Advances in Personalized Oncology
Abstract
Simple Summary
Abstract
1. Introduction
2. Genomics, Proteomics, and the Use of Next Generation Sequencing
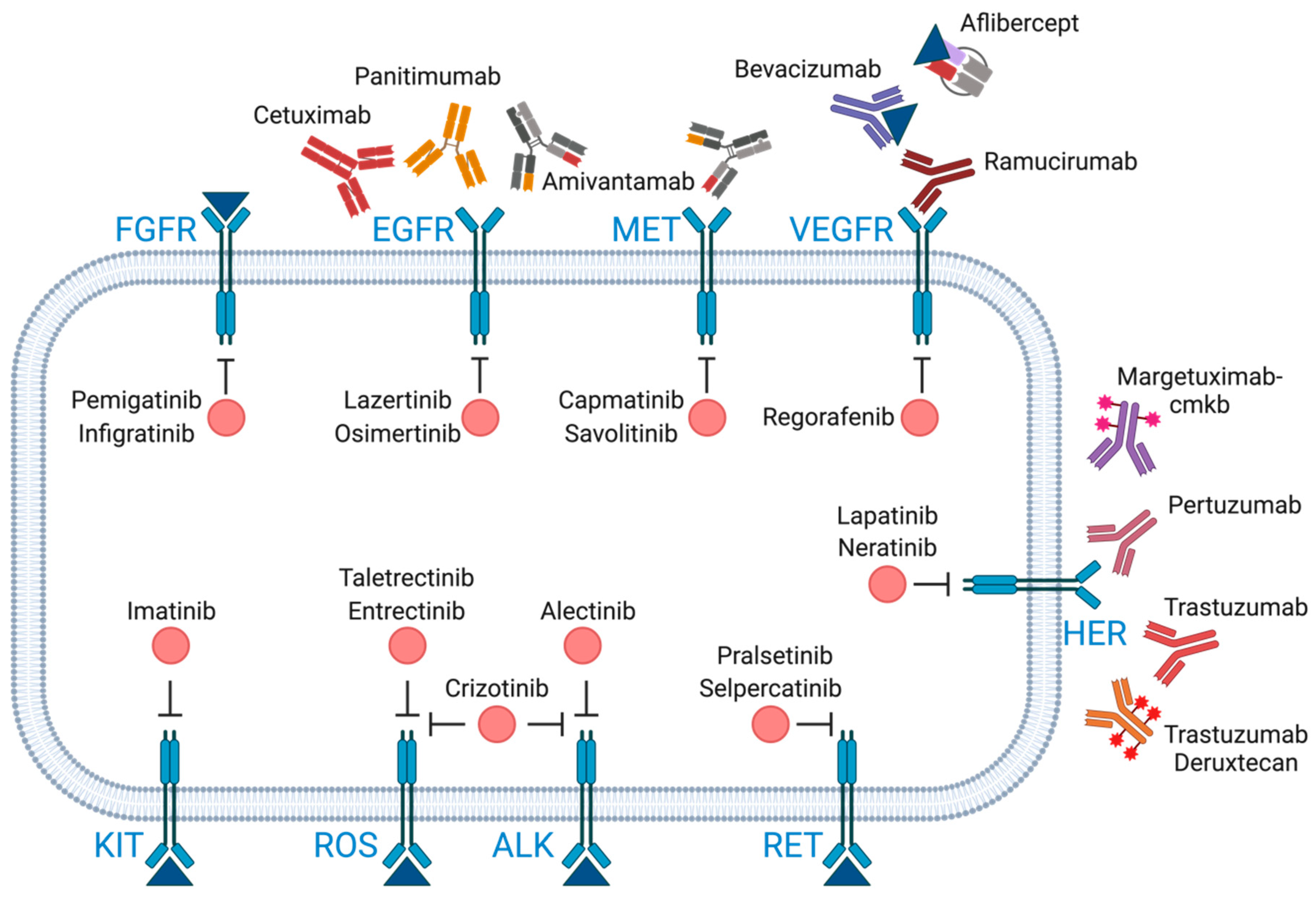
3. Molecular Targets and Mechanisms of Action
3.1. Mitotic Cycle and DNA Repair Targeting
3.2. Monoclonal Antibodies
3.3. Tyrosine Kinase Receptor Inhibitors
3.4. Downstream Signaling Effectors
3.5. Epigenetic Targets
3.6. Hedgehog Pathway Inhibitors
3.7. Circulating Tumor Markers, ctDNA
4. Selective Examples of Targeted Therapies in Cancers
4.1. Targeted Therapies in Breast Cancer
4.1.1. HER2 Targeting Strategy
4.1.2. CDK4/6 Targeting Strategy
4.1.3. PIK3CA/AKT Inhibitors
PARP Inhibitors
mTOR Inhibitors
4.2. Targeted Therapies in Cholangiocarcinoma
4.2.1. FGFR Targeting Strategy
4.2.2. IDH1/2 Targeting Strategy
4.2.3. RAS-MEK-ERK Targeting Strategy
4.3. Targeted Therapies in NSCLC
4.3.1. EGFR and MET Targeting Strategy
4.3.2. ALK
4.3.3. ROS1
4.3.4. RET
4.3.5. MET
4.3.6. KRAS-G12C
4.3.7. KRAS Non-G12C Targeting Strategy
4.4. Targeted Therapies in Melanoma
4.4.1. BRAF/MEK Targeting Strategy
4.4.2. KIT Targeting Strategy
4.5. Targeted Therapies in Colon Cancer
4.5.1. EGFR Targeting Strategy
4.5.2. BRAF Targeting Strategy
4.5.3. VEGF/VEGFR
4.5.4. KRAS-G12C Targeting Strategy
4.5.5. HER2
4.6. Targeted Therapies in Gastric Cancer
4.6.1. HER2 Targeting Strategy
4.6.2. Claudin-18 Targeting Strategy
4.6.3. VEGF Targeting Strategies
4.6.4. Rare Occurrences: EFGR and MET Targeting Strategies
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.F.; Mardis, E.R. The Emerging Clinical Relevance of Genomics in Cancer Medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef]
- Thiery, J.; Fahrner, M. Integration of Proteomics in the Molecular Tumor Board. Proteomics 2023, 24, 2300002. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Soria, J.-C.; Berger, R.; Miller, W.H.; Rubin, E.; Kugel, A.; Tsimberidou, A.; Saintigny, P.; Ackerstein, A.; Braña, I.; et al. Genomic and Transcriptomic Profiling Expands Precision Cancer Medicine: The WINTHER Trial. Nat. Med. 2019, 25, 751–758. [Google Scholar] [CrossRef]
- Cerneckis, J.; Ming, G.-L.; Song, H.; He, C.; Shi, Y. The Rise of Epitranscriptomics: Recent Developments and Future Directions. Trends Pharmacol. Sci. 2023, 45, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Sig. Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Vyas, S.; Chang, P. New PARP Targets for Cancer Therapy. Nat. Rev. Cancer 2014, 14, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.P.; Adonin, L.S.; Zvereva, S.D.; Guschin, D.Y.; Korneenko, T.V.; Telegina, A.V.; Kondratieva, O.K.; Frolova, S.E.; Pestov, N.B.; Barlev, N.A. BRCA Mutations—The Achilles Heel of Breast, Ovarian and Other Epithelial Cancers. Int. J. Mol. Sci. 2023, 24, 4982. [Google Scholar] [CrossRef]
- Lim, D.V.; Woo, W.H.; Lim, J.X.; Loh, X.Y.; Soh, H.T.; Lim, S.Y.A.; Lee, Z.Y.; Yow, H.Y.; Hamzah, S.B.; Sellappans, R.; et al. Targeting Mutant-P53 for Cancer Treatment: Are We There Yet? CMP 2023, 17, e140923221042. [Google Scholar] [CrossRef]
- Zhu, G.; Pan, C.; Bei, J.-X.; Li, B.; Liang, C.; Xu, Y.; Fu, X. Mutant P53 in Cancer Progression and Targeted Therapies. Front. Oncol. 2020, 10, 595187. [Google Scholar] [CrossRef]
- Kang, J.C.; Poovassery, J.S.; Bansal, P.; You, S.; Manjarres, I.M.; Ober, R.J.; Ward, E.S. Engineering Multivalent Antibodies to Target Heregulin-Induced HER3 Signaling in Breast Cancer Cells. mAbs 2014, 6, 340–353. [Google Scholar] [CrossRef][Green Version]
- Castelli, M.S.; McGonigle, P.; Hornby, P.J. The Pharmacology and Therapeutic Applications of Monoclonal Antibodies. Pharmacol. Res. Perspec 2019, 7, e00535. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Chiachio, M.; Sarrió, D.; Moreno-Bueno, G. Novel Therapies and Strategies to Overcome Resistance to Anti-HER2-Targeted Drugs. Cancers 2022, 14, 4543. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Giordano, G.; Parcesepe, P.; Bruno, G.; Piscazzi, A.; Lizzi, V.; Remo, A.; Pancione, M.; D’Andrea, M.R.; De Santis, E.; Coppola, L.; et al. Evidence-Based Second-Line Treatment in RAS Wild-Type/Mutated Metastatic Colorectal Cancer in the Precision Medicine Era. Int. J. Mol. Sci. 2021, 22, 7717. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, R.; Takahashi, N.; Yamada, Y. Claudin18.2 in Advanced Gastric Cancer. Cancers 2023, 15, 5742. [Google Scholar] [CrossRef]
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 Is a Novel Molecular Biomarker for Tumor-Targeted Immunotherapy. Biomark. Res. 2022, 10, 38. [Google Scholar] [CrossRef]
- Friedlaender, A.; Drilon, A.; Banna, G.L.; Peters, S.; Addeo, A. The METeoric Rise of MET in Lung Cancer. Cancer 2020, 126, 4826–4837. [Google Scholar] [CrossRef]
- Tan, D.S.-W.; Kim, T.M.; Guarneri, V.; Voon, P.J.; Lim, B.K.; Wislez, M.; Huang, C.; Liam, C.K.; Mazieres, J.; Tho, L.M.; et al. Tepotinib + Osimertinib for EGFR Mutant (EGFR m) NSCLC with MET Amplification (MET Amp) after First-Line (1L) Osimertinib. JCO 2023, 41, 9021. [Google Scholar] [CrossRef]
- Cho, B.C.; Simi, A.; Sabari, J.; Vijayaraghavan, S.; Moores, S.; Spira, A. Amivantamab, an Epidermal Growth Factor Receptor (EGFR) and Mesenchymal-Epithelial Transition Factor (MET) Bispecific Antibody, Designed to Enable Multiple Mechanisms of Action and Broad Clinical Applications. Clin. Lung Cancer 2023, 24, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.G.; Antman, K.H. Imatinib Mesylate—A New Oral Targeted Therapy. N. Engl. J. Med. 2002, 346, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Amelia, T.; Kartasasmita, R.E.; Ohwada, T.; Tjahjono, D.H. Structural Insight and Development of EGFR Tyrosine Kinase Inhibitors. Molecules 2022, 27, 819. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Taeger, J.; Moser, C.; Hellerbrand, C.; Mycielska, M.E.; Glockzin, G.; Schlitt, H.J.; Geissler, E.K.; Stoeltzing, O.; Lang, S.A. Targeting FGFR/PDGFR/VEGFR Impairs Tumor Growth, Angiogenesis, and Metastasis by Effects on Tumor Cells, Endothelial Cells, and Pericytes in Pancreatic Cancer. Mol. Cancer Ther. 2011, 10, 2157–2167. [Google Scholar] [CrossRef]
- Guo, T.; Ma, S. Recent Advances in the Discovery of Multitargeted Tyrosine Kinase Inhibitors as Anticancer Agents. ChemMedChem 2021, 16, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Sever, R.; Brugge, J.S. Signal Transduction in Cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Agarwala, S.S.; Trefzer, U.; Hogg, D.; Robert, C.; Hersey, P.; Eggermont, A.; Grabbe, S.; Gonzalez, R.; Gille, J.; et al. Results of a Phase III, Randomized, Placebo-Controlled Study of Sorafenib in Combination With Carboplatin and Paclitaxel As Second-Line Treatment in Patients With Unresectable Stage III or Stage IV Melanoma. JCO 2009, 27, 2823–2830. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in Patients with BRAF-Mutant Melanoma (COLUMBUS): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Sig. Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Punekar, S.R.; Velcheti, V.; Neel, B.G.; Wong, K.-K. The Current State of the Art and Future Trends in RAS-Targeted Cancer Therapies. Nat. Rev. Clin. Oncol. 2022, 19, 637–655. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Waarts, M.R.; Stonestrom, A.J.; Park, Y.C.; Levine, R.L. Targeting Mutations in Cancer. J. Clin. Investig. 2022, 132, e154943. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Daoud, A.; Eblen, S.T. Targeting Chromatin Remodeling for Cancer Therapy. CMP 2019, 12, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Du, W.; Guo, W. EZH2: A Novel Target for Cancer Treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH Mutation in Glioma: Molecular Mechanisms and Potential Therapeutic Targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, T.-C.A.; Mukherjee, J.; Viswanath, P.; Ohba, S.; Ronen, S.M.; Bjerkvig, R.; Pieper, R.O. Rapid Conversion of Mutant IDH1 from Driver to Passenger in a Model of Human Gliomagenesis. Mol. Cancer Res. 2016, 14, 976–983. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, J.-S. A Short Guide to Histone Deacetylases Including Recent Progress on Class II Enzymes. Exp. Mol. Med. 2020, 52, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.S.; Johnson, J.R.; He, K.; Sridhara, R.; Abraham, S.; Booth, B.P.; Verbois, L.; Morse, D.E.; Jee, J.M.; Pope, S.; et al. Vorinostat for Treatment of Cutaneous Manifestations of Advanced Primary Cutaneous T-Cell Lymphoma. Clin. Cancer Res. 2007, 13, 2318–2322. [Google Scholar] [CrossRef]
- Pak, E.; Segal, R.A. Hedgehog Signal Transduction: Key Players, Oncogenic Drivers, and Cancer Therapy. Dev. Cell 2016, 38, 333–344. [Google Scholar] [CrossRef]
- Basset-Séguin, N.; Hauschild, A.; Kunstfeld, R.; Grob, J.; Dréno, B.; Mortier, L.; Ascierto, P.A.; Licitra, L.; Dutriaux, C.; Thomas, L.; et al. Vismodegib in Patients with Advanced Basal Cell Carcinoma: Primary Analysis of STEVIE, an International, Open-Label Trial. Eur. J. Cancer 2017, 86, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Raei, N.; Safaralizadeh, R.; Latifi-Navid, S. Clinical Application of Circulating Tumor DNA in Metastatic Cancers. Expert Rev. Mol. Diagn. 2023, 23, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Dong, M.; Zang, X.-Y.; Li, M.-Y.; Zhou, J.-Y.; Ma, J.-J.; Wang, G.-Y. The Emerging Role of Circulating Tumor Cells in Cancer Management. Am. J. Transl. Res. 2020, 12, 332–342. [Google Scholar] [PubMed]
- Ye, F.; Dewanjee, S.; Li, Y.; Jha, N.K.; Chen, Z.-S.; Kumar, A.; Vishakha; Behl, T.; Jha, S.K.; Tang, H. Advancements in Clinical Aspects of Targeted Therapy and Immunotherapy in Breast Cancer. Mol. Cancer 2023, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; De Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA): End-of-Study Results from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.-B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.-M.; Smitt, M.; Yu, R.; Leung, A.C.F.; Wildiers, H. Trastuzumab Emtansine versus Treatment of Physician’s Choice for Pretreated HER2-Positive Advanced Breast Cancer (TH3RESA): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. LBA1 Trastuzumab Deruxtecan (T-DXd) vs Trastuzumab Emtansine (T-DM1) in Patients (Pts) with HER2+ Metastatic Breast Cancer (mBC): Results of the Randomized Phase III DESTINY-Breast03 Study. Ann. Oncol. 2021, 32, S1287–S1288. [Google Scholar] [CrossRef]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. JCO 2020, 38, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.; Sledge, G.; Koehler, M.; Ellis, C.; Casey, M.; Vukelja, S.; Bischoff, J.; et al. Randomized Study of Lapatinib Alone or in Combination With Trastuzumab in Women With ErbB2-Positive, Trastuzumab-Refractory Metastatic Breast Cancer. JCO 2010, 28, 1124–1130. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. JCO 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.-A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573. [Google Scholar] [CrossRef]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in Combination with Endocrine Therapy versus Capecitabine in Hormonal Receptor-Positive, Human Epidermal Growth Factor 2-Negative, Aromatase Inhibitor-Resistant Metastatic Breast Cancer: A Phase III Randomised Controlled Trial—PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, T.-Y.; Kim, G.M.; Kang, S.Y.; Park, I.H.; Kim, J.H.; Lee, K.E.; Ahn, H.K.; Lee, M.H.; Kim, H.-J.; et al. Palbociclib plus Exemestane with Gonadotropin-Releasing Hormone Agonist versus Capecitabine in Premenopausal Women with Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer (KCSG-BR15-10): A Multicentre, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 2019, 20, 1750–1759. [Google Scholar] [CrossRef]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2− Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. JCO 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Jones, R.H.; Casbard, A.; Carucci, M.; Cox, C.; Butler, R.; Alchami, F.; Madden, T.-A.; Bale, C.; Bezecny, P.; Joffe, J.; et al. Fulvestrant plus Capivasertib versus Placebo after Relapse or Progression on an Aromatase Inhibitor in Metastatic, Oestrogen Receptor-Positive Breast Cancer (FAKTION): A Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2020, 21, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD Final Overall Survival and Tolerability Results: Olaparib versus Chemotherapy Treatment of Physician’s Choice in Patients with a Germline BRCA Mutation and HER2-Negative Metastatic Breast Cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Gonçalves, A.; Rugo, H.S.; Lee, K.-H.; Fehrenbacher, L.; Mina, L.A.; Diab, S.; Blum, J.L.; Chakrabarti, J.; Elmeliegy, M.; et al. Talazoparib in Patients with a Germline BRCA-Mutated Advanced Breast Cancer: Detailed Safety Analyses from the Phase III EMBRACA Trial. Oncol. 2020, 25, e439–e450. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.M.; Al Rabadi, L.; Kaempf, A.J.; Saraceni, M.M.; Savin, M.A.; Mitri, Z.I. Everolimus Plus Exemestane Treatment in Patients with Metastatic Hormone Receptor-Positive Breast Cancer Previously Treated with CDK4/6 Inhibitor Therapy. Oncol. 2021, 26, 101–106. [Google Scholar] [CrossRef]
- Rugo, H.S.; Seneviratne, L.; Beck, J.T.; Glaspy, J.A.; Peguero, J.A.; Pluard, T.J.; Dhillon, N.; Hwang, L.C.; Nangia, C.; Mayer, I.A.; et al. Prevention of Everolimus-Related Stomatitis in Women with Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer Using Dexamethasone Mouthwash (SWISH): A Single-Arm, Phase 2 Trial. Lancet Oncol. 2017, 18, 654–662. [Google Scholar] [CrossRef]
- Jerusalem, G.; De Boer, R.H.; Hurvitz, S.; Yardley, D.A.; Kovalenko, E.; Ejlertsen, B.; Blau, S.; Özgüroglu, M.; Landherr, L.; Ewertz, M.; et al. Everolimus Plus Exemestane vs Everolimus or Capecitabine Monotherapy for Estrogen Receptor–Positive, HER2-Negative Advanced Breast Cancer: The BOLERO-6 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1367. [Google Scholar] [CrossRef]
- Du, J.; Lv, X.; Zhang, Z.; Huang, Z.; Zhang, E. Revisiting Targeted Therapy and Immunotherapy for Advanced Cholangiocarcinoma. Front. Immunol. 2023, 14, 1142690. [Google Scholar] [CrossRef] [PubMed]
- Merz, V.; Zecchetto, C.; Melisi, D. Pemigatinib, a Potent Inhibitor of FGFRs for the Treatment of Cholangiocarcinoma. Future Oncol. 2021, 17, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Bahleda, R.; Italiano, A.; Hierro, C.; Mita, A.; Cervantes, A.; Chan, N.; Awad, M.; Calvo, E.; Moreno, V.; Govindan, R.; et al. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors. Clin. Cancer Res. 2019, 25, 4888–4897. [Google Scholar] [CrossRef]
- Kang, C. Infigratinib: First Approval. Drugs 2021, 81, 1355–1360. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. A Foreword on Biliary Tract Cancers: Emerging Treatments, Drug Targets, and Fundamental Knowledge Gaps. Expert Opin. Investig. Drugs 2021, 30, 279. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in Previously Treated Patients with Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusions or Rearrangements: Mature Results from a Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. JCO 2018, 36, 276–282. [Google Scholar] [CrossRef]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.-Y.; Macarulla, T.; et al. Infigratinib in Patients with Advanced Cholangiocarcinoma with FGFR2 Gene Fusions/Translocations: The PROOF 301 Trial. Future Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in Advanced or Inoperable FGFR2 Gene Fusion-Positive Intrahepatic Cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef]
- Taylor, M.H.; Schmidt, E.V.; Dutcus, C.; Pinheiro, E.M.; Funahashi, Y.; Lubiniecki, G.; Rasco, D. The LEAP Program: Lenvatinib plus Pembrolizumab for the Treatment of Advanced Solid Tumors. Future Oncol. 2021, 17, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Kommalapati, A.; Tella, S.H.; Borad, M.; Javle, M.; Mahipal, A. FGFR Inhibitors in Oncology: Insight on the Management of Toxicities in Clinical Practice. Cancers 2021, 13, 2968. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and Prognostic Significance of Isocitrate Dehydrogenase 1 Mutations in Cholangiocarcinoma: A Systematic Literature Review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Fujimoto, A.; Furuta, M.; Shiraishi, Y.; Gotoh, K.; Kawakami, Y.; Arihiro, K.; Nakamura, T.; Ueno, M.; Ariizumi, S.; Hai Nguyen, H.; et al. Whole-Genome Mutational Landscape of Liver Cancers Displaying Biliary Phenotype Reveals Hepatitis Impact and Molecular Diversity. Nat. Commun. 2015, 6, 6120. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-Mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Hyder, O.; Dodson, R.; Nayar, S.K.; Poling, J.; Beierl, K.; Eshleman, J.R.; Lin, M.-T.; Pawlik, T.M.; Anders, R.A. The Frequency of KRAS and BRAF Mutations in Intrahepatic Cholangiocarcinomas and Their Correlation with Clinical Outcome. Hum. Pathol. 2013, 44, 2768–2773. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Lassen, U.N.; Elez, E.; Italiano, A.; Curigliano, G.; De Braud, F.G.; Prager, G.; Greil, R.; Stein, A.; Fasolo, A.; et al. Efficacy and Safety of Dabrafenib (D) and Trametinib (T) in Patients (Pts) with BRAF V600E–Mutated Biliary Tract Cancer (BTC): A Cohort of the ROAR Basket Trial. JCO 2019, 37, 187. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.W.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-Institutional Phase II Study of Selumetinib in Patients With Metastatic Biliary Cancers. JCO 2011, 29, 2357–2363. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Infante, J.R.; Janku, F.; Wong, D.J.L.; Sosman, J.A.; Keedy, V.; Patel, M.R.; Shapiro, G.I.; Mier, J.W.; Tolcher, A.W.; et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study. Cancer Discov. 2018, 8, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. JCO 2022, 40, 611–625. [Google Scholar] [CrossRef]
- Reck, M.; Mok, T.S.K.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus Bevacizumab and Chemotherapy in Non-Small-Cell Lung Cancer (IMpower150): Key Subgroup Analyses of Patients with EGFR Mutations or Baseline Liver Metastases in a Randomised, Open-Label Phase 3 Trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- White, M.N.; Piper-Vallillo, A.J.; Gardner, R.M.; Cunanan, K.; Neal, J.W.; Das, M.; Padda, S.K.; Ramchandran, K.; Chen, T.T.; Sequist, L.V.; et al. Chemotherapy Plus Immunotherapy Versus Chemotherapy Plus Bevacizumab Versus Chemotherapy Alone in EGFR-Mutant NSCLC After Progression on Osimertinib. Clin. Lung Cancer 2022, 23, e210–e221. [Google Scholar] [CrossRef]
- Cho, B.C.; Lee, K.H.; Cho, E.K.; Kim, D.-W.; Lee, J.-S.; Han, J.-Y.; Kim, S.-W.; Spira, A.; Haura, E.B.; Sabari, J.K.; et al. 1258O Amivantamab (JNJ-61186372), an EGFR-MET Bispecific Antibody, in Combination with Lazertinib, a 3rd-Generation Tyrosine Kinase Inhibitor (TKI), in Advanced EGFR NSCLC. Ann. Oncol. 2020, 31, S813. [Google Scholar] [CrossRef]
- Shu, C.A.; Goto, K.; Ohe, Y.; Besse, B.; Lee, S.-H.; Wang, Y.; Griesinger, F.; Yang, J.C.-H.; Felip, E.; Sanborn, R.E.; et al. Amivantamab and Lazertinib in Patients with EGFR-Mutant Non–Small Cell Lung (NSCLC) after Progression on Osimertinib and Platinum-Based Chemotherapy: Updated Results from CHRYSALIS-2. JCO 2022, 40, 9006. [Google Scholar] [CrossRef]
- Cho, B.C.; Felip, E.; Hayashi, H.; Thomas, M.; Lu, S.; Besse, B.; Sun, T.; Martinez, M.; Sethi, S.N.; Shreeve, S.M.; et al. MARIPOSA: Phase 3 Study of First-Line Amivantamab + Lazertinib versus Osimertinib in EGFR-Mutant Non-Small-Cell Lung Cancer. Future Oncol. 2022, 18, 639–647. [Google Scholar] [CrossRef]
- Singal, G.; Miller, P.G.; Agarwala, V.; Li, G.; Kaushik, G.; Backenroth, D.; Gossai, A.; Frampton, G.M.; Torres, A.Z.; Lehnert, E.M.; et al. Association of Patient Characteristics and Tumor Genomics With Clinical Outcomes Among Patients With Non–Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA 2019, 321, 1391. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-J.; Zhou, J.; Cheng, Y.; Li, M.; Zhao, Q.; Zhang, Z.; Zang, A.; Fan, Y.; Hui, A.-M.; Zhou, Y.; et al. SAF-189s in Advanced, ALK-Positive, Non–Small Cell Lung Cancer: Results from a First-in-Human Phase 1/2, Multicenter Study. JCO 2022, 40, 9076. [Google Scholar] [CrossRef]
- Cho, B.C.; Doebele, R.C.; Lin, J.; Nagasaka, M.; Baik, C.; Van Der Wekken, A.; Velcheti, V.; Lee, K.H.; Liu, S.; Solomon, B.; et al. MA11.07 Phase 1/2 TRIDENT-1 Study of Repotrectinib in Patients with ROS1+ or NTRK+ Advanced Solid Tumors. J. Thorac. Oncol. 2021, 16, S174–S175. [Google Scholar] [CrossRef]
- Li, W.; Yang, N.; Ma, H.; Fan, H.; Li, K.; Wu, H.; Yu, Q.; Wang, Y.; Meng, X.; Wang, X.; et al. The Efficacy and Safety of Taletrectinib in Patients with TKI-Naïve or Crizotinib-Pretreated ROS1-Positive Non–Small Cell Lung Cancer (NSCLC). JCO 2022, 40, 8572. [Google Scholar] [CrossRef]
- Drilon, A.; Besse, B.; Camidge, D.R.; Ou, S.H.I.; Gadgeel, S.M.; Johnson, M.L.; Calles, A.; De Miguel, M.J.; Spira, A.I.; Felip, E.; et al. Safety and Preliminary Clinical Activity of NVL-520, a Highly Selective ROS1 Inhibitor, in Patients with Advanced ROS1 Fusion-Positive Solid Tumors. Eur. J. Cancer 2022, 174, S6–S7. [Google Scholar] [CrossRef]
- Fang, D.D.; Tao, R.; Wang, G.; Li, Y.; Zhang, K.; Xu, C.; Zhai, G.; Wang, Q.; Wang, J.; Tang, C.; et al. Discovery of a Novel ALK/ROS1/FAK Inhibitor, APG-2449, in Preclinical Non-Small Cell Lung Cancer and Ovarian Cancer Models. BMC Cancer 2022, 22, 752. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-Agnostic Efficacy and Safety of Selpercatinib in Patients with RET Fusion-Positive Solid Tumours Other than Lung or Thyroid Tumours (LIBRETTO-001): A Phase 1/2, Open-Label, Basket Trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET Fusion-Positive Non-Small-Cell Lung Cancer (ARROW): A Multi-Cohort, Open-Label, Phase 1/2 Study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Zhou, C.; Solomon, B.; Loong, H.H.; Park, K.; Pérol, M.; Arriola, E.; Novello, S.; Han, B.; Zhou, J.; Ardizzoni, A.; et al. First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion–Positive NSCLC. N. Engl. J. Med. 2023, 389, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Non-Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; De Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14–Mutated or MET-Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.-H.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef]
- Sacher, A.; LoRusso, P.; Patel, M.R.; Miller, W.H.; Garralda, E.; Forster, M.D.; Santoro, A.; Falcon, A.; Kim, T.W.; Paz-Ares, L.; et al. Single-Agent Divarasib (GDC-6036) in Solid Tumors with a KRAS G12C Mutation. N. Engl. J. Med. 2023, 389, 710–721. [Google Scholar] [CrossRef]
- Lu, S.; Jian, H.; Zhang, Y.; Song, Z.; Zhao, Y.; Wang, P.; Jiang, L.; Gong, Y.; Zhou, J.; Dong, X.; et al. OA03.07 Safety and Efficacy of D-1553 in Patients with KRAS G12C Mutated Non-Small Cell Lung Cancer: A Phase 1 Trial. J. Thorac. Oncol. 2022, 17, S11. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, L.; Maldonato, B.J.; Wang, Y.; Holderfield, M.; Aronchik, I.; Winters, I.P.; Salman, Z.; Blaj, C.; Menard, M.; et al. Translational and Therapeutic Evaluation of RAS-GTP Inhibition by RMC-6236 in RAS-Driven Cancers. Cancer Discov. 2024, 14, OF1–OF24. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chénard-Poirier, M.; Roda, D.; De Miguel, M.; Harris, S.J.; Candilejo, I.M.; Sriskandarajah, P.; Xu, W.; Scaranti, M.; Constantinidou, A.; et al. Intermittent Schedules of the Oral RAF–MEK Inhibitor CH5126766/VS-6766 in Patients with RAS/RAF-Mutant Solid Tumours and Multiple Myeloma: A Single-Centre, Open-Label, Phase 1 Dose-Escalation and Basket Dose-Expansion Study. Lancet Oncol. 2020, 21, 1478–1488. [Google Scholar] [CrossRef]
- Yang, T.-T.; Yu, S.; Ke, C.-L.K.; Cheng, S.-T. The Genomic Landscape of Melanoma and Its Therapeutic Implications. Genes 2023, 14, 1021. [Google Scholar] [CrossRef] [PubMed]
- McArthur, G.A.; Maio, M.; Arance, A.; Nathan, P.; Blank, C.; Avril, M.-F.; Garbe, C.; Hauschild, A.; Schadendorf, D.; Hamid, O.; et al. Vemurafenib in Metastatic Melanoma Patients with Brain Metastases: An Open-Label, Single-Arm, Phase 2, Multicentre Study. Ann. Oncol. 2017, 28, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Minor, D.; Ribas, A.; Lebbe, C.; O’Hagan, A.; Arya, N.; Guckert, M.; Schadendorf, D.; Kefford, R.F.; Grob, J.-J.; et al. Phase II Trial (BREAK-2) of the BRAF Inhibitor Dabrafenib (GSK2118436) in Patients With Metastatic Melanoma. JCO 2013, 31, 3205–3211. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Dummer, R.; Schadendorf, D.; Ascierto, P.A.; Arance, A.; Dutriaux, C.; Di Giacomo, A.M.; Rutkowski, P.; Del Vecchio, M.; Gutzmer, R.; Mandala, M.; et al. Binimetinib versus Dacarbazine in Patients with Advanced NRAS-Mutant Melanoma (NEMO): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2017, 18, 435–445. [Google Scholar] [CrossRef]
- Lebbé, C.; Dutriaux, C.; Lesimple, T.; Kruit, W.; Kerger, J.; Thomas, L.; Guillot, B.; De Braud, F.; Garbe, C.; Grob, J.-J.; et al. Pimasertib Versus Dacarbazine in Patients With Unresectable NRAS-Mutated Cutaneous Melanoma: Phase II, Randomized, Controlled Trial with Crossover. Cancers 2020, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for Melanomas Harboring Mutationally Activated or Amplified KIT Arising on Mucosal, Acral, and Chronically Sun-Damaged Skin. JCO 2013, 31, 3182–3190. [Google Scholar] [CrossRef]
- Carvajal, R.D. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA 2011, 305, 2327. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Carvajal, R.D.; Dummer, R.; Hauschild, A.; Daud, A.; Bastian, B.C.; Markovic, S.N.; Queirolo, P.; Arance, A.; Berking, C.; et al. Efficacy and Safety of Nilotinib in Patients with KIT-Mutated Metastatic or Inoperable Melanoma: Final Results from the Global, Single-Arm, Phase II TEAM Trial. Ann. Oncol. 2017, 28, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Bauer, S.; Shoumariyeh, K.; Jones, R.L.; Spreafico, A.; Jennings, J.; Psoinos, C.; Meade, J.; Ruiz-Soto, R.; Chi, P. Efficacy and Safety of Ripretinib in Patients with KIT-Altered Metastatic Melanoma. ESMO Open 2022, 7, 100520. [Google Scholar] [CrossRef] [PubMed]
- Wieduwilt, M.J.; Moasser, M.M. The Epidermal Growth Factor Receptor Family: Biology Driving Targeted Therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.M.; Ottaiano, A.; Romano, C.; Nasti, G.; Nappi, A.; De Divitiis, C.; Napolitano, M.; Zanotta, S.; Casaretti, R.; D’Alterio, C.; et al. Prospective Evaluation of Cetuximab-Mediated Antibody-Dependent Cell Cytotoxicity in Metastatic Colorectal Cancer Patients Predicts Treatment Efficacy. Cancer Immunol. Res. 2016, 4, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Lo Nigro, C.; Macagno, M.; Sangiolo, D.; Bertolaccini, L.; Aglietta, M.; Merlano, M.C. NK-Mediated Antibody-Dependent Cell-Mediated Cytotoxicity in Solid Tumors: Biological Evidence and Clinical Perspectives. Ann. Transl. Med. 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Meropol, N.J.; Loehrer, P.J.; Needle, M.N.; Kopit, J.; Mayer, R.J. Phase II Trial of Cetuximab in Patients With Refractory Colorectal Cancer That Expresses the Epidermal Growth Factor Receptor. JCO 2004, 22, 1201–1208. [Google Scholar] [CrossRef]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Panitumumab: A Review of Its Use in Metastatic Colorectal Cancer. Drugs 2010, 70, 1059–1078. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Final Results from PRIME: Randomized Phase III Study of Panitumumab with FOLFOX4 for First-Line Treatment of Metastatic Colorectal Cancer. Ann. Oncol. 2014, 25, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Price, T.J.; Peeters, M.; Kim, T.W.; Li, J.; Cascinu, S.; Ruff, P.; Suresh, A.S.; Thomas, A.; Tjulandin, S.; Zhang, K.; et al. Panitumumab versus Cetuximab in Patients with Chemotherapy-Refractory Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer (ASPECCT): A Randomised, Multicentre, Open-Label, Non-Inferiority Phase 3 Study. Lancet Oncol. 2014, 15, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Kaley, K.; Chu, E.; Copur, M.S. Safety and Efficacy of Panitumumab Therapy After Progression With Cetuximab: Experience at Two Institutions. Clin. Color. Cancer 2010, 9, 315–318. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Köhne, C.-H.; Ciardiello, F.; Lenz, H.-J.; Heinemann, V.; Klinkhardt, U.; Beier, F.; Duecker, K.; Van Krieken, J.H.; Tejpar, S. FOLFOX4 plus Cetuximab Treatment and RAS Mutations in Colorectal Cancer. Eur. J. Cancer 2015, 51, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Lenz, H.-J.; Köhne, C.-H.; Heinemann, V.; Tejpar, S.; Melezínek, I.; Beier, F.; Stroh, C.; Rougier, P.; Van Krieken, J.H.; et al. Fluorouracil, Leucovorin, and Irinotecan Plus Cetuximab Treatment and RAS Mutations in Colorectal Cancer. JCO 2015, 33, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.Y.; Tolias, P. Recent Advances in Cancer Drug Discovery Targeting RAS. Drug Discov. Today 2016, 21, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Tejpar, S.; Celik, I.; Schlichting, M.; Sartorius, U.; Bokemeyer, C.; Van Cutsem, E. Association of KRAS G13D Tumor Mutations With Outcome in Patients With Metastatic Colorectal Cancer Treated With First-Line Chemotherapy With or Without Cetuximab. JCO 2012, 30, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Huang, Y.; Yang, Z.; Zheng, D.; Chen, J.; Tang, J. KRAS p.G13D Mutation and Codon 12 Mutations Are Not Created Equal in Predicting Clinical Outcomes of Cetuximab in Metastatic Colorectal Cancer: A Systematic Review and Meta-analysis. Cancer 2013, 119, 714–721. [Google Scholar] [CrossRef]
- Cremolini, C.; Rossini, D.; Dell’Aquila, E.; Lonardi, S.; Conca, E.; Del Re, M.; Busico, A.; Pietrantonio, F.; Danesi, R.; Aprile, G.; et al. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-Line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 343. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating Tumor DNA to Guide Rechallenge with Panitumumab in Metastatic Colorectal Cancer: The Phase 2 CHRONOS Trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Thorrez, L.; Siegfried, G.; Meulemans, S.; Evrard, S.; Tejpar, S.; Khatib, A.-M.; Creemers, J.W.M. The Proprotein Convertase Furin Is a Pro-Oncogenic Driver in KRAS and BRAF Driven Colorectal Cancer. Oncogene 2020, 39, 3571–3587. [Google Scholar] [CrossRef]
- Jones, J.C.; Renfro, L.A.; Al-Shamsi, H.O.; Schrock, A.B.; Rankin, A.; Zhang, B.Y.; Kasi, P.M.; Voss, J.S.; Leal, A.D.; Sun, J.; et al. Non-V600BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. JCO 2017, 35, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.E.; Johnson, B.; Kugathasan, L.; Morris, V.K.; Raghav, K.; Swanson, L.; Lim, H.J.; Renouf, D.J.; Gill, S.; Wolber, R.; et al. Population-Based Screening for BRAF V600E in Metastatic Colorectal Cancer Reveals Increased Prevalence and Poor Prognosis. Clin. Cancer Res. 2020, 26, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Sunakawa, Y. Management of BRAF Gene Alterations in Metastatic Colorectal Cancer: From Current Therapeutic Strategies to Future Perspectives. Front. Oncol. 2021, 11, 602194. [Google Scholar] [CrossRef] [PubMed]
- Delord, J.-P.; Robert, C.; Nyakas, M.; McArthur, G.A.; Kudchakar, R.; Mahipal, A.; Yamada, Y.; Sullivan, R.; Arance, A.; Kefford, R.F.; et al. Phase I Dose-Escalation and -Expansion Study of the BRAF Inhibitor Encorafenib (LGX818) in Metastatic BRAF-Mutant Melanoma. Clin. Cancer Res. 2017, 23, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Principles and Mechanisms of Vessel Normalization for Cancer and Other Angiogenic Diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Wada, S.; Tsunoda, T.; Baba, T.; Primus, F.J.; Kuwano, H.; Shibuya, M.; Tahara, H. Rationale for Antiangiogenic Cancer Therapy with Vaccination Using Epitope Peptides Derived from Human Vascular Endothelial Growth Factor Receptor 2. Cancer Res. 2005, 65, 4939–4946. [Google Scholar] [CrossRef]
- Ishizaki, H.; Tsunoda, T.; Wada, S.; Yamauchi, M.; Shibuya, M.; Tahara, H. Inhibition of Tumor Growth with Antiangiogenic Cancer Vaccine Using Epitope Peptides Derived from Human Vascular Endothelial Growth Factor Receptor 1. Clin. Cancer Res. 2006, 12, 5841–5849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fei, D.; Vanderlaan, M.; Song, A. Biological Activity of Bevacizumab, a Humanized Anti-VEGF Antibody in Vitro. Angiogenesis 2004, 7, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; Von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of Bevacizumab after First Progression in Metastatic Colorectal Cancer (ML18147): A Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Flick, E.D.; Cohn, A.L.; Bekaii-Saab, T.S.; Bendell, J.C.; Kozloff, M.; Roach, N.; Mun, Y.; Fish, S.; Hurwitz, H.I. Bevacizumab Exposure beyond First Disease Progression in Patients with Metastatic Colorectal Cancer: Analyses of the ARIES Observational Cohort Study. Pharmacoepidemiol. Drug 2014, 23, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; Von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab as First-Line Treatment for Patients with Metastatic Colorectal Cancer (FIRE-3): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.-L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A Randomized, Multicenter Phase II Study of Panitumumab Plus Modified Fluorouracil, Leucovorin, and Oxaliplatin (mFOLFOX6) or Bevacizumab Plus mFOLFOX6 in Patients With Previously Untreated, Unresectable, Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer. JCO 2014, 32, 2240–2247. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; Van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients With Metastatic Colorectal Cancer Previously Treated With an Oxaliplatin-Based Regimen. JCO 2012, 30, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus Placebo in Combination with Second-Line FOLFIRI in Patients with Metastatic Colorectal Carcinoma That Progressed during or after First-Line Therapy with Bevacizumab, Oxaliplatin, and a Fluoropyrimidine (RAISE): A Randomised, Double-Blind, Multicentre, Phase 3 Study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Cutsem, E.V.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.-H.; Shen, L.; Xu, J.; Bai, Y.; Yang, L.; Deng, Y.; Chen, Z.; Zhong, H.; et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018, 319, 2486. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.E.; El-Refai, S.M.; Sha, W.; Puccini, A.; Grothey, A.; George, T.J.; Hwang, J.J.; O’Neil, B.; Barrett, A.S.; Kadakia, K.C.; et al. Landscape of KRASG12C, Associated Genomic Alterations, and Interrelation With Immuno-Oncology Biomarkers in KRAS-Mutated Cancers. JCO Precis. Oncol. 2022, 6, e2100245. [Google Scholar] [CrossRef]
- Fakih, M.G.; Kopetz, S.; Kuboki, Y.; Kim, T.W.; Munster, P.N.; Krauss, J.C.; Falchook, G.S.; Han, S.-W.; Heinemann, V.; Muro, K.; et al. Sotorasib for Previously Treated Colorectal Cancers with KRASG12C Mutation (CodeBreaK100): A Prespecified Analysis of a Single-Arm, Phase 2 Trial. Lancet Oncol. 2022, 23, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.-W.; Kuboki, Y.; et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 389, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-Targeted Therapy with Trastuzumab and Lapatinib in Treatment-Refractory, KRAS Codon 12/13 Wild-Type, HER2-Positive Metastatic Colorectal Cancer (HERACLES): A Proof-of-Concept, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular Analysis of Gastric Cancer Identifies Subtypes Associated with Distinct Clinical Outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Haffner, I.; Schierle, K.; Raimúndez, E.; Geier, B.; Maier, D.; Hasenauer, J.; Luber, B.; Walch, A.; Kolbe, K.; Riera Knorrenschild, J.; et al. HER2 Expression, Test Deviations, and Their Impact on Survival in Metastatic Gastric Cancer: Results From the Prospective Multicenter VARIANZ Study. JCO 2021, 39, 1468–1478. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the Treatment of Advanced Gastric Cancer. Tumour Biol. 2017, 39, 101042831771462. [Google Scholar] [CrossRef]
- Smyth, E.C.; Moehler, M. Late-Line Treatment in Metastatic Gastric Cancer: Today and Tomorrow. Ther. Adv. Med. Oncol. 2019, 11, 175883591986752. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A Randomised Phase II Study of Zolbetuximab (IMAB362) plus EOX versus EOX Alone for First-Line Treatment of Advanced CLDN18.2-Positive Gastric and Gastro-Oesophageal Adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus Paclitaxel versus Placebo plus Paclitaxel in Patients with Previously Treated Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma (RAINBOW): A Double-Blind, Randomised Phase 3 Trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Nagatsuma, A.K.; Aizawa, M.; Kuwata, T.; Doi, T.; Ohtsu, A.; Fujii, H.; Ochiai, A. Expression Profiles of HER2, EGFR, MET and FGFR2 in a Large Cohort of Patients with Gastric Adenocarcinoma. Gastric Cancer 2015, 18, 227–238. [Google Scholar] [CrossRef]
- Australasian Gastro-Intestinal Trials Group (AGITG); Tebbutt, N.C.; Price, T.J.; Ferraro, D.A.; Wong, N.; Veillard, A.-S.; Hall, M.; Sjoquist, K.M.; Pavlakis, N.; Strickland, A.; et al. Panitumumab Added to Docetaxel, Cisplatin and Fluoropyrimidine in Oesophagogastric Cancer: ATTAX3 Phase II Trial. Br. J. Cancer 2016, 114, 505–509. [Google Scholar] [CrossRef]
- Waddell, T.; Chau, I.; Cunningham, D.; Gonzalez, D.; Okines, A.F.C.; Wotherspoon, A.; Saffery, C.; Middleton, G.; Wadsley, J.; Ferry, D.; et al. Epirubicin, Oxaliplatin, and Capecitabine with or without Panitumumab for Patients with Previously Untreated Advanced Oesophagogastric Cancer (REAL3): A Randomised, Open-Label Phase 3 Trial. Lancet Oncol. 2013, 14, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Kang, Y.-K.; Chung, H.-C.; Salman, P.; Oh, S.C.; Bodoky, G.; Kurteva, G.; Volovat, C.; Moiseyenko, V.M.; Gorbunova, V.; et al. Capecitabine and Cisplatin with or without Cetuximab for Patients with Previously Untreated Advanced Gastric Cancer (EXPAND): A Randomised, Open-Label Phase 3 Trial. Lancet Oncol. 2013, 14, 490–499. [Google Scholar] [CrossRef]
- Chen, C.-T.; Kim, H.; Liska, D.; Gao, S.; Christensen, J.G.; Weiser, M.R. MET Activation Mediates Resistance to Lapatinib Inhibition of HER2-Amplified Gastric Cancer Cells. Mol. Cancer Ther. 2012, 11, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vande Woude, G.F. HGF/SF-met Signaling in the Control of Branching Morphogenesis and Invasion. J. Cell. Biochem. 2003, 88, 408–417. [Google Scholar] [CrossRef]
- Spigel, D.R.; Ervin, T.J.; Ramlau, R.A.; Daniel, D.B.; Goldschmidt, J.H.; Blumenschein, G.R.; Krzakowski, M.J.; Robinet, G.; Godbert, B.; Barlesi, F.; et al. Randomized Phase II Trial of Onartuzumab in Combination With Erlotinib in Patients With Advanced Non–Small-Cell Lung Cancer. JCO 2013, 31, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Cho, J.-Y.; Tan, I.B.; Tebbutt, N.C.; Yen, C.-J.; Kang, A.; Shames, D.S.; Bu, L.; Kang, Y.-K. A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncol. 2016, 21, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Iveson, T.; Donehower, R.C.; Davidenko, I.; Tjulandin, S.; Deptala, A.; Harrison, M.; Nirni, S.; Lakshmaiah, K.; Thomas, A.; Jiang, Y.; et al. Rilotumumab in Combination with Epirubicin, Cisplatin, and Capecitabine as First-Line Treatment for Gastric or Oesophagogastric Junction Adenocarcinoma: An Open-Label, Dose de-Escalation Phase 1b Study and a Double-Blind, Randomised Phase 2 Study. Lancet Oncol. 2014, 15, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Fox, C.; Peng, S.; Pusung, M.; Pectasides, E.; Matthee, E.; Hong, Y.S.; Do, I.-G.; Jang, J.; Thorner, A.R.; et al. Preexisting Oncogenic Events Impact Trastuzumab Sensitivity in ERBB2-Amplified Gastroesophageal Adenocarcinoma. J. Clin. Investig. 2014, 124, 5145–5158. [Google Scholar] [CrossRef] [PubMed]
- Malka, D.; François, E.; Penault-Llorca, F.; Castan, F.; Bouché, O.; Bennouna, J.; Ghiringhelli, F.; De La Fouchardière, C.; Borg, C.; Samalin, E.; et al. FOLFOX Alone or Combined with Rilotumumab or Panitumumab as First-Line Treatment for Patients with Advanced Gastroesophageal Adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): A Randomised, Open-Label, Three-Arm Phase II Trial. Eur. J. Cancer 2019, 115, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Bang, Y.-J.; Lordick, F.; Alsina, M.; Chen, M.; Hack, S.P.; Bruey, J.M.; Smith, D.; McCaffery, I.; Shames, D.S.; et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol. 2017, 3, 620. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Ott, P.A.; Korytowsky, B.; Le, H.; Le, T.K.; Zhang, Y.; Maglinte, G.A.; Abraham, P.; Patel, D.; Shangguan, T.; et al. Real-World Treatment Patterns and Clinical Outcomes Across Lines of Therapy in Patients With Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer. Clin. Color. Cancer 2020, 19, 32–38.e3. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Cho, B.C.; Ahn, M.-J.; Kang, J.H.; Soo, R.A.; Reungwetwattana, T.; Yang, J.C.-H.; Cicin, I.; Kim, D.-W.; Wu, Y.-L.; Lu, S.; et al. Lazertinib Versus Gefitinib as First-Line Treatment in Patients With EGFR-Mutated Advanced Non–Small-Cell Lung Cancer: Results From LASER301. JCO 2023, 41, 4208–4217. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Shaw, A.T.; Riely, G.J.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer (NSCLC): Updated Results, Including Overall Survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef]
- Drilon, A.; Chiu, C.-H.; Fan, Y.; Cho, B.C.; Lu, S.; Ahn, M.-J.; Krebs, M.G.; Liu, S.V.; John, T.; Otterson, G.A.; et al. Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion–Positive NSCLC. JTO Clin. Res. Rep. 2022, 3, 100332. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Paik, P.K.; Garassino, M.C.; Le, X.; Sakai, H.; Veillon, R.; Smit, E.F.; Cortot, A.B.; Raskin, J.; Viteri, S.; et al. Tepotinib Treatment in Patients With MET Exon 14–Skipping Non–Small Cell Lung Cancer: Long-Term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Dy, G.K.; Govindan, R.; Velcheti, V.; Falchook, G.S.; Italiano, A.; Wolf, J.; Sacher, A.G.; Takahashi, T.; Ramalingam, S.S.; Dooms, C.; et al. Long-Term Outcomes and Molecular Correlates of Sotorasib Efficacy in Patients With Pretreated KRAS G12C-Mutated Non–Small-Cell Lung Cancer: 2-Year Analysis of CodeBreaK 100. JCO 2023, 41, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Yao, W.; Duruisseaux, M.; Doucet, L.; Azkárate Martínez, A.; Gregorc, V.; Juan-Vidal, O.; Lu, S.; De Bondt, C.; De Marinis, F.; et al. KRYSTAL-12: Phase 3 Study of Adagrasib versus Docetaxel in Patients with Previously Treated Advanced/Metastatic Non-Small Cell Lung Cancer (NSCLC) Harboring a KRAS G12C Mutation. JCO 2024, 42, LBA8509. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Huober, J.; Sohn, J.; Trédan, O.; Park, I.H.; Campone, M.; Chen, S.-C.; Manso, L.M.; Paluch-Shimon, S.; et al. Abemaciclib plus a Nonsteroidal Aromatase Inhibitor as Initial Therapy for HR+, HER2− Advanced Breast Cancer: Final Overall Survival Results of MONARCH 3. Ann. Oncol. 2024, 35, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Diéras, V.; Rugo, H.S.; Harbeck, N.; Im, S.-A.; Gelmon, K.A.; Lipatov, O.N.; Walshe, J.M.; Martin, M.; Chavez-MacGregor, M.; et al. Overall Survival With Palbociclib Plus Letrozole in Advanced Breast Cancer. JCO 2024, 42, 994–1000. [Google Scholar] [CrossRef]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus Exemestane for Hormone-Receptor-Positive, Human Epidermal Growth Factor Receptor-2-Negative Advanced Breast Cancer: Overall Survival Results from BOLERO-2. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef]
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, S.; Borad, M.; Gallinson, D.; Murphy, A.; et al. O-2 Pemigatinib for Previously Treated Locally Advanced or Metastatic Cholangiocarcinoma: Final Results from FIGHT-202. Ann. Oncol. 2022, 33, S379. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Borbath, I.; Roychowdhury, S.; Goyal, L.; Lamarca, A.; Macarulla, T.; Shroff, R.T.; Oh, D.-Y.; Javle, M.M.; Tamas, C.; et al. PROOF 301: Results of an Early Discontinued Randomized Phase 3 Trial of the Oral FGFR Inhibitor Infigratinib vs. Gemcitabine plus Cisplatin in Patients with Advanced Cholangiocarcinoma (CCA) with an FGFR2 Gene Fusion/Rearrangement. JCO 2024, 42, 516. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, Phase III Trial of Panitumumab With Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone As First-Line Treatment in Patients With Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. JCO 2010, 28, 4697–4705. [Google Scholar] [CrossRef]
- Cunningham, D.; Lang, I.; Marcuello, E.; Lorusso, V.; Ocvirk, J.; Shin, D.B.; Jonker, D.; Osborne, S.; Andre, N.; Waterkamp, D.; et al. Bevacizumab plus Capecitabine versus Capecitabine Alone in Elderly Patients with Previously Untreated Metastatic Colorectal Cancer (AVEX): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 1077–1085. [Google Scholar] [CrossRef]
- Robert, C.; Flaherty, K.; Nathan, P.; Hersey, P.; Garbe, C.; Milhem, M.; Demidov, L.; Mohr, P.; Hassel, J.C.; Rutkowski, P.; et al. Five-Year Outcomes from a Phase 3 METRIC Study in Patients with BRAF V600 E/K–Mutant Advanced or Metastatic Melanoma. Eur. J. Cancer 2019, 109, 61–69. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dréno, B.; Larkin, J.; Ribas, A.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. 5-Year Outcomes with Cobimetinib plus Vemurafenib in BRAF V600 Mutation–Positive Advanced Melanoma: Extended Follow-up of the coBRIM Study. Clin. Cancer Res. 2021, 27, 5225–5235. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab Monotherapy for Previously Treated Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma (REGARD): An International, Randomised, Multicentre, Placebo-Controlled, Phase 3 Trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in Patients with CLDN18.2-Positive, HER2-Negative, Untreated, Locally Advanced Unresectable or Metastatic Gastric or Gastro-Oesophageal Junction Adenocarcinoma (SPOTLIGHT): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef] [PubMed]

| Tumor | Molecular Alteration | Targeted Options | Validation Trial | Median OS (Months) | Median PFS (Months) | FDA Approval | ESCAT | Reference |
|---|---|---|---|---|---|---|---|---|
| NSCLC | EGFR ex 19 et 21 | Osimertinib | FLAURA | - | 8.7 | 18 April 2018 | IA | [188] |
| Lazertinib | LASER301 | - | 10.9 | - | [189] | |||
| EGFR ex20ins | Amivantamab + Lazertinib | MARIPOSA | - | 4.5 | - | IB | [97] | |
| ALK | Alectinib | ALEX | - | 23.9 | 6 November 2017 | IA | [190] | |
| ROS1 | Crizotinib | PROFILE 1001 | 51.4 | 19.3 | 11 March 2016 | IB | [191] | |
| Entrectinib | ALKA-372-001, STARTRK-1, and STARTRK-2 | 47.8 | 15.7 | 15 August 2019 | [192] | |||
| MET exon 14 | Capmatinib | GEOMETRY | 20.8 | 10.8 | 10 August 2022 | IB | [108] | |
| Tepotinib | VISION | 19.6 | 11.2 | 15 February 2024 | [193] | |||
| KRAS G12C | Sotorasib | CodeBreaK100 | 12.5 | 6.3 | 28 May 2021 | IB | [194] | |
| Adagrasib | KRYSTAL-12 | - | 5.49 | 12 December 2022 | [195] | |||
| RET | Selpercatinib | LIBRETTO001 | - | 16.5 | 21 September 2022 | IB | [196] | |
| Breast cancer | BRCA | Olaparib | OlympiAD | - | 7 | 12 January 2018 | IA | [63] |
| HER2 | Pertuzumab+ trastuzumab + docetaxel | CLEOPATRA | 56.5 | 18.7 | 8 June 2012 | [47] | ||
| Trastuzumab deruxtecan | DESTINY | 23.4 | 9.9 | 20 December 2019 | [197] | |||
| TDM1 | EMILIA1 | 30.9 | 9.6 | 22 February 2013 | [48] | |||
| CDK | Abemaciclib | MONARCH 3 | 66.8 | 29 | 28 September 2017 | IA | [198] | |
| Ribociclib | MONALEESA 2 | 63.9 | 25.3 | 18 July 2018 | [59] | |||
| Palbociclib | PALOMA | 53.9 | 24.8 | 19 February 2016 | [199] | |||
| PIK3CA | Alpelisib | SOLAR-1 | 39.3 | 11.1 | 24 May 2019 | IA | [61] | |
| mTor | Everolimus | BOLERO-21 | 31 | 7.8 | 20 June 2012 | IA | [200] | |
| Cholangiocarcinoma | IDH | Ivosidenib | ClarlDHy | 10.3 | 6.9 | 25 August 2021 | IA | [87] |
| FGFR2 mutation | Pemigatinib | FIGHT-202 | 17.5 | 7.0 | 17 April 2020 | IB | [201] | |
| Infigratinib | PROOF 301 | - | 7.4 | 28 May 2021 | [202] | |||
| Colon | EFGR | Cetuximab | BOND | 8.6 | 4.1 | 12 February 2004 | IA | [131] |
| Panitumumab (+FOLFOX) | PRIME 3 | 23.9 | 9.6 | 1 October 2006 | [203] | |||
| VEGF | Bevacizumab (+capecitabine) | AVEX | - | 9.1 | 26 February 2004 | IA | [204] | |
| Aflibercept (+FOLFIRI) | VELOUR | 13.5 | 6.9 | 3 August 2012 | [157] | |||
| Regorafenib | CORRECT | 6.4 | 1.9 | 27 September 2012 | [159] | |||
| Ramucirumab (+FOLFIRI) | RAISE | 13.3 | 5.7 | 24 April 2015 | [158] | |||
| BRAF/MEK | Encorafenib + Cetuximab | BEACON | 9.0 | 4.3 | 8 April 2020 | IA | [148] | |
| Melanoma | BRAF/MEK | Dabrafenib-trametinib | COMBI-D/COMBI-V (pooled analysis) | 25.9 | 11.1 | 9 January 2014 | IA | [205] |
| 25 November 2015 | ||||||||
| Vemurafenib-Cobimetinib | coBRIM | 22.5 | 12.6 | [206] | ||||
| Encorafenib-binimetinib | COLOMBUS | 36.8 | 14.9 | 27 June 2018 | [28] | |||
| c-KIT | Imatinib | Hodi et al. | 12.5 months | 3.7 months | - | N/A | [123] | |
| Gastric | HER2 | Trastuzumab (+chemotherapy) | ToGa | 13.8 | 6.7 | 21 October 2010 | IA | [168] |
| VEGF | Ramucirumab | REGARD | 5.2 | 2.1 | 21 April 2014 | N/A | [207] | |
| CLDN18.2 | Zolbetuximab | FAST | 18.23 | 10.61 | - | N/A | [208] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechahougui, H.; Gutmans, J.; Colarusso, G.; Gouasmi, R.; Friedlaender, A. Advances in Personalized Oncology. Cancers 2024, 16, 2862. https://doi.org/10.3390/cancers16162862
Mechahougui H, Gutmans J, Colarusso G, Gouasmi R, Friedlaender A. Advances in Personalized Oncology. Cancers. 2024; 16(16):2862. https://doi.org/10.3390/cancers16162862
Chicago/Turabian StyleMechahougui, Hiba, James Gutmans, Gina Colarusso, Roumaïssa Gouasmi, and Alex Friedlaender. 2024. "Advances in Personalized Oncology" Cancers 16, no. 16: 2862. https://doi.org/10.3390/cancers16162862
APA StyleMechahougui, H., Gutmans, J., Colarusso, G., Gouasmi, R., & Friedlaender, A. (2024). Advances in Personalized Oncology. Cancers, 16(16), 2862. https://doi.org/10.3390/cancers16162862





