Non-Small-Cell Lung Cancers (NSCLCs) Harboring RET Gene Fusion, from Their Discovery to the Advent of New Selective Potent RET Inhibitors: “Shadows and Fogs”
Abstract
Simple Summary
Abstract
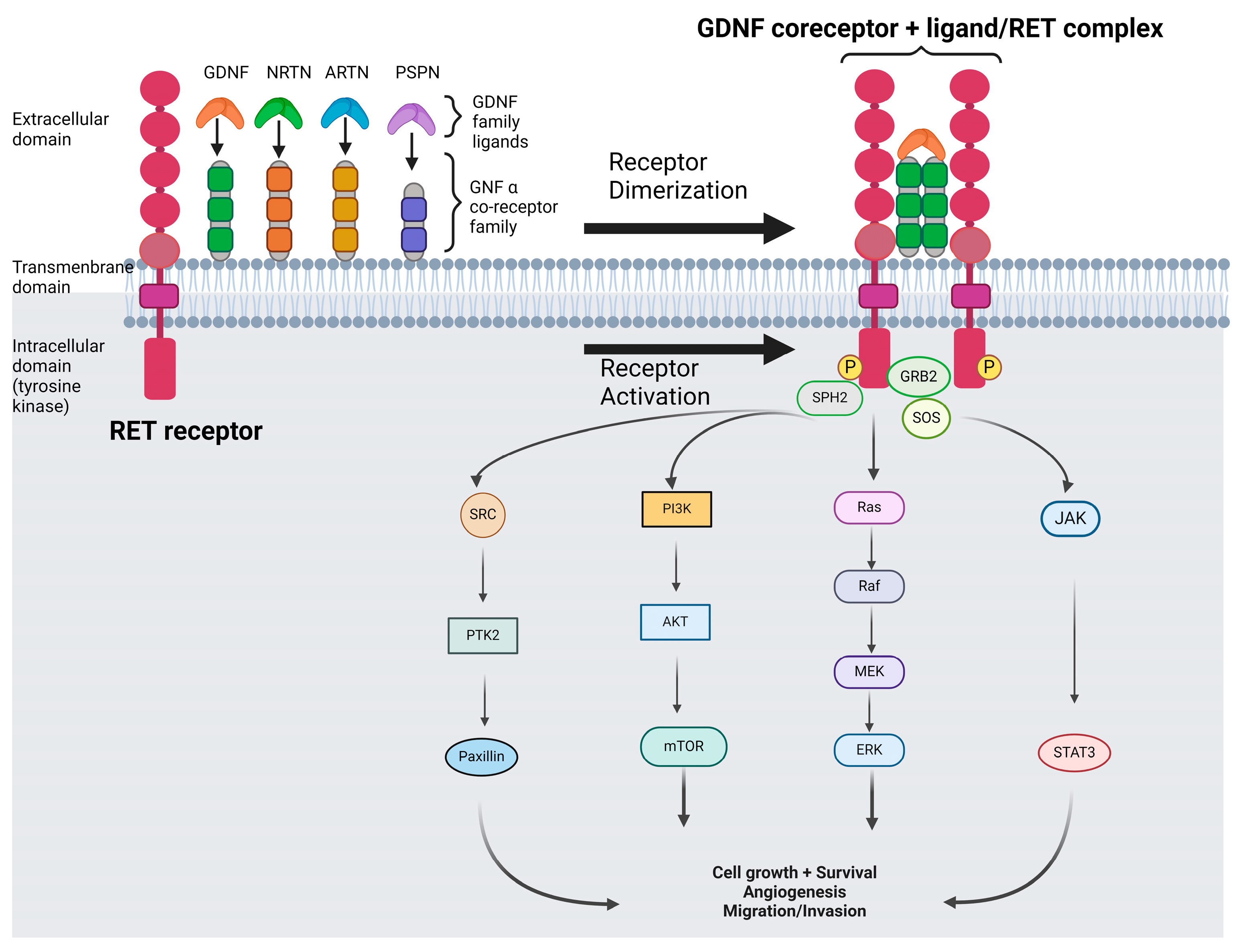
1. Introduction
2. Immunotherapy
3. Multitargeted Agents
3.1. Cabozantinib
3.2. Vandetanib
3.3. Lenvatinib
3.4. Ponatinib
| Reference | Drug | Targets | Pts | Line | ORR | mPFS (Months) | OS (Months) | AE G3/4 | Red. | Disc. |
|---|---|---|---|---|---|---|---|---|---|---|
| Drilon 2016 [45] | Cabozantinib | RET, MET, AXL, FLT3, c-KIT | 26 | ≥1 L% | 28% | 5.5 | 9.9 | 71% | 73% | 8% |
| Yoh 2017/2021 [47,48] | Vandetanib | RET, EGFR, VEGFR2/3 | 19 | ≥2 L | 53% | 4.7 | 13.5 | 84% | 58% | 21% |
| Lee 2017 [49] | Vandetanib | RET, EGFR, VEGFR2/3 | 18 | ≥2 L | 18% | 4.5 | 11.6 | 34% | 28% | NR |
| Hida 2019 [51] | Lenvatinib | RET, VEGFR1-3, FGFR1-4, PDGFRa, cKIT | 25 | ≥1 L * | 16% | 7.3 | NE | 92% | 64% | 24% |
| Gainor 2020 [53] | Ponatinib | BCR-ABL-RET | 9 | ≥2 L | 0 | 3.8 | 17.5 | 11% | 56% | NR |
3.5. Other Multi-TKIs
4. New and Selective RET Inhibitors (RET-Is)
4.1. Pralsetinib
4.2. Selpercatinib
5. Resistance Mechanisms to TKIs
5.1. On-Target Mechanisms
5.2. Off-Target Mechanisms
6. New Drugs
6.1. Next Generation RET TKIs
6.2. New Strategies
6.3. Early Stage
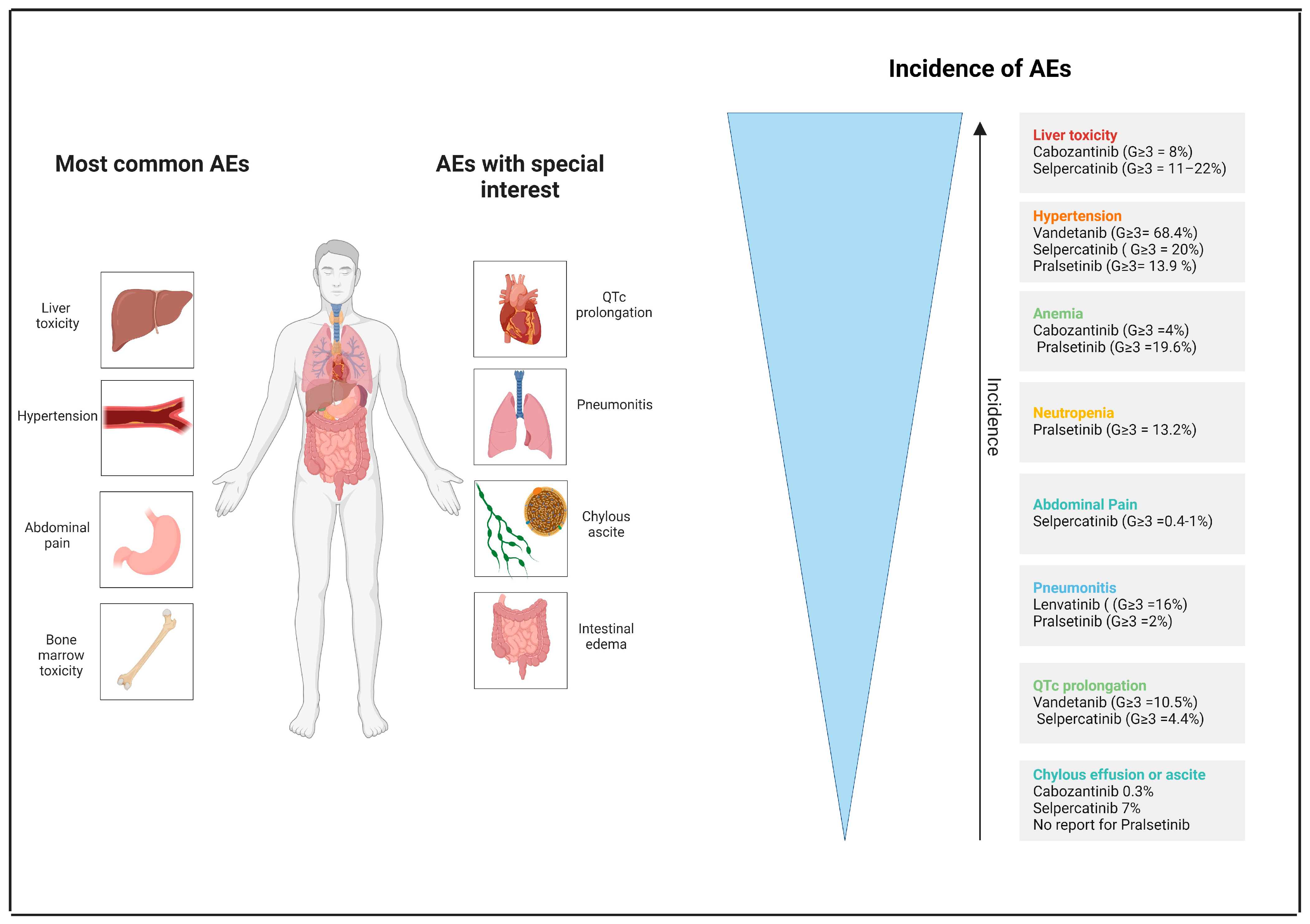
7. Toxicity
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takahashi, M.; Ritz, J.; Cooper, G.M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985, 42, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Buma, Y.; Iwamoto, T.; Inaguma, Y.; Ikeda, H.; Hiai, H. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene 1988, 3, 571–578. [Google Scholar] [PubMed]
- Rossel, M.; Pasini, A.; Chappuis, S.; Geneste, O.; Fournier, L.; Schuffenecker, I.; Takahashi, M.; van Grunsven, L.A.; Urdiales, J.L.; Rudkin, B.B.; et al. Distinct biological properties of two RET isoforms activated by MEN 2A and MEN 2B mutations. Oncogene 1997, 14, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lian, E.Y.; Maritan, S.M.; Cockburn, J.G.; Kasaian, K.; Crupi, M.J.; Hurlbut, D.; Jones, S.J.; Wiseman, S.M.; Mulligan, L.M. Differential roles of RET isoforms in medullary and papillary thyroid carcinomas. Endocr. Relat. Cancer 2017, 24, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.M.; Kjær, S.; Beuron, F.; Knowles, P.P.; Nawrotek, A.; Burns, E.M.; Purkiss, A.G.; George, R.; Santoro, M.; Morris, E.P.; et al. RET recognition of GDNF-GFRα1 ligand by a composite binding site promotes membrane-proximal self-association. Cell Rep. 2014, 8, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.A.; Vega, Q.C.; Chao, H.H.; Seasholtz, A.F.; Thompson, R.C.; Dixon, J.E. Identification and characterization of GFRalpha-3, a novel Co-receptor belonging to the glial cell line-derived neurotrophic receptor family. J. Biol. Chem. 1998, 273, 3502–3508. [Google Scholar] [CrossRef] [PubMed]
- Besset, V.; Scott, R.P.; Ibáñez, C.F. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J. Biol. Chem. 2000, 275, 39159–39166. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Ichihara, M.; Iwashita, T.; Murakami, H.; Shimono, Y.; Kawai, K.; Kurokawa, K.; Murakumo, Y.; Imai, T.; Funahashi, H.; et al. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neu-rotrophic factor. Oncogene 2000, 19, 4469–4475. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vega, Q.C.; Decker, R.A.; Pandey, A.; Worby, C.A.; Dixon, J.E. Oncogenic RET receptors display different autophosphorylation sites and substrate binding specificities. J. Biol. Chem. 1996, 271, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Borrello, M.G.; Alberti, L.; Arighi, E.; Bongarzone, I.; Battistini, C.; Bardelli, A.; Pasini, B.; Piutti, C.; Rizzetti, M.G.; Mondellini, P.; et al. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cgamma. Mol. Cell Biol. 1996, 16, 2151–2163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schuringa, J.J.; Wojtachnio, K.; Hagens, W.; Vellenga, E.; Buys, C.H.; Hofstra, R.; Kruijer, W. MEN2A-RET-induced cellular transfor-mation by activation of STAT3. Oncogene 2001, 20, 5350–5358. [Google Scholar] [CrossRef] [PubMed]
- Perrinjaquet, M.; Vilar, M.; Ibáñez, C.F. Protein-tyrosine phosphatase SHP2 contributes to GDNF neurotrophic activity through direct binding to phospho-Tyr687 in the RET receptor tyrosine kinase. J. Biol. Chem. 2010, 285, 31867–31875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Encinas, M.; Crowder, R.J.; Milbrandt, J.; Johnson, E.M., Jr. Tyrosine 981, a novel ret autophosphorylation site, binds c-Src to mediate neuronal survival. J. Biol. Chem. 2004, 279, 18262–18269. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, A.; D’Agati, V.; Larsson-Blomberg, L.; Costantini, F.; Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994, 367, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Shakya, R.; Watanabe, T.; Costantini, F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev. Cell. 2005, 8, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pachnis, V.; Mankoo, B.; Costantini, F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 1993, 119, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.W.; Yonemura, S.; Oppenheim, R.W.; Ohmori, S.; Enomoto, H. The neurotrophic effects of glial cell line-derived neu-rotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J. Neurosci. 2008, 28, 2131–2146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kramer, E.R.; Knott, L.; Su, F.; Dessaud, E.; Krull, C.E.; Helmbacher, F.; Klein, R. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb. Neuron 2006, 50, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Naughton, C.K.; Yang, M.; Strickland, A.; Vij, K.; Encinas, M.; Golden, J.; Gupta, A.; Heuckeroth, R.; Johnson, E.M., Jr.; et al. Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development 2004, 131, 5503–5513. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Pereira, D.; Arroz-Madeira, S.; Rodrigues-Campos, M.; Barbosa, I.A.; Domingues, R.G.; Bento, T.; Almeida, A.R.; Ribeiro, H.; Potocnik, A.J.; Enomoto, H.; et al. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature 2014, 514, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Yang, D.; Velcheti, V.; Drilon, A.; Meric-Bernstam, F. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. J. Clin. Oncol. 2020, 38, 1209–1221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipson, D.; Capelletti, M.; Yelensky, R.; Otto, G.; Parker, A.; Jarosz, M.; Curran, J.A.; Balasubramanian, S.; Bloom, T.; Brennan, K.W.; et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat. Med. 2012, 18, 382–384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ju, Y.S.; Lee, W.C.; Shin, J.Y.; Lee, S.; Bleazard, T.; Won, J.K.; Kim, Y.T.; Kim, J.I.; Kang, J.H.; Seo, J.S. A transforming KIF5B and RET gene fu-sion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012, 22, 436–445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeuchi, K.; Soda, M.; Togashi, Y.; Suzuki, R.; Sakata, S.; Hatano, S.; Asaka, R.; Hamanaka, W.; Ninomiya, H.; Uehara, H.; et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 2012, 18, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550, Erratum in Nature 2014, 514, 262; Erratum in Nature 2018, 559, E12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belli, C.; Penault-Llorca, F.; Ladanyi, M.; Normanno, N.; Scoazec, J.Y.; Lacroix, L.; Reis-Filho, J.S.; Subbiah, V.; Gainor, J.F.; Endris, V.; et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann. Oncol. 2021, 32, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, R.; Hu, H.; Pan, Y.; Li, Y.; Ye, T.; Li, C.; Luo, X.; Wang, L.; Li, H.; Zhang, Y.; et al. RET fusions define a unique molecular and clinico-pathologic subtype of non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 4352–4359. [Google Scholar] [CrossRef] [PubMed]
- Tsuta, K.; Kohno, T.; Yoshida, A.; Shimada, Y.; Asamura, H.; Furuta, K.; Kushima, R. RET-rearranged non-small-cell lung carcinoma: A clinicopathological and molecular analysis. Br. J. Cancer. 2014, 110, 1571–1578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drilon, A.; Lin, J.J.; Filleron, T.; Ni, A.; Milia, J.; Bergagnini, I.; Hatzoglou, V.; Velcheti, V.; Offin, M.; Li, B.; et al. Frequency of Brain Metas-tases and Multikinase Inhibitor Outcomes in Patients With RET-Rearranged Lung Cancers. J. Thorac. Oncol. 2018, 13, 1595–1601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sereno, M.; Hernandez de Córdoba, I.; Gutiérrez-Gutiérrez, G.; Casado, E. Brain metastases and lung cancer: Molecular biology, natural history, prediction of response and efficacy of immunotherapy. Front. Immunol. 2024, 14, 1297988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drilon, A.; Bergagnini, I.; Delasos, L.; Sabari, J.; Woo, K.M.; Plodkowski, A.; Wang, L.; Hellmann, M.D.; Joubert, P.; Sima, C.S.; et al. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann. Oncol. 2016, 27, 1286–1291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gautschi, O.; Milia, J.; Filleron, T.; Wolf, J.; Carbone, D.P.; Owen, D.; Camidge, R.; Narayanan, V.; Doebele, R.C.; Besse, B.; et al. Targeting RET in Patients with RET-Rearranged Lung Cancers: Results from the Global, Multicenter RET Registry. J. Clin. Oncol. 2017, 35, 1403–1410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, T.; Pu, X.; Wang, L.; Yu, Z.; Li, J.; Zhang, Y.; Liang, X.; Chen, H.; Xu, C.; Song, Z.; et al. Association Between RET Fusions and Efficacy of Pemetrexed-based Chemotherapy for Patients with Advanced NSCLC in China: A Multicenter Retrospective Study. Clin. Lung Cancer 2020, 21, e349–e354. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Sakai, K.; Nishio, K.; Nakagawa, K. Successful long-term treatment of non-small cell lung cancer positive for RET re-arrangement with pemetrexed. Onco Targets Ther. 2019, 12, 5355–5358. [Google Scholar] [CrossRef] [PubMed]
- Roque, K.; Ruiz, R.; Mas, L.; Pozza, D.H.; Vancini, M.; Silva Júnior, J.A.; de Mello, R.A. Update in Immunotherapy for Advanced Non-Small Cell Lung Cancer: Optimizing Treatment Sequencing and Identifying the Best Choices. Cancers 2023, 15, 4547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hegde, A.; Andreev-Drakhlin, A.Y.; Roszik, J.; Huang, L.; Liu, S.; Hess, K.; Cabanillas, M.; Hu, M.I.; Busaidy, N.L.; Sherman, S.I.; et al. Re-sponsiveness to immune checkpoint inhibitors versus other systemic therapies in RET-aberrant malignancies. ESMO Open 2020, 5, e000799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Offin, M.; Guo, R.; Wu, S.L.; Sabari, J.; Land, J.D.; Ni, A.; Montecalvo, J.; Halpenny, D.F.; Buie, L.W.; Pak, T.; et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, N.; Zhang, H.; Shen, S.; Guo, S.; Li, X. Response to immune checkpoint inhibitor combination therapy in metastatic RET-mutated lung cancer from real-world retrospective data. BMC Cancer 2024, 24, 178. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.J.; McLaughlin, R.; Naidoo, J. Is Immunotherapy Beneficial in Patients with Oncogene-Addicted Non-Small Cell Lung Cancers? A Narrative Review. Cancers 2024, 16, 527. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNO-TARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dudnik, E.; Bshara, E.; Grubstein, A.; Fridel, L.; Shochat, T.; Roisman, L.C.; Ilouze, M.; Rozenblum, A.B.; Geva, S.; Zer, A.; et al. Rare tar-getable drivers (RTDs) in non-small cell lung cancer (NSCLC): Outcomes with immune check-point inhibitors (ICPi). Lung Cancer 2018, 124, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Guisier, F.; Dubos-Arvis, C.; Viñas, F.; Doubre, H.; Ricordel, C.; Ropert, S.; Janicot, H.; Bernardi, M.; Fournel, P.; Lamy, R.; et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients with Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J. Thorac. Oncol. 2020, 15, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Maroto, P.; Porta, C.; Capdevila, J.; Apolo, A.B.; Viteri, S.; Rodriguez-Antona, C.; Martin, L.; Castellano, D. Cabozantinib for the treat-ment of solid tumors: A systematic review. Ther. Adv. Med. Oncol. 2022, 14, 17588359221107112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drilon, A.; Rekhtman, N.; Arcila, M.; Wang, L.; Ni, A.; Albano, M.; Van Voorthuysen, M.; Somwar, R.; Smith, R.S.; Montecalvo, J.; et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, sin-gle-arm trial. Lancet Oncol. 2016, 17, 1653–1660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fallahi, P.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Materazzi, G.; Antonelli, A. Evaluating vandetanib in the treatment of medullary thyroid cancer: Patient-reported outcomes. Cancer Manag. Res. 2019, 11, 7893–7907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoh, K.; Seto, T.; Satouchi, M.; Nishio, M.; Yamamoto, N.; Murakami, H.; Nogami, N.; Matsumoto, S.; Kohno, T.; Tsuta, K.; et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): An open-label, multicentre phase 2 trial. Lancet Respir. Med. 2017, 5, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Seto, T.; Satouchi, M.; Nishio, M.; Yamamoto, N.; Murakami, H.; Nogami, N.; Nosaki, K.; Kohno, T.; Tsuta, K.; et al. Final survival results for the LURET phase II study of vandetanib in previously treated patients with RET-rearranged advanced non-small cell lung cancer. Lung Cancer 2021, 155, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.K.; Ahn, M.J.; Kim, D.W.; Sun, J.M.; Keam, B.; Kim, T.M.; Heo, D.S.; Ahn, J.S.; Choi, Y.L.; et al. Vandetanib in pretreated pa-tients with advanced non-small cell lung cancer-harboring RET rearrangement: A phase II clinical trial. Ann. Oncol. 2017, 28, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Singla, A. Lenvatinib: A narrative drug review. Cancer Res. Stat. Treat. 2021, 4, 709–720. [Google Scholar] [CrossRef]
- Hida, T.; Velcheti, V.; Reckamp, K.L.; Nokihara, H.; Sachdev, P.; Kubota, T.; Nakada, T.; Dutcus, C.E.; Ren, M.; Tamura, T. A phase 2 study of lenvatinib in patients with RET fusion-positive lung adenocarcinoma. Lung Cancer. 2019, 138, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A novel multi-tyrosine kinase inhibitor against human malignancies. Onco Targets Ther. 2019, 12, 635–645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gainor, J.F.; Gadgeel, S.; Ou, S.I.; Yeap, B.; Otterson, G.A.; Shaw, A.T. A Phase II Study of the Multikinase Inhibitor Ponatinib in Patients with Advanced, RET-Rearranged NSCLC. JTO Clin. Res. Rep. 2020, 1, 100045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nokihara, H.; Nishio, M.; Yamamoto, N.; Fujiwara, Y.; Horinouchi, H.; Kanda, S.; Horiike, A.; Ohyanagi, F.; Yanagitani, N.; Nguyen, L.; et al. Phase 1 Study of Cabozantinib in Japanese Patients with Expansion Cohorts in Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, e317–e328. [Google Scholar] [CrossRef] [PubMed]
- Platt, A.; Morten, J.; Ji, Q.; Elvin, P.; Womack, C.; Su, X.; Donald, E.; Gray, N.; Read, J.; Bigley, G.; et al. A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized Phase III studies. BMC Cancer 2015, 15, 171. [Google Scholar] [CrossRef]
- Drilon, A.; Fu, S.; Patel, M.R.; Fakih, M.; Wang, D.; Olszanski, A.J.; Morgensztern, D.; Liu, S.V.; Cho, B.C.; Bazhenova, L.; et al. A Phase I/Ib Trial of the VEGFR-Sparing Multikinase RET Inhibitor RXDX-105. Cancer Discov. 2019, 9, 384–395. [Google Scholar] [CrossRef]
- Horiike, A.; Takeuchi, K.; Uenami, T.; Kawano, Y.; Tanimoto, A.; Kaburaki, K.; Tambo, Y.; Kudo, K.; Yanagitani, N.; Ohyanagi, F.; et al. So-rafenib treatment for patients with RET fusion-positive non-small cell lung cancer. Lung Cancer 2016, 93, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.F.S.A.; Alessi, J.V.M.; Oliveira, L.J.C.; Gongora, A.B.L.; Sacardo, K.P.; Zucchetti, B.M.; Shimada, A.K.; de Galiza Barbosa, F.; Feher, O.; Katz, A. Alectinib activity in chemotherapy-refractory metastatic RET-rearranged non-small cell lung carcinomas: A case series. Lung Cancer 2020, 139, 9–12. [Google Scholar] [CrossRef]
- Lin, J.J.; Kennedy, E.; Sequist, L.V.; Brastianos, P.K.; Goodwin, K.E.; Stevens, S.; Wanat, A.C.; Stober, L.L.; Digumarthy, S.R.; Engelman, J.A.; et al. Clinical Activity of Alectinib in Advanced RET-Rearranged Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 2027–2032. [Google Scholar] [CrossRef]
- Subbiah, V.; Gainor, J.F.; Rahal, R.; Brubaker, J.D.; Kim, J.L.; Maynard, M.; Hu, W.; Cao, Q.; Sheets, M.P.; Wilson, D.; et al. Precision Tar-geted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov. 2018, 8, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, F.; Guida, T.; Anaganti, S.; Vecchio, G.; Fusco, A.; Ryan, A.J.; Billaud, M.; Santoro, M. Disease associated mutations at va-line 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 2004, 23, 6056–6063. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Curigliano, G.; Kim, D.W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969, Erratum in Lancet Oncol. 2021, 22, e347. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.; Hu, W.; Cao, F.; Hoeflich, K.; Dorsch, M. BLU-667 demonstrates robust activity in RET fusion-driven intracranial tumor models. J. Thorac. Oncol. 2019, 14 (Suppl. S10), S701. [Google Scholar] [CrossRef]
- Griesinger, F.; Curigliano, G.; Thomas, M.; Subbiah, V.; Baik, C.S.; Tan, D.S.W.; Lee, D.H.; Misch, D.; Garralda, E.; Kim, D.W.; et al. Safety and efficacy of pralsetinib in RET fusion-positive non-small-cell lung cancer including as first-line therapy: Update from the AR-ROW trial. Ann. Oncol. 2022, 33, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Griesinger, F.; Curigliano, G.; Thomas, M.; Subbiah, V.; Baik, C.S.; Tan, D.S.W.; Lee, D.H.; Garralda, E.; Kim, D.-W.; et al. 1170P Updated efficacy and safety data from the phase I/II ARROW study of pralsetinib in patients (pts) with advanced RET fusion + non-small cell lung cancer (NSCLC). Ann. Oncol. 2022, 33 (Suppl. S7), S1083–S1084. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Gainor, J.; Cappuzzo, F.; Garralda, E.; Lee, D.-H.; Mazieres, J.; Kim, D.-W.; Zhu, V.; Lopes, G.; Miller, S.; et al. 984P Relation-ship between RET fusion partner and treatment outcomes in patients (pts) with non-small cell lung cancer (NSCLC) from the phase I/II ARROW study and real-world data (RWD). Ann. Oncol. 2022, 33 (Suppl. S7), S1001–S1002. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, Y.-L.; Zhao, J.; Chang, J.; Wang, H.; Fan, Y.; Wang, K.; Wu, G.; Nian, W.; Gong, Y.; et al. Updated efficacy and safety of pralsetinib in Chinese patients with advanced RET fusion + non-small cell lung cancer. Ann. Oncol. 2022, 33 (Suppl. S9), S1593. [Google Scholar] [CrossRef]
- FDA Approves Pralsetinib for Non-Small Cell Lung Cancer with RET Gene Fusions. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pralsetinib-non-small-cell-lung-cancer-ret-gene-fusions#:~:text=On%20August%209%2C%202023%2C%20the,by%20an%20FDA%2Dapproved%20test (accessed on 15 June 2024).
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/gavreto (accessed on 15 June 2024).
- Passaro, A.; Russo, G.L.; Passiglia, F.; D’Arcangelo, M.; Sbrana, A.; Russano, M.; Bonanno, L.; Giusti, R.; Metro, G.; Bertolini, F.; et al. Pralsetinib in RET fusion-positive non-small-cell lung cancer: A real-world data (RWD) analysis from the Italian expanded ac-cess program (EAP). Lung Cancer 2022, 174, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Felip, E.; Kim, E.S.; de Marinis, F.; Cho, B.C.; Wermke, M.; De Langen, A.; Ferrara, R.; Kanzler, S.; Cecere, F.L.; et al. AcceleRET Lung: A phase 3 study of first-line pralsetinib in patients with RET fusion–positive advanced/metastatic NSCLC. J. Clin. Oncol. 2022, 40, TPS9159. [Google Scholar] [CrossRef]
- Available online: https://ir.blueprintmedicines.com/news-releases/news-release-details/blueprint-medicines-highlights-2024-corporate-strategy-and (accessed on 15 June 2024).
- Subbiah, V.; Velcheti, V.; Tuch, B.B.; Ebata, K.; Busaidy, N.L.; Cabanillas, M.E.; Wirth, L.J.; Stock, S.; Smith, S.; Lauriault, V.; et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol. 2018, 29, 1869–1876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drilon, A.E.; Subbiah, V.; Oxnard, G.R.; Bauer, T.M.; Velcheti, V.; Lakhani, N.J.; Besse, B.; Park, K.; Patel, J.D.; Cabanillas, M.E.; et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J. Clin. Oncol. 2018, 36 (Suppl. S15), 102. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Subbiah, V.; Gainor, J.F.; Oxnard, G.R.; Tan, D.S.W.; Owen, D.H.; Cho, B.C.; Loong, H.H.; McCoach, C.E.; Weiss, J.; Kim, Y.J.; et al. Intracranial Efficacy of Selpercatinib in RET Fusion-Positive Non-Small Cell Lung Cancers on the LIBRETTO-001 Trial. Clin. Cancer Res. 2021, 27, 4160–4167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, R.; Schreyer, M.; Chang, J.C.; Rothenberg, S.M.; Henry, D.; Cotzia, P.; Kris, M.G.; Rekhtman, N.; Young, R.J.; Hyman, D.M.; et al. Response to Selective RET Inhibition With LOXO-292 in a Patient with RET Fusion-Positive Lung Cancer with Leptomeninge-al Metastases. JCO Precis. Oncol. 2019, 3, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gautschi, O.; Drilon, A.; Solomon, B.; Tomasini, P.; Loong, H.H.F.; De Braud, F.G.M.; Goto, K.; Peterson, P.; Barker, S.; Liming, K.; et al. 35P—Final data from phase I/II LIBRETTO-001 trial of selpercatinib in RET fusion-positive non-small cell lung cancer. Ann. Oncol. 2024, 9 (Suppl. S3), 1–53. [Google Scholar] [CrossRef]
- Drilon, A.; Subbiah, V.; Gautschi, O.; Tomasini, P.; de Braud, F.; Solomon, B.J.; Shao-Weng Tan, D.; Alonso, G.; Wolf, J.; Park, K.; et al. Selp-ercatinib in Patients with RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy from the Registra-tional LIBRETTO-001 Phase I/II Trial. J. Clin. Oncol. 2023, 41, 385–394, Erratum in J. Clin. Oncol. 2023, 41, 4941. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-locally-advanced-or-metastatic-ret-fusion-positive-non-small-cell-lung (accessed on 17 June 2024).
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/retsevmo (accessed on 17 June 2024).
- Zhou, C.; Solomon, B.; Loong, H.H.; Park, K.; Pérol, M.; Arriola, E.; Novello, S.; Han, B.; Zhou, J.; Ardizzoni, A.; et al. LIBRETTO-431 Trial Investigators. First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RETFusion—Positive NSCLC. N. Engl. J. Med. 2023, 389, 1839–1850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérol, M.; Solomon, B.J.; Goto, K.; Park, K.; Nadal, E.; Bria, E.; Martin, C.; Bar, J.; Williams, J.N.; Puri, T.; et al. CNS Protective Effect of Selpercatinib in First-Line RET Fusion-Positive Advanced Non-Small Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 2500. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Goroff, Y.R.; Falcon, C.J.; Lin, S.T.; Chacko, C.; Grimaldi, G.; Liu, D.; Wilhelm, C.; Iasonos, A.; Drilon, A. Central Nervous Sys-tem Disease in Patients with RET Fusion-Positive NSCLC Treated with Selpercatinib. J. Thorac. Oncol. 2023, 18, 620–627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Shen, T.; Mooers, B.H.M.; Hilberg, F.; Wu, J. Drug resistance profiles of mutations in the RET kinase domain. Br. J. Pharmacol. 2018, 175, 3504–3515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dagogo-Jack, I.; Stevens, S.E.; Lin, J.J.; Nagy, R.; Ferris, L.; Shaw, A.T.; Gainor, J.F. Emergence of a RET V804M Gatekeeper Mutation During Treatment with Vandetanib in RET-Rearranged NSCLC. J. Thorac. Oncol. 2018, 13, e226–e227. [Google Scholar] [CrossRef] [PubMed]
- Nakaoku, T.; Kohno, T.; Araki, M.; Niho, S.; Chauhan, R.; Knowles, P.P.; Tsuchihara, K.; Matsumoto, S.; Shimada, Y.; Mimaki, S.; et al. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat. Commun. 2018, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.Y.; Won, H.H.; Zheng, Y.; Cocco, E.; Selcuklu, D.; Gong, Y.; Friedman, N.D.; de Bruijn, I.; Sumer, O.; Bielski, C.M.; et al. The evolu-tion of RET inhibitor resistance in RET-driven lung and thyroid cancers. Nat. Commun. 2022, 13, 1450, Erratum in Nat. Commun. 2022, 13, 1936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solomon, B.J.; Tan, L.; Lin, J.J.; Wong, S.Q.; Hollizeck, S.; Ebata, K.; Tuch, B.B.; Yoda, S.; Gainor, J.F.; Sequist, L.V.; et al. Ret solvent front muta-tions mediate acquired resistance to selective ret inhibition in ret-driven malignancies. J. Thorac. Oncol. 2020, 15, 541–549. [Google Scholar] [CrossRef]
- Lin, J.J.; Liu, S.V.; McCoach, C.E.; Zhu, V.W.; Tan, A.C.; Yoda, S.; Peterson, J.; Do, A.; Prutisto-Chang, K.; Dagogo-Jack, I.; et al. Mechanisms of resistance to selective ret tyrosine kinase inhibitors in ret fusion-positive non-Small-Cell lung cancer. Ann. Oncol. 2020, 31, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Shen, T.; Terzyan, S.S.; Liu, X.; Hu, X.; Patel, K.P.; Hu, M.; Cabanillas, M.; Behrang, A.; Meric-Bernstam, F.; et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET muta-tions. Ann. Oncol. 2021, 32, 261–268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, T.; Hu, X.; Liu, X.; Subbiah, V.; Mooers, B.H.M.; Wu, J. The L730v/I ret roof mutations display different activities toward pralsetinib and selpercatinib. NPJ Precis. Oncol. 2021, 5, 48. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Cheng, Y.; Zhou, C.; Ohe, Y.; Imamura, F.; Cho, B.C.; Lin, M.-C.; Majem, M.; Shah, R.; Rukazenkov, Y.; et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann. Oncol. 2018, 29 (Suppl. S8), viii740. [Google Scholar] [CrossRef]
- Vaishnavi, A.; Schubert, L.; Rix, U.; Marek, L.A.; Le, A.T.; Keysar, S.B.; Glogowska, M.J.; Smith, M.A.; Kako, S.; Sumi, N.J.; et al. EGFR Medi-ates Responses to Small-Molecule Drugs Targeting Oncogenic Fusion Kinases. Cancer Res. 2017, 77, 3551–3563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, H.; Sung, J.H.; Moon, S.U.; Kim, H.S.; Kim, J.W.; Lee, J.S. EGF Induced RET Inhibitor Resistance in CCDC6-RET Lung Cancer Cells. Yonsei Med. J. 2017, 58, 9–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Somwar, R.; Smith, R.; Hayashi, T.; Ishizawa, K.; Charen, A.S.; Khodos, I.; Mattar, M.; He, J.; Balasubramanian, S.; Stephens, P.; et al. MDM2 amplification (Amp) to mediate cabozantinib resistance in patients (Pts) with advanced RET-rearranged lung cancers. J. Clin. Oncol. 2016, 34 (Suppl. S15), 9068. [Google Scholar] [CrossRef]
- Zhu, V.W.; Madison, R.; Schrock, A.B.; Ou, S.-H.I. Emergence of High Level of MET Amplification as Off-Target Re-sistance to Selpercatinib Treatment in KIF5B-RET NSCLC. J. Thorac. Oncol. 2020, 15, e124–e127. [Google Scholar] [CrossRef]
- Subbiah, V.; Shen, T.; Tetzlaff, M.; Weissferdt, A.; Byers, L.A.; Cascone, T.; Behrang, A.; Meric-Bernstam, F.; Mooers, B.H.M.; Rothenberg, S.M.; et al. Patient-driven discovery and post-clinical validation of NTRK3 fusion as an acquired resistance mechanism to selp-ercatinib in RET fusion-positive lung cancer. Ann. Oncol. 2021, 32, 817–819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dimou, A.; Lo, Y.C.; Merrell, K.W.; Halling, K.C.; Mansfield, A.S. Small Cell Transformation in a Patient with RET Fusion-Positive Lung Adenocarcinoma on Pralsetinib. JCO Precis. Oncol. 2022, 6, e2200478. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Drilon, A.E.; Rotow, J.K.; Liu, S.V.; Gautschi, O.; Rhoades Smith, K.E.; Lee, D.H.; Nagasaka, M.; Loong, H.H.F.; Pennell, N.A.; et al. First results from the RETgistry: A global consortium for the study of resistance to RET inhibition in RET-altered solid tu-mors. J. Clin. Oncol. 2023, 41, 9065. [Google Scholar] [CrossRef]
- Drilon, A.E.; Zhai, D.; Rogers, E.; Deng, W.; Zhang, X.; Ung, J.; Lee, D.; Rodon, L.; Graber, A.; Zimmerman, Z.F.; et al. The next-generation RET inhibitor TPX-0046 is active in drug-resistant and naïve RET-driven cancer models. J. Clin. Oncol. 2020, 38 (Suppl. S15), 3616. [Google Scholar] [CrossRef]
- Kolakowski, G.R.; Anderson, E.D.; Ballard, J.A.; Brandhuber, B.J.; Condroski, K.R.; Gomez, E.B.; Irvin, T.C.; Kumar, M.; Patel, N.A.; Watson, F.D.; et al. Abstract 1464: Pre-clinical characterization of potent and selective next-generation RET inhibitors. Cancer Res. 2021, 81 (Suppl. S13), 1464. [Google Scholar] [CrossRef]
- Pennell, N.A.; Wirth, L.J.; Gainor, J.F.; Rotow, J.K.; Johnson, M.L.; Bauer, T.M.; Kroiss, M.; Sukrithan, V.; Kang, H.; Worden, F.P.; et al. A first-in-human phase 1 study of the next-generation RET inhibitor, LOXO-260, in RET inhibitor refractory patients with RET-altered cancers (trial in progress). J. Clin. Oncol. 2022, 40, TPS8595. [Google Scholar] [CrossRef]
- Schöffski, P.; Cho, B.C.; Italiano, A.; Loong, H.H.; Massard, C.; Rodriguez, L.M.; Shih, J.-H.; Subbiah, V.; Verlingue, L.; Andreas, K.; et al. BOS172738, a highly potent and selective RET inhibitor, for the treatment of RET-altered tumors including RET-fusion + NSCLC and RET-mutant MTC: Phase 1 study results. J. Clin. Oncol. 2021, 39 (Suppl. S15), 3008. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, X.; Huang, Y.; Yang, Y.; Fang, W.; Ma, Y.; Chen, L.; Chen, D.; Wang, F.; Peng, R.; et al. 1329P A single-arm, open-label, mul-ti-center, phase I study of HA121-28 in patients with advanced solid tumors. Ann. Oncol. 2021, 32 (Suppl. S5), S1018. [Google Scholar] [CrossRef]
- Xiong, A.; Li, X.Y.; Yang, N.; Meng, X.; Yu, Q.; Tan, L.; Wang, Q.; Wang, Y.; Liu, B.; Luo, H.; et al. Efficacy and safety of SY-5007, a highly potent and selective RET inhibitor, in Chinese patients with advanced RET-fusion positive non-small cell lung cancer (NSCLC): Results from a multicenter, single-arm, phase II study. J. Clin. Oncol. 2024, 42 (Suppl. S16), 3106. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Q.; Wu, L.; Xing, L.; Li, Y.; Han, L.; Dong, X.; Wei, H.; Xu, W.; Li, C.; et al. HS-10365, a highly potent and selective RET tyrosine kinase inhibitor, demonstrates robust activity in RET fusion positive NSCLC patients. Cancer Res. 2023, 83 (Suppl. S8), CT201. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, Y.-L.; Zheng, X.; Li, D.; Huang, D.; Li, X.; Liu, A.; Song, X.; Jing, S.; Wang, M.; et al. A phase I study of KL590586, a next-generation selective RET inhibitor, in patients with RET-altered solid tumors. J. Clin. Oncol. 2023, 41, 3007. [Google Scholar] [CrossRef]
- Garralda, E.; Guzman, A.; Garrido, P.; Gianoukakis, A.G.; Taylor, M.; Piha-Paul, S.; Krebs, M.G.; Italiano, A.; Clark, L.; Fisher, G.; et al. Preliminary results from a phase I/II study evaluating the safety, tolerability, and efficacy of EP0031, a next generation selective RET inhibitor, in patients with advanced RET-altered malignancies. Mol. Cancer Ther. 2023, 22 (Suppl. S12), B043. [Google Scholar] [CrossRef]
- Garralda, E.; Wang, J.S.; Gianoukakis, A.G.; Taylor, M.H.; Spigel, D.R.; Piha-Paul, S.A.; Morgensztern, D.; Arnold, S.M.; Alonso, G.; Gar-cía-Pardo, M.; et al. A phase-1 dose escalation and expansion study of EP0031, a next-generation selective RET inhibitor (SRI), in patients with SRI-naïve or pretreated advanced RET-altered NSCLC and other tumors. J. Clin. Oncol. 2024, 42 (Suppl. S16), 8556. [Google Scholar] [CrossRef]
- Niu, C.; Zheng, M.; Wang, H.; Ji, K.; Li, M.; Wang, G.; Ni, R.; Liang, A.; Gong, A.; Zhang, Y.; et al. TY-1091, a highly selective and potent second-generation RET inhibitor, demonstrates superior antitumor activity in multiple RET-mutant models. Cancer Res. 2023, 83 (Suppl. S7), 3419. [Google Scholar] [CrossRef]
- Odintsov, I.; Lui, A.J.W.; Ishizawa, K.; Miyazaki, I.; Khodos, I.; Wakayama, K.; Vojnic, M.; Hagen, C.J.; Chang, Q.; Bonifacio, A.; et al. Comparison of TAS0953/HM06 and selpercatinib in RET fusion-driven preclinical disease models of intracranial metastases. J. Clin. Oncol. 2022, 40 (Suppl. S16), 2024. [Google Scholar] [CrossRef]
- Alqahtani, T.; Kumarasamy, V.; Alghamdi, S.S.; Suliman, R.S.; Bin Saleh, K.; Alrashed, M.A.; Aldhaeefi, M.; Sun, D. Adefovir Dipivoxil as a Therapeutic Candidate for Medullary Thyroid Carcinoma: Targeting RET and STAT3 Proto-Oncogenes. Cancers 2023, 15, 2163. [Google Scholar] [CrossRef]
- Aldea, M.; Marinello, A.; Monnet, I.; de Saint Basile, H.; Cousin, S.; Duruisseaux, M.; Calles, A.; Metro, G.; Zugazagoitia, J.; Massa, G.; et al. Bevacizumab in combination with chemotherapy for treating patients with advanced RET + non-small cell lung cancer. J. Clin. Oncol. 2024, 42 (Suppl. S16), 8647. [Google Scholar] [CrossRef]
- Patil, T.; Martin, A.C.; Wise, H.K.; Schmitt, E.; Kuykendall, H.; Reventaite, E.; Filar, E.; Miller, E.; Yoder, B.A.; Qin, A.; et al. A phase 1/2, open label study of amivantamab in combination with tyrosine kinase inhibitors among participants with advanced NSCLC harboring ALK, ROS1, and RET gene fusions. J. Clin. Oncol. 2024, 42 (Suppl. S16), TPS8661J. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/search?cond=NSCLC&term=RET&aggFilters=status:not%20rec&viewType=Table (accessed on 27 June 2024).
- Goldman, J.W.; Sands, J.; Hallqvist, A.; Kim, H.R.; Li, G.; Wu, L.; Su, W.; Bayt, T.; Yang, X.-N.; Hochmair, M. LIBRETTO-432: A phase 3 study of adjuvant selpercatinib or placebo in stage IB-IIIARET fusion-positive (RET +) NSCLC. J. Clin. Oncol. 2024, 42 (Suppl. S16), TPS8115. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Gay, C.M.; Zhou, C.; Kato, T.; Corrales, L.; Redhead, K.; Rahman, A.; Bradley, D.; Theogaraj, E.; Hutchinson, K.E.; et al. A phase I-III platform study evaluating the safety and efficacy of multiple therapies in patients with biomarker-defined locally advanced, unresectable stage III non–small-cell lung cancer (NSCLC). J. Clin. Oncol. 2023, 41, TPS8605. [Google Scholar] [CrossRef]
- Mingard, C.; Paech, F.; Bouitbir, J.; Krähenbühl, S. Mechanisms of toxicity associated with six tyrosine kinase inhibitors in human hepatocyte cell lines. J. Appl. Toxicol. 2018, 38, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.L.; Ho, H.K.; Chan, A. Formation of reactive metabolites and management of tyrosine kinase inhibitor-induced hepato-toxicity: A literature review. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Studentova, H.; Volakova, J.; Spisarova, M.; Zemankova, A.; Aiglova, K.; Szotkowski, T.; Melichar, B. Severe tyrosine-kinase inhibitor induced liver injury in metastatic renal cell carcinoma patients: Two case reports assessed for causality using the updated RUCAM and review of the literature. BMC Gastroenterol. 2022, 22, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horowitz, J.R.; Rivard, A.; van der Zee, R.; Hariawala, M.; Sheriff, D.D.; Esakof, D.D.; Chaudhry, G.M.; Symes, J.F.; Isner, J.M. Vascular en-dothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a mainte-nance role in quiescent adult endothelium. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Rini, B.I.; Bukowski, R.M.; Curti, B.D.; George, D.J.; Hudes, G.R.; Redman, B.G.; Margolin, K.A.; Merchan, J.R.; Wilding, G.; et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006, 295, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.C.; Farrell, A.T.; Saber, H.; Tang, S.; Williams, G.; Jee, J.M.; Liang, C.; Booth, B.; Chidambaram, N.; Morse, D.; et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. 2006, 12, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Eremina, V.; Sood, M.; Haigh, J.; Nagy, A.; Lajoie, G.; Ferrara, N.; Gerber, H.P.; Kikkawa, Y.; Miner, J.H.; Quaggin, S.E. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Investig. 2003, 111, 707–716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scappaticci, F.A.; Fehrenbacher, L.; Cartwright, T.; Hainsworth, J.D.; Heim, W.; Berlin, J.; Kabbinavar, F.; Novotny, W.; Sarkar, S.; Hurwitz, H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J. Surg. Oncol. 2005, 91, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kamba, T.; Tam, B.Y.; Hashizume, H.; Haskell, A.; Sennino, B.; Mancuso, M.R.; Norberg, S.M.; O’Brien, S.M.; Davis, R.B.; Gowen, L.C.; et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H560–H576. [Google Scholar] [CrossRef] [PubMed]
- Scattolin, D.; Scagliori, E.; Scapinello, A.; Fantin, A.; Guarneri, V.; Pasello, G. Small bowel edema and lymphocytic duodenitis as se-vere reversible gastrointestinal toxicity of selpercatinib in RET fusion-positive non-small cell lung cancer: A case report. Front. Oncol. 2023, 13, 1201599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsang, V.; Gill, A.; Gild, M.; Lurie, B.; Blumer, L.; Siddall, R.; Clifton-Bligh, R.; Robinson, B. Selpercatinib Treatment of RET-Mutated Thyroid Cancers Is Associated with Gastrointestinal Adverse Effects. J. Clin. Endocrinol. Metab. 2022, 107, e3824–e3829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prete, A.; Gambale, C.; Cappagli, V.; Bottici, V.; Rossi, P.; Caciagli, M.; Papini, P.; Taddei, D.; Ortori, S.; Gabbrielli, L.; et al. Chylous effu-sions in advanced medullary thyroid cancer patients treated with selpercatinib. Eur. J. Endocrinol. 2022, 187, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Kalchiem-Dekel, O.; Falcon, C.J.; Bestvina, C.M.; Liu, D.; Kaplanis, L.A.; Wilhelm, C.; Eichholz, J.; Harada, G.; Wirth, L.J.; Digumarthy, S.R.; et al. Brief Report: Chylothorax and Chylous Ascites During RET Tyrosine Kinase Inhibitor Therapy. J. Thorac. Oncol. 2022, 17, 1130–1136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fricke, J.; Wang, J.; Gallego, N.; Mambetsariev, I.; Kim, P.; Babikian, R.; Chen, B.T.; Afkhami, M.; Subbiah, V.; Salgia, R. Selpercatinib and Pralsetinib Induced Chylous Ascites in RET-Rearranged Lung Adenocarcinoma: A Case Series. Clin. Lung Cancer 2023, 24, 666–671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Busafi, S.A.; Ghali, P.; Deschênes, M.; Wong, P. Chylous Ascites: Evaluation and Management. ISRN Hepatol. 2014, 2014, 240473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeetu, G.; Anusha, G. Pharmacovigilance: A worldwide master key for drug safety monitoring. J. Young Pharm. 2010, 2, 315–320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desilets, A.; Repetto, M.; Yang, S.-R.; Sherman, E.J.; Drilon, A. RET-Altered Cancers—A Tumor-Agnostic Review of Biology, Diagnosis and Targeted Therapy Activity. Cancers 2023, 15, 4146. [Google Scholar] [CrossRef]
- Hamidi, S.; Hofmann, M.C.; Iyer, P.C.; Cabanillas, M.E.; Hu, M.I.; Busaidy, N.L.; Dadu, R. Review article: New treatments for advanced differentiated thyroid cancers and potential mechanisms of drug resistance. Front. Endocrinol. 2023, 14, 1176731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Study (Reference) | Patient Number | Line | ORR (%) | mPFS (Months) | OS (Months) |
|---|---|---|---|---|---|
| Offin 2019 [38] | 16 | ≥1 L | 0 | 3.4 | NR |
| Yan 2024 [39] | 38 | 1 L + 2 L * | 26.3 | 5.0 | 19.0 |
| Immunotarget (Mazieres 2019) [41] | 16 | ≥2 L | 6 | 2.1 | 21.3 |
| Dudnik 2018 [42] | 13 | ≥1 L | 0 | 3.0 | 14.9 |
| GFPC 01-2018 (Guisier 2020) [43] | 9 | ≥2 L | 37.5 | 7.6 | NE |
| Drug | Trial | Setting | Pt N | ORR | mPFS (Months) | OS (Months) | IC ORR * | G ≥ 3 | Reduction Rate | Discontinuation Rate | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pralsetinib | Ph I/II ARROW (281 pts) | ≥2 L | 141 | 59.6% | 16.4 | 44.3 | 53.3% | 62.6% | 38% | 10% | ||
| 1 L | 116 | 72.4% | 12.6 | NE | - | |||||||
| B 47 | A 69 | 68% | 75% | 10.9 | 13.2 | NE I NE | - | |||||
| Selpercatinib | Ph I/II Libretto001 (316 pts) | ≥2 L | 247 | 61.5% | 26.2 | 47.6 | 84.6% | 42% | 48.9% | 11% | ||
| Trial | Arm | Pt N | ORR | mDoR (Months) | mPFS (Months) | IC-ORR | 12-mo CNS inc * | G ≥ 3 | Reduction Rate | Discontinuation Rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Libretto-431 Zhou 2023 [82] | Selpercatinib | 129 | 84% | 24.2 | 24.8 | 82% | 6% | 70% | 51% | 10% |
| HR 0.46 | ||||||||||
| CT + pembro | 83 | 65% | 11.5 | 11.2 | 58% | 20% | 57% | 29% | 2% |
| Drug | Target(s) | Phase | Tumor | N. Patient | ORR | Trial Identifier | Comment |
|---|---|---|---|---|---|---|---|
| TPX-0046 | RET (G810R+) SRC | I/II | RET + advanced solid tumors | 41 | - | NCT04161391 | Drug withdrawal for toxicity. |
| Loxo-260 | RET (G810X+) | I | Unresectable locally advanced or metastatic cancers RET + | 110 | - | NCT05241834 | Active, not recruiting. |
| RET (G810X+) | EAP | RET + advanced solid tumors | NA | - | NCT05225259 | Expanded access program no longer available. | |
| BOS172738 Zeteletinib Schöffski 2021 [104] | RET | I | RET + advanced solid tumors | 67 | 33% | NCT03780517 | Dose: 150 mg QD. Trial completed. Last update 30 October 2023. No further trials planned. |
| HA121-28 Zhao 2021 [105] | RET/EGFR/VEGFR | I/II | RET + advanced solid tumors | 41 | 41% | NCT03994484 | Chinese study. Included 11 patients with NSCLC in phase II. |
| HA121-28 | RET/EGFR/VEGFR | II | RET + aNSCLC (No prior RET-Is) | 83 * | - | NCT05117658 | Primary endpoint: ORR. Location: China. Unknown status enrollment. |
| SY-5007 Xiong 2024 [106] | RET | II | RET + aNSCLC | All pts (105 pts) Treatment naïve (56 pts) Pretreated pts (49) | 77.1% 83.9% 69.4% | NCT05278364 | In phase I, included all metastatic solid tumors. |
| SY-5007 | RET | III | RET + aNSCLC (Treatment naïve, RET fusion on tissue or lx) | 120 * | - | NCT06031558 | Chinese non-randomized trial in first-line setting. Active, recruiting. |
| HS-10365 Lu 2023 [107] | RET | I/II | RET + advanced solid tumors | All pts (30) # Pretreated pts (24) Treatment naïve pts (6) | 70% 66.7% 83.3% | NCT05207787 | An amount of 160 mg BID was the RP2D. Active, recruiting. |
| EP0031 (A400/KL590586) Zhou 2023 [108] | RET | I | RET + advanced solid tumors | All pts (57) # Treatment naïve (25 pts) Pretreated (32pts) | 80.8% 69.7% 50% | NCT05265091 | Chinese population. Active, not recruiting. |
| EP0031 Garralda 2024 [110] | RET | I/II | RET + advanced solid tumors | 12 pts with NSCL (10 pts preteated) | 50% | NCT05443126 | Western population. Active and recruiting. |
| TY-1091 | RET | I/I | RET + advanced solid tumors | 248 * | - | NCT05675605 | Primary endpoint: DLT/ORR. Location: China. Active and recruiting. |
| TAS0953/HM06 (Vepafestinib) | RET | I/II | RET + advanced solid tumors (prior RET-Is allowed) | 202 * | - | NCT04683250 (MARGARET) | Primary endpoint: RP2D/ORR. Location: Japan–USA. Active and recruiting. |
| HEC169096 | RET | I/II | RET + advanced solid tumors (no restriction on prior treatment lines) | 456 * | - | NCT05451602 | Primary endpoint: RP2D/ORR. Location: China. Active and recruiting. |
| HS-10365 | RET | I/II | RET + aNSCLC (no prior RETIs) | 62 * | - | NCT06147570 | Primary endpoint: RP2D/ORR. Location: China. Active and recruiting. |
| APS03118 | RET | I | RET + advanced solid tumors | 35 * | - | NCT05653869 | Location: China. Active and recruiting. |
| Parameter | Multi-TKIs | RET-Is |
|---|---|---|
| G ≥ 3 AE rate (mean) | 82% | 66.3% |
| Gain | −15.7% | |
| Dose reduction (mean) | 55.8% | 44.5% |
| Gain | −11.3% | |
| Drug discontinuation (mean) | 17.6% | 10.5% |
| Gain | −7.1% | |
| Specific AEs | ||
| G3 ≥ liver toxicity | 8% (cabozantinib) | 13–22% (selpercatinib) |
| G ≥ 3 hypertension | 68.4% (vandetanib) | 13.9–20% (pralsetinib/selpercatinib) |
| G ≥ 3 Anemia | 4% (cabozantinib) | 19.6% (pralsetinib) |
| G ≥ 3 Neutropenia | - | 13.2% (pralsetinib) |
| G ≥ 3 Thrombocytopenia | 4%/8% (lenvatinib/cabozantinib) | 3%/4% (selpercatinib/pralsetinib) |
| Pneumonitis (G ≥ 3) | 16% (16%) (lenvatinib) | 12% (2%) (pralsetinib) |
| QTc prolongation (G ≥ 3) | 47.4% (10.5%) (vandetanib) | 16.3% (4.4%) (selpercatinib) |
| Chylous ascites (G: NA) | - | 7% (selpercatinib) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spitaleri, G.; Trillo Aliaga, P.; Attili, I.; Del Signore, E.; Corvaja, C.; Pellizzari, G.; Katrini, J.; Passaro, A.; de Marinis, F. Non-Small-Cell Lung Cancers (NSCLCs) Harboring RET Gene Fusion, from Their Discovery to the Advent of New Selective Potent RET Inhibitors: “Shadows and Fogs”. Cancers 2024, 16, 2877. https://doi.org/10.3390/cancers16162877
Spitaleri G, Trillo Aliaga P, Attili I, Del Signore E, Corvaja C, Pellizzari G, Katrini J, Passaro A, de Marinis F. Non-Small-Cell Lung Cancers (NSCLCs) Harboring RET Gene Fusion, from Their Discovery to the Advent of New Selective Potent RET Inhibitors: “Shadows and Fogs”. Cancers. 2024; 16(16):2877. https://doi.org/10.3390/cancers16162877
Chicago/Turabian StyleSpitaleri, Gianluca, Pamela Trillo Aliaga, Ilaria Attili, Ester Del Signore, Carla Corvaja, Gloria Pellizzari, Jalissa Katrini, Antonio Passaro, and Filippo de Marinis. 2024. "Non-Small-Cell Lung Cancers (NSCLCs) Harboring RET Gene Fusion, from Their Discovery to the Advent of New Selective Potent RET Inhibitors: “Shadows and Fogs”" Cancers 16, no. 16: 2877. https://doi.org/10.3390/cancers16162877
APA StyleSpitaleri, G., Trillo Aliaga, P., Attili, I., Del Signore, E., Corvaja, C., Pellizzari, G., Katrini, J., Passaro, A., & de Marinis, F. (2024). Non-Small-Cell Lung Cancers (NSCLCs) Harboring RET Gene Fusion, from Their Discovery to the Advent of New Selective Potent RET Inhibitors: “Shadows and Fogs”. Cancers, 16(16), 2877. https://doi.org/10.3390/cancers16162877






