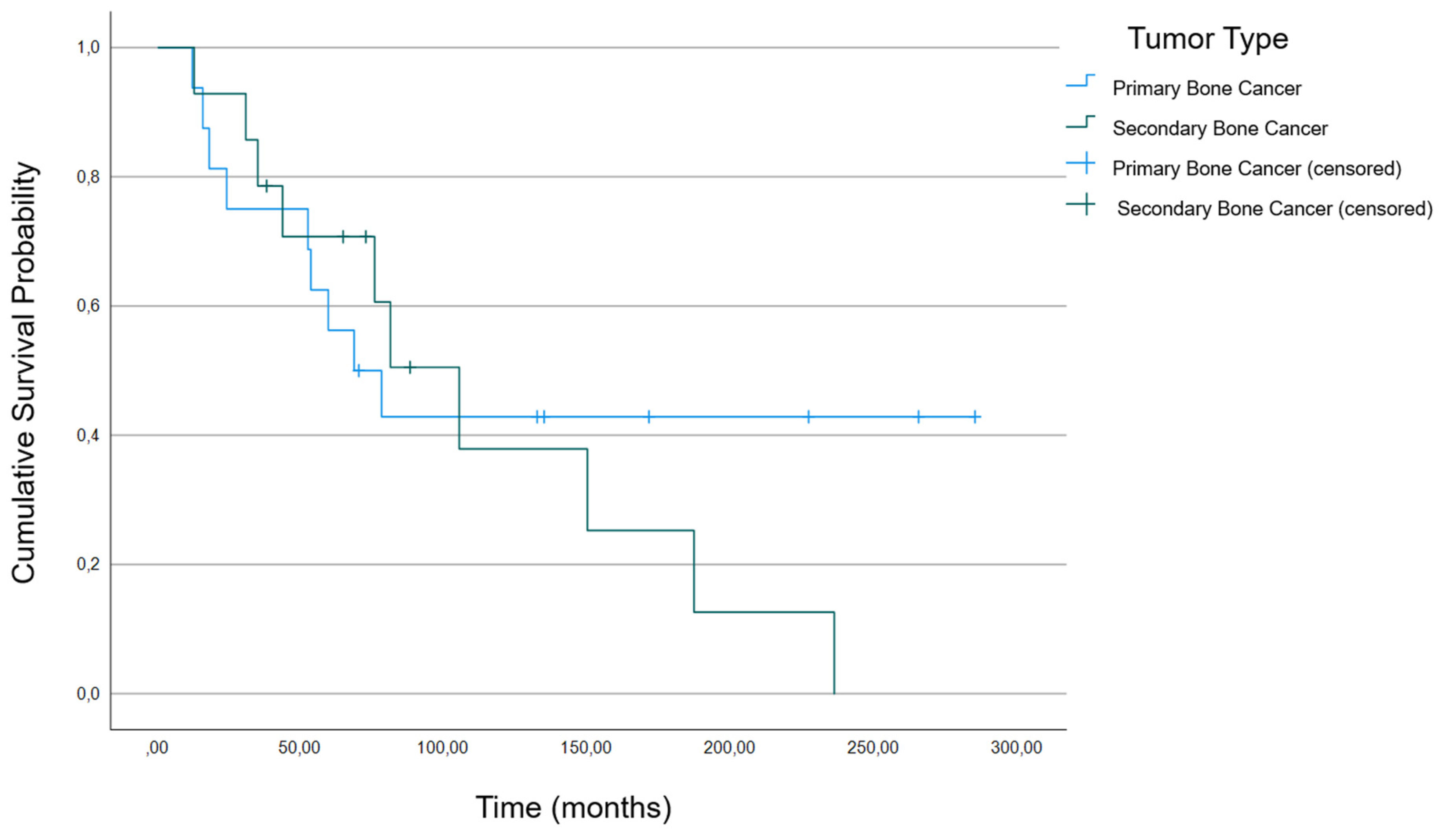
3.2. Association between Patient Characteristics and Survival
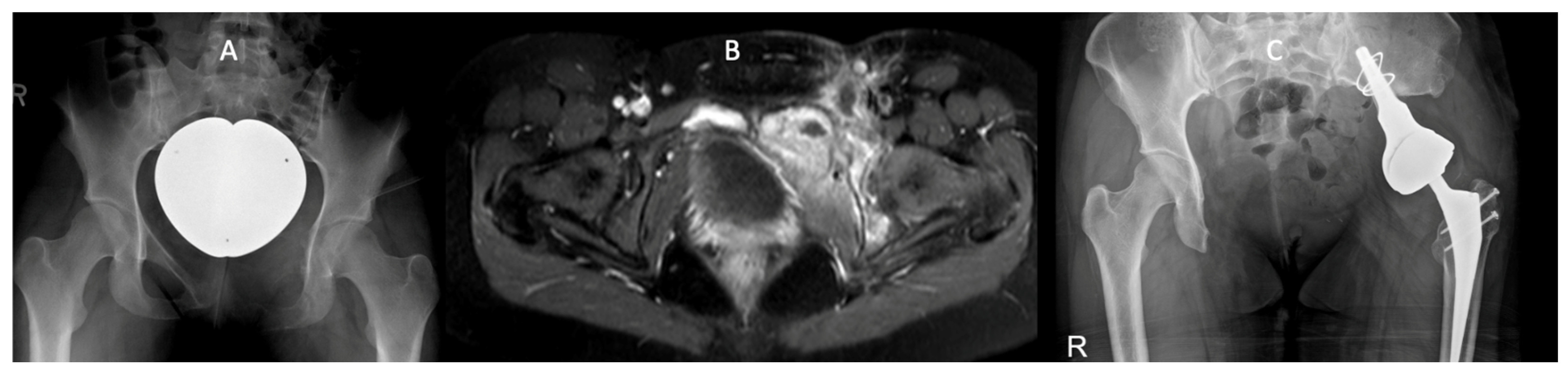
3.3. Example Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williard, W.C.; Collin, C.; Casper, E.S.; Hajdu, S.I.; Brennan, M.F. The changing role of amputation for soft tissue sarcoma of the extremity in adults. Surg. Gynecol. Obstet. 1992, 175, 389–396. [Google Scholar]
- Williard, W.C.; Hajdu, S.I.; Casper, E.S.; Brennan, M.F. Comparison of amputation with limb-sparing operations for adult soft tissue sarcoma of the extremity. Ann. Surg. 1992, 215, 269–275. [Google Scholar] [CrossRef]
- Hardes, J.; Gebert, C.; Schwappach, A.; Ahrens, H.; Streitburger, A.; Winkelmann, W.; Gosheger, G. Characteristics and outcome of infections associated with tumor endoprostheses. Arch. Orthop. Trauma Surg. 2006, 126, 289–296. [Google Scholar] [CrossRef]
- Calabró, T.; Van Rooyen, R.; Piraino, I.; Pala, E.; Trovarelli, G.; Panagopoulos, G.N.; Megaloikonomos, P.D.; Angelini, A.; Mavrogenis, A.F.; Ruggieri, P. Reconstruction of the proximal femur with a modular resection prosthesis. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 415–421. [Google Scholar] [CrossRef]
- Haijie, L.; Dasen, L.; Tao, J.; Yi, Y.; Xiaodong, T.; Wei, G. Implant Survival and Complication Profiles of Endoprostheses for Treating Tumor Around the Knee in Adults: A Systematic Review of the Literature Over the Past 30 Years. J. Arthroplast. 2018, 33, 1275–1287.e3. [Google Scholar] [CrossRef]
- Hobusch, G.M.; Bollmann, J.; Puchner, S.E.; Lang, N.W.; Hofstaetter, J.G.; Funovics, P.T.; Windhager, R. What Sport Activity Levels Are Achieved in Patients after Resection and Endoprosthetic Reconstruction for a Proximal Femur Bone Sarcoma? Clin. Orthop. 2017, 475, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.R.; Keeney, B.J.; Pala, E.; Funovics, P.T.; Eward, W.C.; Groundland, J.S.; Ehrlichman, L.K.; Puchner, S.S.E.; Brigman, B.E.; Ready, J.E.; et al. The stability of the hip after the use of a proximal femoral endoprosthesis for oncological indications: Analysis of variables relating to the patient and the surgical technique. Bone Jt. J. 2017, 99-B, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Gorter, J.; Ploegmakers, J.J.W.; Ten Have, B.L.E.F.; Schreuder, H.W.B.; Jutte, P.C. The push-through total femoral prosthesis offers a functional alternative to total femoral replacement: A case series. Int. Orthop. 2017, 41, 2237–2244. [Google Scholar] [CrossRef]
- Du, Z.; Tang, S.; Yang, R.; Tang, X.; Ji, T.; Guo, W. Use of an Artificial Ligament Decreases Hip Dislocation and Improves Limb Function after Total Femoral Prosthetic Replacement Following Femoral Tumor Resection. J. Arthroplast. 2018, 33, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.J.; Langerhuizen, D.W.G.; Schwab, J.H.; Bramer, J.A.M. Outcome after reconstruction of proximal femoral tumors: A systematic review. J. Surg. Oncol. 2019, 119, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Kumar, A.; Inna, P.; Bakhshi, S.; Rastogi, S. Endoprosthetic replacement for giant cell tumour of the proximal femur. J. Orthop. Surg. 2009, 17, 280–283. [Google Scholar] [CrossRef]
- Liu, L.; Deng, X.-Q.; Zhao, Y.-J.; Ma, R.-X.; Yang, L.; Song, K.-X.; Zhang, J.-Y.; Hu, Y.-C. Modular intercalary prosthetic reconstruction for malignant and metastatic tumours of the proximal femur. Sci. Rep. 2024, 14, 5867. [Google Scholar] [CrossRef] [PubMed]
- Andreani, L.; Ipponi, E.; Falcinelli, F.; Cordoni, M.; Bechini, E.; Vannucci, L.; D’arienzo, A.; Capanna, R. Proximal Femur Megaprostheses in Orthopedic Oncology: Evaluation of a Standardized Post-operative Rehabilitation Protocol. Indian J. Orthop. 2024, 58, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Guzik, G. Treatment Outcomes and Quality of Life after the Implantation of Modular Prostheses of the Proximal Femur in Patients with Cancer Metastases. Ortop. Traumatol. Rehabil. 2016, 18, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Watts, C.D.; Wyles, C.C.; Rose, P.S.; Taunton, M.J.; Sim, F.H. Functional and oncologic outcome of cemented endoprosthesis for malignant proximal femoral tumors. J. Surg. Oncol. 2016, 114, 501–506. [Google Scholar] [CrossRef]
- Pitera, T.; Guzik, G.; Biega, P. Assessment of Post-operative Physical Performance in Patients after Resection Arthroplasty of the Proximal Femur. Ortop. Traumatol. Rehabil. 2017, 19, 333–340. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Wang, B.; Guo, A. Clinical and functional comparison of endoprosthetic replacement with intramedullary nailing for treating proximal femur metastasis. Chin. J. Cancer Res. 2016, 28, 209–214. [Google Scholar] [CrossRef]
- Meermans, G.; Konan, S.; Das, R.; Volpin, A.; Haddad, F.S. The direct anterior approach in total hip arthroplasty: A systematic review of the literature. Bone Jt. J. 2017, 99-B, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gu, J.; Zhou, Y. Primary total hip arthroplasty failure: Aseptic loosening remains the most common cause of revision. Am. J. Transl. Res. 2022, 14, 7080–7089. [Google Scholar] [PubMed] [PubMed Central]
- Zhao, J.; Ma, X.; Feng, H. Innovation in proximal femoral replacement for oncology patients-A novel eggshell procedure. J. Bone Oncol. 2023, 39, 100473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pennekamp, P.H.; Wirtz, D.C.; Dürr, H.R. Proximal and total femur replacement. Oper. Orthop. Traumatol. 2012, 24, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Mahran, M.A.; Khalifa, A.A.; El-Sayed, A. Pelvis reconstruction by proximal femur upshifting and total hip arthroplasty after radical resection of an adolescent patient pelvic Ewing’s sarcoma, a case report, and literature review. Int. J. Surg. Case Rep. 2023, 106, 108146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandrasekar, C.R.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A.; Buckley, L. Modular endoprosthetic replacement for tumours of the proximal femur. J. Bone Jt. Surg. Br. 2009, 91, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Ahlmann, E.R.; Allison, D.C.; Wang, L.; Menendez, L.R. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin. Orthop. Relat. Res. 2012, 470, 684–691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henderson, E.R.; O’Connor, M.I.; Ruggieri, P.; Windhager, R.; Funovics, P.T.; Gibbons, C.L.; Guo, W.; Hornicek, F.J.; Temple, H.T.; Letson, G.D. Classification of failure of limb salvage after reconstructive surgery for bone tumours: A modified system Including biological and expandable reconstructions. Bone Jt. J. 2014, 96-B, 1436–1440. [Google Scholar] [CrossRef]
- Funovics, P.T.; Hipfl, C.; Hofstaetter, J.G.; Puchner, S.; Kotz, R.I.; Dominkus, M. Management of septic complications following modular endoprosthetic reconstruction of the proximal femur. Int. Orthop. 2011, 35, 1437–1444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Streitbuerger, A.; Henrichs, M.P.; Hauschild, G.; Nottrott, M.; Guder, W.; Hardes, J. Silver-coated megaprostheses in the proximal femur in patients with sarcoma. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Theil, C.; Schwarze, J.; Gosheger, G.; Moellenbeck, B.; Schneider, K.N.; Deventer, N.; Klingebiel, S.; Grammatopoulos, G.; Boettner, F.; Schmidt-Braekling, T. Implant Survival, Clinical Outcome and Complications of Megaprosthetic Reconstructions Following Sarcoma Resection. Cancers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simard, S.; Thewes, B.; Humphris, G.; Dixon, M.; Hayden, C.; Mireskandari, S.; Ozakinci, G. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J. Cancer Surviv. Res. Pract. 2013, 7, 300–322. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Wen, Y.; Wang, H.; Sun, H.; Liang, W.; Zhang, B.; Humphris, G. Fear of cancer recurrence in adolescent and young adult cancer survivors: A systematic review of the literature. Psychooncology 2019, 28, 675–686. [Google Scholar] [CrossRef]
| Patients (n) | 30 |
| Average age ± SD (range) | 60.34 ± 15.20 (20–83) |
| Years of treatment | 2010–2018 |
| Sex male: female (n) | 17:13 |
| Age adjusted CCI ± SD (range) | 6.4 ± 2.8 (2–10) |
| Follow-up (years) | 3.16 (±2.51) |
| 4.62 (±2.82) |
| 2.31 (±2.02) |
| Cancer-related death (n) | 19 |
| Number of patients with revision surgery (n) | 5 |
| 1 |
| 4 |
| 2 (2/2 loosening) |
| 2 (½: 1. Loosening 2. Infection, ½: 1. Loosening 2. Loosening) |
| 1 (1. Loosening 2. Infection 3. Infection) |
| Amputations (n) | 0 |
| Local cancer recurrence (n) | 1 |
| Systemic cancer recurrence (n) | 8 |
| Tumor Type (n) | |
|---|---|
| 1 |
| 9 |
| 1 |
| 7 |
| 1 |
| 1 |
| 1 |
| 1 |
| 2 |
| 1 |
| 3 |
| 2 |
| Pathological fracture (n) | 10 |
| Arthroplasty type (n): proximal femur | 30 |
| PROM | Mean ± SD (Range) |
|---|---|
| HHS | 75.3 ± 15.3 (49–96) |
| MSTS | 18 ± 7 (7–28) |
| KPS | 74.55 ± 16.95 (50–90) |
| SF-36 overall | 64.3 ± 14.9 (33.2–88.6) |
| PF | 50.45 ± 28.06 (15–90) |
| PR | 61.36 ± 49.20 (0–100) |
| BP | 61.64 ± 31.15 (10–100) |
| GH | 58.64 ± 19.38 (25–85) |
| VIT | 50.09 ± 16.4 (30–80) |
| SR | 92.18 ± 15.03 (50–100) |
| ER | 78.73 ± 30.95 (33–100) |
| MH | 75.27 ± 14.06 (48–92) |
| No Revision (A1) | Revision (A2) | p-Value | Age < 57 (B1) | Age > 57 (B2) | p-Value | |
|---|---|---|---|---|---|---|
| Number | 8 | 3 | - | 6 | 5 | - |
| HHS | 81.9 ± 11.0 | 60 ± 17.3 | 0.183 | 79.2 ± 20.2 | 71.4 ± 11.7 | 0.257 |
| MSTS | 20.0 ± 7.1 | 12.7 ± 3.2 | 0.133 | 20.5 ± 8.2 | 15.0 ± 4.3 | 0.030 |
| KPS | 75.0 ± 16.9 | 73.3 ± 20.8 | 0.921 | 80 ± 15.5 | 68 ± 17.9 | 0.052 |
| SF-36 overall | 0.68 ± 0.14 | 0.55 ± 0.19 | 0.279 | 0.69 ± 0.15 | 59 ± 0.16 | 0.247 |
| PF | 0.55 ± 0.29 | 0.38 ± 0.25 | 0.497 | 0.64 ± 0.26 | 0.34 ± 0.22 | 0.030 |
| PR | 0.72 ± 0.45 | 0.33 ± 0.58 | 0.376 | 0.79 ± 0.40 | 0.87 ± 0.30 | 0.126 |
| BP | 0.71 ± 0.26 | 0.37 ± 0.36 | 0.133 | 0.69 ± 0.32 | 0.53 ± 0.31 | 0.329 |
| GH | 0.61 ± 0.17 | 0.52 ± 0.28 | 0.630 | 0.63 ± 0.19 | 0.53 ± 0.21 | 0.537 |
| VIT | 0.52 ± 0.20 | 0.37 ± 0.16 | 0.133 | 0.56 ± 0.18 | 0.45 ± 0.17 | 0.662 |
| SR | 0.91 ± 0.17 | 0.96 ± 0.07 | 0.999 | 0.88 ± 0.19 | 0.98 ± 0.05 | 0.792 |
| ER | 0.79 ± 0.31 | 0.78 ± 0.39 | 0.999 | 0.72 ± 0.33 | 0.87 ± 0.30 | 0.792 |
| MH | 0.56 ± 0.16 | 0.71 ± 0.20 | 0.776 | 0.77 ± 0.11 | 0.74 ± 0.19 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khakzad, T.; Putzier, M.; Paksoy, A.; Rau, D.; Thielscher, L.; Taheri, N.; Wittenberg, S.; Märdian, S. Outcome of Endoprosthetic Hip Reconstruction Following Resection of Malignant Bone Tumors. Cancers 2024, 16, 2890. https://doi.org/10.3390/cancers16162890
Khakzad T, Putzier M, Paksoy A, Rau D, Thielscher L, Taheri N, Wittenberg S, Märdian S. Outcome of Endoprosthetic Hip Reconstruction Following Resection of Malignant Bone Tumors. Cancers. 2024; 16(16):2890. https://doi.org/10.3390/cancers16162890
Chicago/Turabian StyleKhakzad, Thilo, Michael Putzier, Alp Paksoy, Daniel Rau, Leonard Thielscher, Nima Taheri, Silvan Wittenberg, and Sven Märdian. 2024. "Outcome of Endoprosthetic Hip Reconstruction Following Resection of Malignant Bone Tumors" Cancers 16, no. 16: 2890. https://doi.org/10.3390/cancers16162890
APA StyleKhakzad, T., Putzier, M., Paksoy, A., Rau, D., Thielscher, L., Taheri, N., Wittenberg, S., & Märdian, S. (2024). Outcome of Endoprosthetic Hip Reconstruction Following Resection of Malignant Bone Tumors. Cancers, 16(16), 2890. https://doi.org/10.3390/cancers16162890






