Resistance to Anti-HER2 Therapies in Gastrointestinal Malignancies
Abstract
Simple Summary
Abstract
1. Introduction
2. HER2 Targeting Modalities
2.1. Monoclonal Antibodies
2.2. Tyrosine Kinase Inhibitors
2.3. Antibody–Drug Conjugates
2.4. Bispecific Antibodies
3. HER2 in GI Malignancies: Specific Insights into Different Gastrointestinal Cancers
3.1. Gastric and GEJ Adenocarcinoma
| Cancer Type | Stage | Treatment | Targeting Modalities | Indications | Trial |
|---|---|---|---|---|---|
| Gastric/GEJ | Unresectable, locally advanced or metastatic | Trastuzumab + Fluoropyrimidine (5-FU or capecitabine) + Cisplatin | Monoclonal antibody | 1st line | ToGA [20] |
| Unresectable, locally advanced or metastatic | Fam-trastuzumab deruxtecan-nxki | ADC | 2nd line | DESTINY-Gastric01 [70] | |
| Colorectal Cancer | Chemotherapy refractory RAS wild-type unresectable or metastatic | Tucatinib + trastuzumab | TKI (tucatinib), monoclonal antibody (trastuzumab) | 2nd line | MOUNTAINEER [72] |
| Unresectable or metastatic | Fam-trastuzumab deruxtecan-nxki | ADC | 3rd line | DESTINY-CRC01 [73] DESTINY-CRC02 [74] |
3.2. Colorectal Cancer
3.3. Biliary Tract Cancers
3.4. Small Bowel Adenocarcinoma
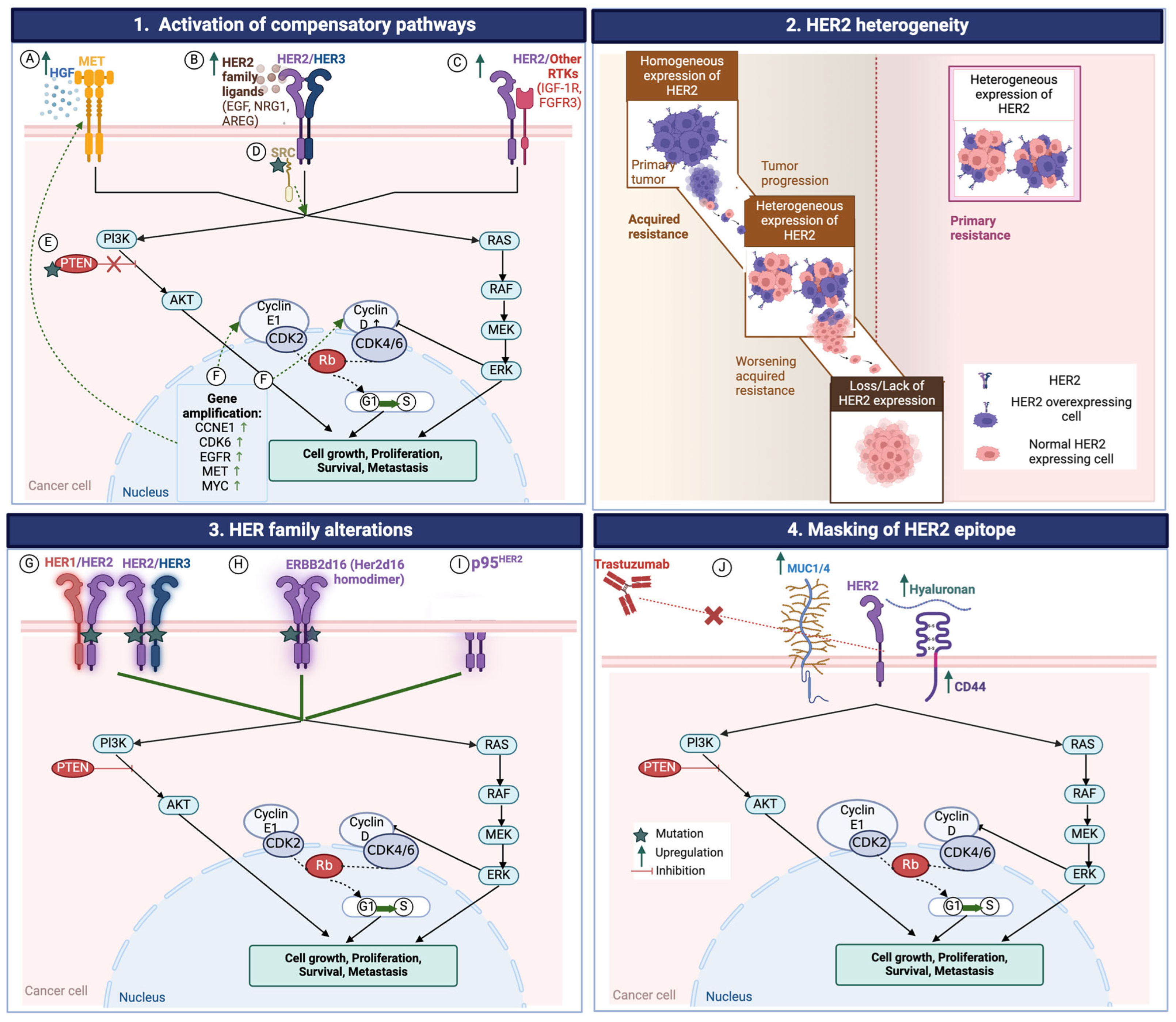
4. Resistance to HER2-Targeted Therapies
4.1. HER2 Heterogeneity
4.2. Loss of HER2 Expression
4.3. Changes in Intracellular Signaling
4.3.1. MET Amplification
4.3.2. PTEN Mutation
4.3.3. PIK3CA Mutation
4.3.4. Overexpression of Tyrosine Kinases and Their Ligands
4.3.5. Amplification of Cell-Cycle Mediators
4.3.6. Activation of Bypass Pathways
4.4. Epithelial to Mesenchymal Transition (EMT)
4.5. HER2 Receptor Variants
4.6. Masking of HER2 Epitopes
4.7. ADC-Specific Resistance Mechanisms
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ménard, S.; Pupa, S.M.; Campiglio, M.; Tagliabue, E. Biologic and therapeutic role of HER2 in cancer. Oncogene 2003, 22, 6570–6578. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Liao, X.F.; Zhang, J. Clinicopathological factors associated with HER2-positive gastric cancer: A meta-analysis. Medicine 2017, 96, e8437. [Google Scholar] [CrossRef]
- El-Deiry, W.S.; Vijayvergia, N.; Xiu, J.; Scicchitano, A.; Lim, B.; Yee, N.S.; Harvey, H.A.; Gatalica, Z.; Reddy, S. Molecular profiling of 6,892 colorectal cancer samples suggests different possible treatment options specific to metastatic sites. Cancer Biol. Ther. 2015, 16, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Ishige, K.; Thomas, M.; Yamashita-Kashima, Y.; Shu, S.; Ishikura, N.; Ariizumi, S.; Yamamoto, M.; Kurosaki, K.; Shoda, J. Overexpression and gene amplification of EGFR, HER2, and HER3 in biliary tract carcinomas, and the possibility for therapy with the HER2-targeting antibody pertuzumab. J. Gastroenterol. 2015, 50, 467–479. [Google Scholar] [CrossRef]
- Laforest, A.; Aparicio, T.; Zaanan, A.; Silva, F.P.; Didelot, A.; Desbeaux, A.; Le Corre, D.; Benhaim, L.; Pallier, K.; Aust, D.; et al. ERBB2 gene as a potential therapeutic target in small bowel adenocarcinoma. Eur. J. Cancer 2014, 50, 1740–1746. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Wang, Z. ErbB Receptors and Cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.Y.; Chipuk, J.E. Survival of HER2-Positive Breast Cancer Cells: Receptor Signaling to Apoptotic Control Centers. Genes Cancer 2013, 4, 187–195. [Google Scholar] [CrossRef]
- Albarello, L.; Pecciarini, L.; Doglioni, C. HER2 testing in gastric cancer. Adv. Anat. Pathol. 2011, 18, 53–59. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, H.J.; Yang, H.K.; Bang, Y.J.; Kim, W.H. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology 2011, 59, 822–831. [Google Scholar] [CrossRef]
- Hofmann, M.; Stoss, O.; Shi, D.; Büttner, R.; van de Vijver, M.; Kim, W.; Ochiai, A.; Rüschoff, J.; Henkel, T. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 2008, 52, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Abrahao-Machado, L.F.; Scapulatempo-Neto, C. HER2 testing in gastric cancer: An update. World J. Gastroenterol. 2016, 22, 4619–4625. [Google Scholar] [CrossRef]
- Sharaf, B.; Abu-Fares, H.; Tamimi, F.; Al-Sawajneh, S.; Salama, O.; Daoud, R.; Alhajahjeh, A.; Al-Lababidi, S.; Abdel-Razeq, H. Differences in Treatment Outcomes Between Patients with HER2-Low versus HER2-Zero, Hormone Receptor-Positive Advanced-Stage Breast Cancer Treated with Ribociclib. Breast Cancer 2023, 15, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yamaguchi, Y.; Inukai, N.; Okamoto, S.; Mura, T.; Handa, H. P-TEFb-mediated phosphorylation of hSpt5 C-terminal repeats is critical for processive transcription elongation. Mol. Cell 2006, 21, 227–237. [Google Scholar] [CrossRef]
- Cenaj, O.; Ligon, A.H.; Hornick, J.L.; Sholl, L.M. Detection of ERBB2 Amplification by Next-Generation Sequencing Predicts HER2 Expression in Colorectal Carcinoma. Am. J. Clin. Pathol. 2019, 152, 97–108. [Google Scholar] [CrossRef]
- Takegawa, N.; Yonesaka, K.; Sakai, K.; Ueda, H.; Watanabe, S.; Nonagase, Y.; Okuno, T.; Takeda, M.; Maenishi, O.; Tsurutani, J.; et al. HER2 genomic amplification in circulating tumor DNA from patients with cetuximab-resistant colorectal cancer. Oncotarget 2016, 7, 3453–3460. [Google Scholar] [CrossRef]
- Niu, D.; Li, L.; Yu, Y.; Zang, W.; Li, Z.; Zhou, L.; Jia, L.; Rao, G.; Gao, L.; Cheng, G.; et al. Evaluation of Next Generation Sequencing for Detecting HER2 Copy Number in Breast and Gastric Cancers. Pathol. Oncol. Res. 2020, 26, 2577–2585. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat. Med. 2021, 27, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Pavlick, D.; Klempner, S.J.; Chung, J.H.; Forcier, B.; Welsh, A.; Young, L.; Leyland-Jones, B.; Bordoni, R.; Carvajal, R.D.; et al. Hybrid Capture-Based Genomic Profiling of Circulating Tumor DNA from Patients with Advanced Cancers of the Gastrointestinal Tract or Anus. Clin. Cancer Res. 2018, 24, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Ricotta, R.; Verrioli, A.; Ghezzi, S.; Porcu, L.; Grothey, A.; Falcone, A.; Van Cutsem, E.; Argiles, G.; Adenis, A.; Ychou, M.; et al. Radiological imaging markers predicting clinical outcome in patients with metastatic colorectal carcinoma treated with regorafenib: Post hoc analysis of the CORRECT phase III trial (RadioCORRECT study). ESMO Open 2016, 1, e000111. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Cercek, A.; Siena, S.; Andre, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.S.; Hubbard, J.M.; Coveler, A.L.; et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Arnould, L.; Gelly, M.; Penault-Llorca, F.; Benoit, L.; Bonnetain, F.; Migeon, C.; Cabaret, V.; Fermeaux, V.; Bertheau, P.; Garnier, J.; et al. Trastuzumab-based treatment of HER2-positive breast cancer: An antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer 2006, 94, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Varchetta, S.; Gibelli, N.; Oliviero, B.; Nardini, E.; Gennari, R.; Gatti, G.; Silva, L.S.; Villani, L.; Tagliabue, E.; Menard, S.; et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007, 67, 11991–11999. [Google Scholar] [CrossRef] [PubMed]
- Petricevic, B.; Laengle, J.; Singer, J.; Sachet, M.; Fazekas, J.; Steger, G.; Bartsch, R.; Jensen-Jarolim, E.; Bergmann, M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J. Transl. Med. 2013, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Xu, L.; di Tomaso, E.; Fukumura, D.; Jain, R.K. Tumour biology: Herceptin acts as an anti-angiogenic cocktail. Nature 2002, 416, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Albanell, J.; Baselga, J. Unraveling resistance to trastuzumab (Herceptin): Insulin-like growth factor-I receptor, a new suspect. J. Natl. Cancer Inst. 2001, 93, 1830–1832. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Albanell, J.; Molina, M.A.; Arribas, J. Mechanism of action of trastuzumab and scientific update. Semin. Oncol. 2001, 28 (Suppl. S16), 4–11. [Google Scholar] [CrossRef]
- Nami, B.; Maadi, H.; Wang, Z. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers 2018, 10, 342. [Google Scholar] [CrossRef]
- Spector, N.L.; Blackwell, K.L. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2009, 27, 5838–5847. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, M.; King, P.; Perera, T.; Parker, P.J.; Harris, A.L.; Larijani, B.; Kong, A. HER2 phosphorylation is maintained by a PKB negative feedback loop in response to anti-HER2 herceptin in breast cancer. PLoS Biol. 2010, 8, e1000563. [Google Scholar] [CrossRef] [PubMed]
- Cuello, M.; Ettenberg, S.A.; Clark, A.S.; Keane, M.M.; Posner, R.H.; Nau, M.M.; Dennis, P.A.; Lipkowitz, S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001, 61, 4892–4900. [Google Scholar] [PubMed]
- Valabrega, G.; Montemurro, F.; Sarotto, I.; Petrelli, A.; Rubini, P.; Tacchetti, C.; Aglietta, M.; Comoglio, P.M.; Giordano, S. TGFalpha expression impairs Trastuzumab-induced HER2 downregulation. Oncogene 2005, 24, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Pietras, R.J.; Fendly, B.M.; Chazin, V.R.; Pegram, M.D.; Howell, S.B.; Slamon, D.J. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 1994, 9, 1829–1838. [Google Scholar] [PubMed]
- Pietras, R.J.; Pegram, M.D.; Finn, R.S.; Maneval, D.A.; Slamon, D.J. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 1998, 17, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Pietras, R.J.; Poen, J.C.; Gallardo, D.; Wongvipat, P.N.; Lee, H.J.; Slamon, D.J. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res. 1999, 59, 1347–1355. [Google Scholar] [PubMed]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Giaccone, G.; Im, S.A.; Oh, D.Y.; Bauer, T.M.; Nordstrom, J.L.; Li, H.; Chichili, G.R.; Moore, P.A.; Hong, S.; et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 2017, 28, 855–861. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortes, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Bachelot, T.; Wright, G.S.; Saura, C.; et al. Margetuximab Versus Trastuzumab in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results from a Randomized Phase 3 Trial. J. Clin. Oncol. 2023, 41, 198–205. [Google Scholar] [CrossRef]
- Nordstrom, J.L.; Gorlatov, S.; Zhang, W.; Yang, Y.; Huang, L.; Burke, S.; Li, H.; Ciccarone, V.; Zhang, T.; Stavenhagen, J.; et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011, 13, R123. [Google Scholar] [CrossRef] [PubMed]
- Stavenhagen, J.B.; Gorlatov, S.; Tuaillon, N.; Rankin, C.T.; Li, H.; Burke, S.; Huang, L.; Vijh, S.; Johnson, S.; Bonvini, E.; et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007, 67, 8882–8890. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.; Rosales, M.; Chung, H.C.; Shen, H.L.; Moehler, M.; Kang, Y.K. MAHOGANY: Margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021, 17, 1155–1164. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, D.W.; Lackey, K.; Affleck, K.; Wood, E.R.; Alligood, K.J.; Rhodes, N.; Keith, B.R.; Murray, D.M.; Knight, W.B.; Mullin, R.J.; et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer Ther. 2001, 1, 85–94. [Google Scholar] [PubMed]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; de Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef] [PubMed]
- A Phase 2 Basket Study of Tucatinib in Combination with Trastuzumab in Subjects with Previously Treated, Locally Advanced Unresectable or Metastatic Solid Tumors Driven by HER2 Alterations. 2020. Available online: https://clinicaltrials.gov/study/NCT04579380 (accessed on 4 May 2024).
- Yin, Y.; Yang, H.; Liu, Z.; Tan, J.; Zhu, C.; Chen, M.; Zhou, R.; Wang, L.; Qian, J. Studies on the Safety and Efficacy of Pyrotinib in the Treatment of HER2- Positive Advanced Solid Tumors Excluding Breast Cancer. Cancer Manag. Res. 2020, 12, 13479–13487. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kou, F.; Gong, J.; Wang, Z.; Zhang, X.; Li, J.; Li, Y.; Li, J.; Zhou, J.; Lu, M.; et al. Pyrotinib alone or in combination with docetaxel in refractory HER2-positive gastric cancer: A dose-escalation phase I study. Cancer Med. 2023, 12, 10704–10714. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Xu, M.; Wang, C.; Huang, D.; Zhang, Z.; Chen, Z.; Zhu, X.; Li, W. Dual HER2 Targeted Therapy With Pyrotinib and Trastuzumab in Refractory HER2 Positive Metastatic Colorectal Cancer: A Result From HER2-FUSCC-G Study. Clin. Color. Cancer 2022, 21, 347–353. [Google Scholar] [CrossRef]
- Kostova, V.; Désos, P.; Starck, J.B.; Kotschy, A. The Chemistry Behind ADCs. Pharmaceuticals 2021, 14, 442. [Google Scholar] [CrossRef]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nakagawa, K.; Tsurutani, J. DS-8201a, a new HER2-targeting antibody–drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer 2017, 141, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- A Phase 2, Multicenter, Open-Label Study to Evaluate the Efficacy and Safety of Trastuzumab Deruxtecan (T-DXd, DS-8201a) for the Treatment of Selected HER2 Expressing Tumors (DESTINY-PanTumor02). L. Daiichi Sankyo Co. 2020. Available online: https://www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/clinical-trials-index/clinical-trials-detail.ID2020-0654.html (accessed on 4 May 2024).
- AstraZeneca. A Phase 2, Multicenter, Randomized, Study of Trastuzumab Deruxtecan in Participants with HER2-Overexpressing Locally Advanced, Unresectable or Metastatic Colorectal Cancer (DESTINY-CRC02). 2021. Available online: https://jrct.niph.go.jp/en-latest-detail/jRCT2051200124 (accessed on 4 May 2024).
- Randomized, Controlled, Multicenter Phase III Clinical Study Evaluating the Efficacy and Safety of RC48-ADC for the Treatment of Locally Advanced or Metastatic Gastric Cancer with HER2-overexpression. 2021. Available online: https://clinicaltrials.gov/study/NCT04714190 (accessed on 4 May 2024).
- Madsen, A.V.; Pedersen, L.E.; Kristensen, P.; Goletz, S. Design and engineering of bispecific antibodies: Insights and practical considerations. Front. Bioeng. Biotechnol. 2024, 12, 1352014. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, X.; Wei, X.; Tang, C.; Zhang, W. HER2-targeted therapies in gastric cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188549. [Google Scholar] [CrossRef] [PubMed]
- Phase 2 Study of ZW25 Plus First-Line Combination Chemotherapy in HER2-Expressing Gastrointestinal (GI) Cancers, Including Gastroesophageal Adenocarcinoma (GEA), Biliary Tract Cancer (BTC), and Colorectal Cancer (CRC). 2019. Available online: https://adisinsight.springer.com/trials/700306473 (accessed on 4 May 2024).
- Phase 1b/2 Study Investigating Safety, Tolerability, Pharmacokinetics and Preliminary Antitumor Activity of Anti-HER2 Bispecific Antibody ZW25 in Combination with Chemotherapy with/without Tislelizumab in Patients with Advanced HER2-positive Breast Cancer or Gastric/Gastroesophageal Junction Adenocarcinoma. 2020. Available online: https://www.medifind.com/articles/clinical-trial/217275992 (accessed on 4 May 2024).
- An Open-label Randomized Trial of the Efficacy and Safety of Zanidatamab with Standard-of-care Therapy against Standard-of-care Therapy Alone for Advanced HER2-positive Biliary Tract Cancer. 2024. Available online: https://clinicaltrials.gov/study/NCT06282575 (accessed on 4 May 2024).
- Van Cutsem, E.; Bang, Y.J.; Feng-Yi, F.; Xu, J.M.; Lee, K.W.; Jiao, S.C.; Chong, J.L.; López-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, M.; Li, X.; Song, S.; Liu, N.; Du, H.; Ye, J.; Li, H.; Zhang, Z.; Zhang, L. HER2 copy number as predictor of disease-free survival in HER2-positive resectable gastric adenocarcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Makiyama, A.; Sukawa, Y.; Kashiwada, T.; Kawada, J.; Hosokawa, A.; Horie, Y.; Tsuji, A.; Moriwaki, T.; Tanioka, H.; Shinozaki, K.; et al. Randomized, Phase II Study of Trastuzumab Beyond Progression in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction Cancer: WJOG7112G (T-ACT Study). J. Clin. Oncol. 2020, 38, 1919–1927. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef]
- Satoh, T.; Xu, R.H.; Chung, H.C.; Sun, G.P.; Doi, T.; Xu, J.M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.W.; et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—A randomized, phase III study. J. Clin. Oncol. 2014, 32, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Catenacci, D.V. MOUNTAINEER-02: Phase 2/3 Study of Tucatinib, Trastuzumab, Ramucirumab, and Paclitaxel in Previously Treated HER2+ Gastric or Gastroesophageal Junction Adenocarcinoma—Trial in Progress. 2022. Available online: https://ascopubs.org/doi/10.1200/JCO.2022.40.4_suppl.TPS371 (accessed on 16 May 2024).
- Casak, S.J.; Horiba, M.N.; Yuan, M.; Cheng, J.; Lemery, S.J.; Shen, Y.L.; Fu, W.; Moore, J.N.; Li, Y.; Bi, Y.; et al. FDA Approval Summary: Tucatinib with Trastuzumab for Advanced Unresectable or Metastatic, Chemotherapy Refractory, HER2-Positive RAS Wild-Type Colorectal Cancer. Clin. Cancer Res. 2023, 29, 4326–4330. [Google Scholar] [CrossRef]
- Yoshino, T.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Wainberg, Z.; Elez, E.; et al. Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat. Commun. 2023, 14, 3332. [Google Scholar] [CrossRef]
- Raghav, K.P.S. Trastuzumab Deruxtecan (T-DXd) in Patients (pts) with HER2-Overexpressing/Amplified (HER2+) Metastatic Colorectal Cancer (mCRC): Primary Results from the Multicenter, Randomized, Phase 2 DESTINY-CRC02 Study. 2023. Available online: https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.3501 (accessed on 13 May 2024).
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef] [PubMed]
- Raghav, K.; Loree, J.M.; Morris, J.S.; Overman, M.J.; Yu, R.; Meric-Bernstam, F.; Menter, D.; Korphaisarn, K.; Kee, B.; Muranyi, A.; et al. Validation of HER2 Amplification as a Predictive Biomarker for Anti-Epidermal Growth Factor Receptor Antibody Therapy in Metastatic Colorectal Cancer. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S. MOUNTAINEER-03: Phase 3 Study of Tucatinib, Trastuzumab, and mFOLFOX6 as First-Line Treatment in HER2+ Metastatic Colorectal Cancer—Trial in Progress. 2023. Available online: https://ascopubs.org/doi/10.1200/JCO.2023.41.4_suppl.TPS261 (accessed on 13 May 2024).
- Sweeney, C.J.; Hainsworth, J.D.; Bose, R.; Burris, H.A.; Kurzrock, R.; Swanton, C.; Friedman, C.F.; Spigel, D.R.; Szado, T.; Schulze, K.; et al. MyPathway Human Epidermal Growth Factor Receptor 2 Basket Study: Pertuzumab + Trastuzumab Treatment of a Tissue-Agnostic Cohort of Patients with Human Epidermal Growth Factor Receptor 2-Altered Advanced Solid Tumors. J. Clin. Oncol. 2024, 42, 258–265. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Jacobi, O.; Ross, J.S.; Goshen-Lago, T.; Haddad, R.; Moore, A.; Sulkes, A.; Brenner, B.; Ben-Aharon, I. ERBB2 Pathway in Biliary Tract Carcinoma: Clinical Implications of a Targetable Pathway. Oncol. Res. Treat. 2021, 44, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ten Haaft, B.H.; Pedregal, M.; Prato, J.; Klümpen, H.J.; Moreno, V.; Lamarca, A. Revolutionizing anti-HER2 therapies for extrahepatic cholangiocarcinoma and gallbladder cancer: Current advancements and future perspectives. Eur. J. Cancer 2024, 199, 113564. [Google Scholar] [CrossRef]
- Ostwal, V.; Mandavkar, S.; Bhargava, P.; Srinivas, S.; Kapoor, A.; Shetty, O.; Kannan, S.; Chaugule, D.; Patil, R.; Parulekar, M.; et al. Trastuzumab Plus Gemcitabine-Cisplatin for Treatment-Naïve Human Epidermal Growth Factor Receptor 2-Positive Biliary Tract Adenocarcinoma: A Multicenter, Open-Label, Phase II Study (TAB). J. Clin. Oncol. 2024, 42, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Braga, V.M.; de Oliveira, M.B.; Netto, C.C.; Ibrahim, R.E.; Peixoto, R.D. Human Epidermal Growth Factor Receptor 2-Positive Duodenal Adenocarcinoma: A Case Report and Review of the Literature. Case Rep. Oncol. 2015, 8, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Shan, G.D.; Zhang, H.; Li, L.; Yue, M.; Xiang, Z.; Cheng, Y.; Wu, C.J.; Fang, Y.; Chen, L.H. Double-balloon enteroscopy in small bowel tumors: A Chinese single-center study. World J. Gastroenterol. 2013, 19, 3665–3671. [Google Scholar] [CrossRef]
- Overman, M.J.; Pozadzides, J.; Kopetz, S.; Wen, S.; Abbruzzese, J.L.; Wolff, R.A.; Wang, H. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br. J. Cancer 2010, 102, 144–150. [Google Scholar] [CrossRef]
- Chan, O.T.; Chen, Z.M.; Chung, F.; Kawachi, K.; Phan, D.C.; Himmelfarb, E.; Lin, F.; Perry, A.; Wang, H.L. Lack of HER2 overexpression and amplification in small intestinal adenocarcinoma. Am. J. Clin. Pathol. 2010, 134, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, W.; Wei, Y.; An, L.; Su, S.; Xi, C.; Wang, K.; Hong, D.; Shi, Y. A HER2-mutant patient with late-stage duodenal adenocarcinoma benefited from anti-HER2 therapy and PD-1 inhibition: A case report. J. Gastrointest. Oncol. 2021, 12, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Wang, Y.; Wu, D.; Lin, E.; Xia, Q. Intratumoral and intertumoral heterogeneity of HER2 immunohistochemical expression in gastric cancer. Pathol. Res. Pract. 2020, 216, 153229. [Google Scholar] [CrossRef]
- Yagi, S.; Wakatsuki, T.; Yamamoto, N.; Chin, K.; Takahari, D.; Ogura, M.; Ichimura, T.; Nakayama, I.; Osumi, H.; Shinozaki, E.; et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer 2019, 22, 518–525. [Google Scholar] [CrossRef]
- Grillo, F.; Fassan, M.; Sarocchi, F.; Fiocca, R.; Mastracci, L. HER2 heterogeneity in gastric/gastroesophageal cancers: From benchside to practice. World J. Gastroenterol. 2016, 22, 5879–5887. [Google Scholar] [CrossRef]
- Park, S.R.; Park, Y.S.; Ryu, M.H.; Ryoo, B.Y.; Woo, C.G.; Jung, H.Y.; Lee, J.H.; Lee, G.H.; Kang, Y.K. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: Results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur. J. Cancer 2016, 53, 42–50. [Google Scholar] [CrossRef]
- Bartley, A.N.; Washington, M.K.; Colasacco, C.; Ventura, C.B.; Ismaila, N.; III, A.B.B.; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 446–464. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up☆. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Gullo, I.; Grillo, F.; Molinaro, L.; Fassan, M.; De Silvestri, A.; Tinelli, C.; Rugge, M.; Fiocca, R.; Mastracci, L. Minimum biopsy set for HER2 evaluation in gastric and gastro-esophageal junction cancer. Endosc. Int. Open 2015, 3, E165–E170. [Google Scholar] [CrossRef]
- Tominaga, N.; Gotoda, T.; Hara, M.; Hale, M.D.; Tsuchiya, T.; Matsubayashi, J.; Kono, S.; Kusano, C.; Itoi, T.; Fujimoto, K.; et al. Five biopsy specimens from the proximal part of the tumor reliably determine HER2 protein expression status in gastric cancer. Gastric Cancer 2016, 19, 553–560. [Google Scholar] [CrossRef]
- Kanayama, K.; Imai, H.; Yoneda, M.; Hirokawa, Y.S.; Shiraishi, T. Significant intratumoral heterogeneity of human epidermal growth factor receptor 2 status in gastric cancer: A comparative study of immunohistochemistry, FISH, and dual-color in situ hybridization. Cancer Sci. 2016, 107, 536–542. [Google Scholar] [CrossRef]
- Wang, T.; Hsieh, E.T.; Henry, P.; Hanna, W.; Streutker, C.J.; Grin, A. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum. Pathol. 2014, 45, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; de Boer, W.B.; Fermoyle, S.; Platten, M.; Kumarasinghe, M.P. Human epidermal growth factor receptor 2 testing in gastric carcinoma: Issues related to heterogeneity in biopsies and resections. Histopathology 2011, 59, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Takayanagi, D.; Yonemaru, J.; Naka, T.; Nagashima, K.; Machida, E.; Kohno, T.; Yatabe, Y.; Kanemitsu, Y.; Hamamoto, R.; et al. A comprehensive appraisal of HER2 heterogeneity in HER2-amplified and HER2-low colorectal cancer. Br. J. Cancer 2023, 129, 1176–1183. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Yamamoto, N.; Sano, T.; Chin, K.; Kawachi, H.; Takahari, D.; Ogura, M.; Ichimura, T.; Nakayama, I.; Osumi, H.; et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J. Gastroenterol. 2018, 53, 1186–1195. [Google Scholar] [CrossRef]
- Bang, K.; Cheon, J.; Park, Y.S.; Kim, H.D.; Ryu, M.H.; Park, Y.; Moon, M.; Lee, H.; Kang, Y.K. Association between HER2 heterogeneity and clinical outcomes of HER2-positive gastric cancer patients treated with trastuzumab. Gastric Cancer 2022, 25, 794–803. [Google Scholar] [CrossRef]
- Nagatsuma, A.K.; Aizawa, M.; Kuwata, T.; Doi, T.; Ohtsu, A.; Fujii, H.; Ochiai, A. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 2015, 18, 227–238. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Caporale, M.; Morano, F.; Scartozzi, M.; Gloghini, A.; De Vita, F.; Giommoni, E.; Fornaro, L.; Aprile, G.; Melisi, D.; et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int. J. Cancer 2016, 139, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Sanchez-Vega, F.; Jonsson, P.; Chatila, W.K.; Hechtman, J.F.; Ku, G.Y.; Riches, J.C.; Tuvy, Y.; Kundra, R.; Bouvier, N.; et al. Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer Discov. 2018, 8, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Ryu, M.H.; Park, Y.S.; Ahn, J.Y.; Park, Y.; Park, S.R.; Ryoo, B.Y.; Lee, G.H.; Jung, H.Y.; Kang, Y.K. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: Results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer 2019, 22, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Oki, E.; Kashiwada, T.; Arigami, T.; Makiyama, A.; Iwatsuki, M.; Narita, Y.; Satake, H.; Matsuda, Y.; Sonoda, H.; et al. Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur. J. Cancer 2018, 105, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Fox, C.; Peng, S.; Pusung, M.; Pectasides, E.; Matthee, E.; Hong, Y.S.; Do, I.G.; Jang, J.; Thorner, A.R.; et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J. Clin. Investig. 2014, 124, 5145–5158. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Park, Y.; Nam, S.K.; Kang, E.; Kim, K.K.; Jeong, I.; Kwak, Y.; Yoon, J.; Kim, T.Y.; Lee, K.W.; et al. Genetic and immune microenvironment characterization of HER2-positive gastric cancer: Their association with response to trastuzumab-based treatment. Cancer Med. 2023, 12, 10371–10384. [Google Scholar] [CrossRef] [PubMed]
- Kubicka, S.; Claas, C.; Staab, S.; Kühnel, F.; Zender, L.; Trautwein, C.; Wagner, S.; Rudolph, K.L.; Manns, M. p53 mutation pattern and expression of c-erbB2 and c-met in gastric cancer: Relation to histological subtypes, Helicobacter pylori infection, and prognosis. Dig. Dis. Sci. 2002, 47, 114–121. [Google Scholar] [CrossRef]
- Nakajima, M.; Sawada, H.; Yamada, Y.; Watanabe, A.; Tatsumi, M.; Yamashita, J.; Matsuda, M.; Sakaguchi, T.; Hirao, T.; Nakano, H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 1999, 85, 1894–1902. [Google Scholar] [CrossRef]
- Ha, S.Y.; Lee, J.; Jang, J.; Hong, J.Y.; Do, I.G.; Park, S.H.; Park, J.O.; Choi, M.G.; Sohn, T.S.; Bae, J.M.; et al. HER2-positive gastric cancer with concomitant MET and/or EGFR overexpression: A distinct subset of patients for dual inhibition therapy. Int. J. Cancer 2015, 136, 1629–1635. [Google Scholar] [CrossRef]
- Takahashi, N.; Furuta, K.; Taniguchi, H.; Sasaki, Y.; Shoji, H.; Honma, Y.; Iwasa, S.; Okita, N.; Takashima, A.; Kato, K.; et al. Serum level of hepatocyte growth factor is a novel marker of predicting the outcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancer. Oncotarget 2016, 7, 4925–4938. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Kim, H.; Liska, D.; Gao, S.; Christensen, J.G.; Weiser, M.R. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol. Cancer Ther. 2012, 11, 660–669. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Kim, H.P.; Kang, M.J.; Cho, B.K.; Han, S.W.; Kim, T.Y.; Yi, E.C. Phosphoproteomic analysis identifies activated MET-axis PI3K/AKT and MAPK/ERK in lapatinib-resistant cancer cell line. Exp. Mol. Med. 2013, 45, e64. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Shien, K.; Takeda, T.; Takahashi, Y.; Kurihara, E.; Ogoshi, Y.; Namba, K.; Torigoe, H.; Sato, H.; Tomida, S.; et al. Acquired resistance mechanisms to afatinib in HER2-amplified gastric cancer cells. Cancer Sci. 2019, 110, 2549–2557. [Google Scholar] [CrossRef]
- Ebert, K.; Mattes, J.; Kunzke, T.; Zwingenberger, G.; Luber, B. MET as resistance factor for afatinib therapy and motility driver in gastric cancer cells. PLoS ONE 2019, 14, e0223225. [Google Scholar] [CrossRef]
- Hlobilková, A.; Knillová, J.; Bártek, J.; Lukás, J.; Kolár, Z. The mechanism of action of the tumour suppressor gene PTEN. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2003, 147, 19–25. [Google Scholar] [CrossRef]
- Zhang, X.; Park, J.S.; Park, K.H.; Kim, K.H.; Jung, M.; Chung, H.C.; Rha, S.Y.; Kim, H.S. PTEN deficiency as a predictive biomarker of resistance to HER2-targeted therapy in advanced gastric cancer. Oncology 2015, 88, 76–85. [Google Scholar] [CrossRef]
- Yokoyama, D.; Hisamori, S.; Deguchi, Y.; Nishigori, T.; Okabe, H.; Kanaya, S.; Manaka, D.; Kadokawa, Y.; Hata, H.; Minamiguchi, S.; et al. PTEN is a predictive biomarker of trastuzumab resistance and prognostic factor in HER2-overexpressing gastroesophageal adenocarcinoma. Sci. Rep. 2021, 11, 9013. [Google Scholar] [CrossRef]
- Kim, C.; Lee, C.K.; Chon, H.J.; Kim, J.H.; Park, H.S.; Heo, S.J.; Kim, H.J.; Kim, T.S.; Kwon, W.S.; Chung, H.C.; et al. PTEN loss and level of HER2 amplification is associated with trastuzumab resistance and prognosis in HER2-positive gastric cancer. Oncotarget 2017, 8, 113494–113501. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Liu, J.; Zhang, J.; Wu, M.; Guo, L.; Liao, W. Development of trastuzumab-resistant human gastric carcinoma cell lines and mechanisms of drug resistance. Sci. Rep. 2015, 5, 11634. [Google Scholar] [CrossRef]
- Deguchi, Y.; Okabe, H.; Oshima, N.; Hisamori, S.; Minamiguchi, S.; Muto, M.; Sakai, Y. PTEN loss is associated with a poor response to trastuzumab in HER2-overexpressing gastroesophageal adenocarcinoma. Gastric Cancer 2017, 20, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Mina, S.; Bohn, B.A.; Simon, R.; Krohn, A.; Reeh, M.; Arnold, D.; Bokemeyer, C.; Sauter, G.; Izbicki, J.R.; Marx, A.; et al. PTEN deletion is rare but often homogeneous in gastric cancer. J. Clin. Pathol. 2012, 65, 693–698. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, W.; Zhang, W.; Chen, Y.; Shen, H.; Zhu, H.; Liang, X.; Shen, Z. Tracking of trastuzumab resistance in patients with HER2-positive metastatic gastric cancer by CTC liquid biopsy. Am. J. Cancer Res. 2023, 13, 5684–5697. [Google Scholar]
- Wang, D.S.; Liu, Z.X.; Lu, Y.X.; Bao, H.; Wu, X.; Zeng, Z.L.; Liu, Z.; Zhao, Q.; He, C.Y.; Lu, J.H.; et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 2019, 68, 1152–1161. [Google Scholar] [CrossRef]
- Siravegna, G.; Lazzari, L.; Crisafulli, G.; Sartore-Bianchi, A.; Mussolin, B.; Cassingena, A.; Martino, C.; Lanman, R.B.; Nagy, R.J.; Fairclough, S.; et al. Radiologic and Genomic Evolution of Individual Metastases during HER2 Blockade in Colorectal Cancer. Cancer Cell 2018, 34, 148–162.e7. [Google Scholar] [CrossRef] [PubMed]
- Ebbing, E.A.; Medema, J.P.; Damhofer, H.; Meijer, S.L.; Krishnadath, K.K.; van Berge Henegouwen, M.I.; Bijlsma, M.F.; van Laarhoven, H.W. ADAM10-mediated release of heregulin confers resistance to trastuzumab by activating HER3. Oncotarget 2016, 7, 10243–10254. [Google Scholar] [CrossRef]
- Sampera, A.; Sánchez-Martín, F.J.; Arpí, O.; Visa, L.; Iglesias, M.; Menéndez, S.; Gaye, É.; Dalmases, A.; Clavé, S.; Gelabert-Baldrich, M.; et al. HER-Family Ligands Promote Acquired Resistance to Trastuzumab in Gastric Cancer. Mol. Cancer Ther. 2019, 18, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yashiro, M.; Takakura, N. Heregulin induces resistance to lapatinib-mediated growth inhibition of HER2-amplified cancer cells. Cancer Sci. 2013, 104, 1618–1625. [Google Scholar] [CrossRef]
- Cheng, H.; Terai, M.; Kageyama, K.; Ozaki, S.; McCue, P.A.; Sato, T.; Aplin, A.E. Paracrine Effect of NRG1 and HGF Drives Resistance to MEK Inhibitors in Metastatic Uveal Melanoma. Cancer Res. 2015, 75, 2737–2748. [Google Scholar] [CrossRef]
- Piro, G.; Carbone, C.; Cataldo, I.; Di Nicolantonio, F.; Giacopuzzi, S.; Aprile, G.; Simionato, F.; Boschi, F.; Zanotto, M.; Mina, M.M.; et al. An FGFR3 Autocrine Loop Sustains Acquired Resistance to Trastuzumab in Gastric Cancer Patients. Clin. Cancer Res. 2016, 22, 6164–6175. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ren, F.; Tang, R.; Feng, Z.; Chen, G. Prognostic Value of Expression of Cyclin E in Gastrointestinal Cancer: A Systematic Review and Meta-Analysis. Technol. Cancer Res. Treat. 2016, 15, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Banks, K.C.; Pectasides, E.; Kim, S.Y.; Kim, K.; Lanman, R.B.; Talasaz, A.; An, J.; Choi, M.G.; Lee, J.H.; et al. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann. Oncol. 2018, 29, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hong, M.; Kim, S.T.; Park, S.H.; Kang, W.K.; Kim, K.M.; Lee, J. The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive gastric cancer. Sci. Rep. 2015, 5, 9289. [Google Scholar] [CrossRef] [PubMed]
- Frame, M.C. Src in cancer: Deregulation and consequences for cell behaviour. Biochim. Biophys. Acta 2002, 1602, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.M. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene 2004, 23, 8017–8023. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.H.; Nam, A.R.; Park, J.E.; Bang, J.H.; Bang, Y.J.; Oh, D.Y. Resistance Mechanism against Trastuzumab in HER2-Positive Cancer Cells and Its Negation by Src Inhibition. Mol. Cancer Ther. 2017, 16, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Rojo de la Vega, M.; Chapman, E.; Ooi, A.; Zhang, D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 2018, 57, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Long, M.; Huang, Y.; Zhang, L.; Zhang, R.; Zheng, Y.; Liao, X.; Wang, Y.; Liao, Q.; et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016, 8, 334ra351. [Google Scholar] [CrossRef]
- Gambardella, V.; Gimeno-Valiente, F.; Tarazona, N.; Martinez-Ciarpaglini, C.; Roda, D.; Fleitas, T.; Tolosa, P.; Cejalvo, J.M.; Huerta, M.; Roselló, S.; et al. NRF2 through RPS6 Activation Is Related to Anti-HER2 Drug Resistance in HER2-Amplified Gastric Cancer. Clin. Cancer Res. 2019, 25, 1639–1649. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Ko, Y.S.; Kim, Y.; Pyo, J.S.; Jang, B.G.; Kim, M.A.; Lee, J.S.; Chang, M.S.; Park, J.W.; et al. FOXO1 Suppression is a Determinant of Acquired Lapatinib-Resistance in HER2-Positive Gastric Cancer Cells Through MET Upregulation. Cancer Res. Treat. 2018, 50, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.H.; Kuo, C.C.; Lin, B.X.; Huang, Y.H.; Lin, C.W. Elevation of YAP promotes the epithelial-mesenchymal transition and tumor aggressiveness in colorectal cancer. Exp. Cell Res. 2017, 350, 218–225. [Google Scholar] [CrossRef]
- Kim, H.P.; Han, S.W.; Song, S.H.; Jeong, E.G.; Lee, M.Y.; Hwang, D.; Im, S.A.; Bang, Y.J.; Kim, T.Y. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene 2014, 33, 3334–3341. [Google Scholar] [CrossRef]
- Haskins, J.W.; Nguyen, D.X.; Stern, D.F. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci. Signal 2014, 7, ra116. [Google Scholar] [CrossRef]
- Shi, J.; Li, F.; Yao, X.; Mou, T.; Xu, Z.; Han, Z.; Chen, S.; Li, W.; Yu, J.; Qi, X.; et al. The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene 2018, 37, 3022–3038. [Google Scholar] [CrossRef]
- Oshima, Y.; Tanaka, H.; Murakami, H.; Ito, Y.; Furuya, T.; Kondo, E.; Kodera, Y.; Nakanishi, H. Lapatinib sensitivities of two novel trastuzumab-resistant HER2 gene-amplified gastric cancer cell lines. Gastric Cancer 2014, 17, 450–462. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Song, Y.; Qiao, G.; Di, Y.; Zhao, J.; Sun, P.; Zheng, H.; Huang, H.; Huang, H. ERBB2D16 Expression in HER2 Positive Gastric Cancer Is Associated With Resistance to Trastuzumab. Front. Oncol. 2022, 12, 855308. [Google Scholar] [CrossRef]
- Castagnoli, L.; Ghedini, G.C.; Koschorke, A.; Triulzi, T.; Dugo, M.; Gasparini, P.; Casalini, P.; Palladini, A.; Iezzi, M.; Lamolinara, A.; et al. Pathobiological implications of the d16HER2 splice variant for stemness and aggressiveness of HER2-positive breast cancer. Oncogene 2017, 36, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Mejías-Luque, R.; Lindén, S.K.; Garrido, M.; Tye, H.; Najdovska, M.; Jenkins, B.J.; Iglesias, M.; Ernst, M.; de Bolós, C. Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors. Oncogene 2010, 29, 1753–1762. [Google Scholar] [CrossRef]
- Singh, A.P.; Chaturvedi, P.; Batra, S.K. Emerging roles of MUC4 in cancer: A novel target for diagnosis and therapy. Cancer Res. 2007, 67, 433–436. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Z.; Hu, M.; Liu, D.; Hu, Y.; Qian, L.; Zhang, W.; Chen, H.; Guo, L.; Yu, M.; et al. Catecholamine-Induced β2-adrenergic receptor activation mediates desensitization of gastric cancer cells to trastuzumab by upregulating MUC4 expression. J. Immunol. 2013, 190, 5600–5608. [Google Scholar] [CrossRef]
- Li, G.; Zhao, L.; Li, W.; Fan, K.; Qian, W.; Hou, S.; Wang, H.; Dai, J.; Wei, H.; Guo, Y. Feedback activation of STAT3 mediates trastuzumab resistance via upregulation of MUC1 and MUC4 expression. Oncotarget 2014, 5, 8317–8329. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Xu, Y.; Yang, Y.; Chen, X.; Quan, H.; Lou, L. Aberrant intracellular metabolism of T-DM1 confers T-DM1 resistance in human epidermal growth factor receptor 2-positive gastric cancer cells. Cancer Sci. 2017, 108, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; George, T.J.; Kolevska, T.; Wade, J.L.; Zera, R.; Buchschacher, G.L.; Al Baghdadi, T.; Shipstone, A.; Lin, D.; Yothers, G.; et al. NSABP FC-11: A phase II study of neratinib (N) plus trastuzumab (T) or N plus cetuximab (C) in patients (pts) with “quadruple wild-type” metastatic colorectal cancer (mCRC) based on HER2 status. J. Clin. Oncol. 2022, 40, 3564. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, J.; Giobbie-Hurder, A.; Wargo, J.; Hodi, F.S. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin. Cancer Res. 2013, 19, 598–609. [Google Scholar] [CrossRef]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Niu, L.; Zheng, X.; Xiao, F.; Sun, H.; Deng, W.; Cai, J. PD-L1 expression-related PI3K pathway correlates with immunotherapy efficacy in gastric cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231205853. [Google Scholar] [CrossRef] [PubMed]
- Young Rha, S.; Lee, C.; Kim, H.S.; Kang, B.; Jung, M.; Bae, W.K.; Koo, D.H.; Shin, S.J.; Jeung, H.C.; Zang, D.Y.; et al. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: A multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC). J. Clin. Oncol. 2020, 38, 3081. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Y.; Yang, Y.; Lin, B.; Zhu, M.; Xu, J.; Chen, Y.; Wu, W.; Chen, B.; Chen, X.; et al. Anti-PD-1/Her2 Bispecific Antibody IBI315 Enhances the Treatment Effect of Her2-Positive Gastric Cancer through Gasdermin B-Cleavage Induced Pyroptosis. Adv. Sci. 2023, 10, e2303908. [Google Scholar] [CrossRef] [PubMed]
- A Phase 1b/2 Open-Label Study With Randomization in Phase 2 of IMU-131 HER2/Neu Peptide Vaccine Plus Standard of Care Chemotherapy in Patients With HER2/Neu Overexpressing Metastatic or Advanced Adenocarcinoma of the Stomach or Gastroesophageal Junction. 2016. Available online: https://clinicaltrials.gov/study/NCT02795988 (accessed on 4 May 2024).
- NextHERIZON: An Open-Label, Signal Generating, Phase 2 Study of HER-Vaxx in Combination with Chemotherapy or Pembrolizumab in Patients with Metastatic HER2/Neu Over-Expressing Gastric or Gastroesophageal Junction (GEJ) Adenocarcinomas Who Have Previously Received Trastuzumab and Progressed on This Treatment. 2022. Available online: https://www.cancersa.org.au/research/clinical-trials/nextherizon/ (accessed on 4 May 2024).
- Sartore-Bianchi, A.; Martini, M.; Molinari, F.; Veronese, S.; Nichelatti, M.; Artale, S.; Di Nicolantonio, F.; Saletti, P.; De Dosso, S.; Mazzucchelli, L.; et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009, 69, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Jhawer, M.; Goel, S.; Wilson, A.J.; Montagna, C.; Ling, Y.H.; Byun, D.S.; Nasser, S.; Arango, D.; Shin, J.; Klampfer, L.; et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008, 68, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Mezynski, M.J.; Farrelly, A.M.; Cremona, M.; Carr, A.; Morgan, C.; Workman, J.; Armstrong, P.; McAuley, J.; Madden, S.; Fay, J.; et al. Targeting the PI3K and MAPK pathways to improve response to HER2-targeted therapies in HER2-positive gastric cancer. J. Transl. Med. 2021, 19, 184. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, C.; Sterpi, M.; Jeon, H.; Bteich, F. Resistance to Anti-HER2 Therapies in Gastrointestinal Malignancies. Cancers 2024, 16, 2854. https://doi.org/10.3390/cancers16162854
Mo C, Sterpi M, Jeon H, Bteich F. Resistance to Anti-HER2 Therapies in Gastrointestinal Malignancies. Cancers. 2024; 16(16):2854. https://doi.org/10.3390/cancers16162854
Chicago/Turabian StyleMo, Christiana, Michelle Sterpi, Hyein Jeon, and Fernand Bteich. 2024. "Resistance to Anti-HER2 Therapies in Gastrointestinal Malignancies" Cancers 16, no. 16: 2854. https://doi.org/10.3390/cancers16162854
APA StyleMo, C., Sterpi, M., Jeon, H., & Bteich, F. (2024). Resistance to Anti-HER2 Therapies in Gastrointestinal Malignancies. Cancers, 16(16), 2854. https://doi.org/10.3390/cancers16162854






