Utility of Transpapillary Biopsy and Endoscopic Ultrasound-Guided Tissue Acquisition for Comprehensive Genome Profiling of Unresectable Biliary Tract Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
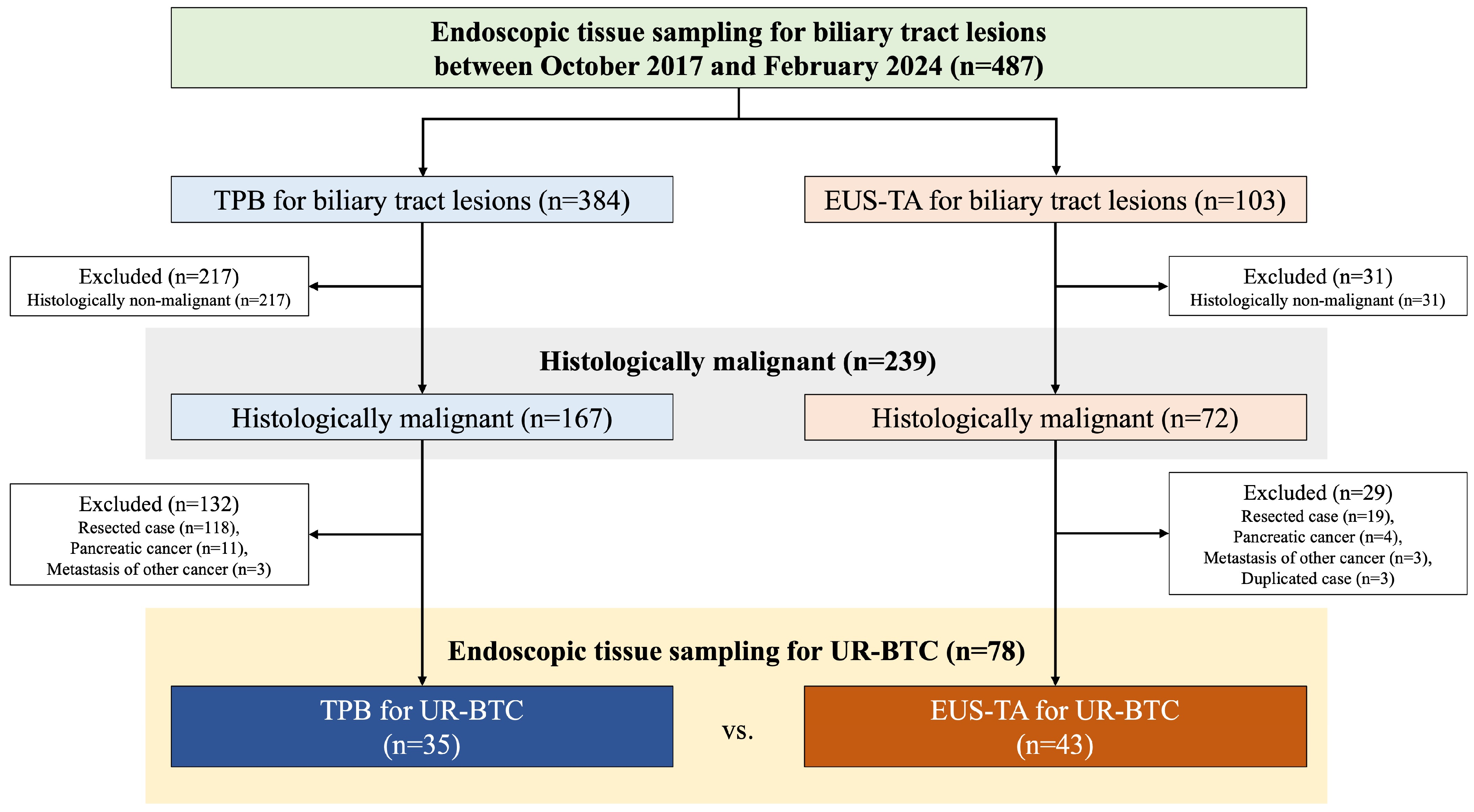
2.1. Study Population
2.2. Endoscopic Procedures
2.3. Endpoints
2.4. Outcome Measures
2.5. Definitions
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Procedure Details
3.3. Outcomes
3.4. Actual CGP Analysis
3.5. Procedural Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, H.; Saurí, T.; Acosta, D.A.; Guardiola, M.; Sierra, A.; Hernando, J.; Nuciforo, P.; Miquel, J.M.; Molero, C.; Peiró, S.; et al. ESMO scale for clinical actionability of molecular targets driving targeted treatment in patients with cholangiocarcinoma. Clin. Cancer Res. 2022, 28, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Doleschal, B.; Taghizadeh, H.; Webersinke, G.; Piringer, G.; Schreil, G.; Decker, J.; Aichberger, K.J.; Kirchweger, P.; Thaler, J.; Petzer, A.; et al. Real world evidence reveals improved survival outcomes in biliary tract cancer through molecular matched targeted treatment. Sci. Rep. 2023, 13, 15421. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Fujisawa, T.; Taniguchi, H.; Bando, H.; Okamoto, W.; Tsuchihara, K.; Yoshino, T.; Ohtsu, A. SCRUM-Japan GI-SCREEN and MONSTAR-SCREEN: Path to the realization of biomarker-guided precision oncology in advanced solid tumors. Cancer Sci. 2021, 112, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Attard, G.; Bidard, F.C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Berchuck, J.E.; Facchinetti, F.; DiToro, D.F.; Baiev, I.; Majeed, U.; Reyes, S.; Chen, C.; Zhang, K.; Sharman, R.; Uson Junior, P.L.S.; et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann. Oncol. 2022, 33, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Maruki, Y.; Morizane, C.; Arai, Y.; Ikeda, M.; Ueno, M.; Ioka, T.; Naganuma, A.; Furukawa, M.; Mizuno, N.; Uwagawa, T.; et al. Molecular detection and clinicopathological characteristics of advanced/recurrent biliary tract carcinomas harboring the FGFR2 rearrangements: A prospective observational study (PRELUDE Study). J. Gastroenterol. 2021, 56, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Verlingue, L.; Malka, D.; Allorant, A.; Massard, C.; Ferté, C.; Lacroix, L.; Rouleau, E.; Auger, N.; Ngo, M.; Nicotra, C.; et al. Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur. J. Cancer 2017, 87, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef] [PubMed]
- De Moura, D.T.H.; Moura, E.G.H.D.; Bernardo, W.M.; De Moura, E.T.H.; Baraca, F.I.; Kondo, A.; Matuguma, S.E.; Almeida Artifon, E.L. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc. Ultrasound 2018, 7, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.B.; Moon, S.H.; Ko, S.W.; Lim, H.; Kang, H.S.; Kim, J.H. Brush cytology, forceps biopsy, or endoscopic ultrasound-guided sampling for diagnosis of bile duct cancer: A meta-analysis. Dig. Dis. Sci. 2022, 67, 3284–3297. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Onda, S.; Ogura, T.; Kurisu, Y.; Masuda, D.; Sano, T.; Takagi, W.; Fukunishi, S.; Higuchi, K. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Ther. Adv. Gastroenterol. 2016, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, S.; Hara, K.; Mizuno, N.; Imaoka, H.; Ogura, T.; Haba, S.; Mekky, M.A.; Bhatia, V.; Hosoda, W.; Yatabe, Y.; et al. Diagnostic yield of endoscopic retrograde cholangiography and of EUS-guided fine needle aspiration sampling in gallbladder carcinomas. J. Hepato-Biliary-Pancreat. Sci. 2012, 19, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Wang, K.; He, J.; Otto, G.; Hawryluk, M.; Zwirco, Z.; Brennan, T.; Nahas, M.; Donahue, A.; Yelensky, R.; et al. Clinical next-generation sequencing successfully applied to fine-needle aspirations of pulmonary and pancreatic neoplasms. Cancer Cytopathol. 2013, 121, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Larson, B.K.; Tuli, R.; Jamil, L.H.; Lo, S.K.; Deng, N.; Hendifar, A.E. Utility of endoscopic ultrasound-guided biopsy for next-generation sequencing of pancreatic exocrine malignancies. Pancreas 2018, 47, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Elhanafi, S.; Mahmud, N.; Vergara, N.; Kochman, M.L.; Das, K.K.; Ginsberg, G.G.; Rajala, M.; Chandrasekhara, V. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J. Gastroenterol. Hepatol. 2019, 34, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Lee, J.H.; Noh, D.H.; Park, J.K.; Lee, K.T.; Lee, J.K.; Lee, K.H.; Jang, K.T.; Cho, J. Factors of endoscopic ultrasound-guided tissue acquisition for successful next-generation sequencing in pancreatic ductal adenocarcinoma. Gut Liver 2020, 14, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Carrara, S.; Soldà, G.; Di Leo, M.; Rahal, D.; Peano, C.; Giunta, M.; Lamonaca, L.; Auriemma, F.; Anderloni, A.; Fugazza, A.; et al. Side-by-side comparison of next-generation sequencing, cytology, and histology in diagnosing locally advanced pancreatic adenocarcinoma. Gastrointest. Endosc. 2021, 93, 597–604.e5. [Google Scholar] [CrossRef] [PubMed]
- Kandel, P.; Nassar, A.; Gomez, V.; Raimondo, M.; Woodward, T.A.; Crook, J.E.; Fares, N.S.; Wallace, M.B. Comparison of endoscopic ultrasound-guided fine-needle biopsy versus fine-needle aspiration for genomic profiling and DNA yield in pancreatic cancer: A randomized crossover trial. Endoscopy 2021, 53, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, G.; Hijioka, S.; Nagashio, Y.; Maruki, Y.; Ohba, A.; Hisada, Y.; Yoshinari, M.; Harai, S.; Kitamura, H.; Koga, T.; et al. Fine-needle biopsy with 19G needle is effective in combination with endoscopic ultrasound-guided tissue acquisition for genomic profiling of unresectable pancreatic cancer. Dig. Endosc. 2023, 35, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, H.; Tanimoto, A.; Toyodome, K.; Kojima, I.; Hinokuchi, M.; Tanoue, S.; Hashimoto, S.; Kawahira, M.; Arima, S.; Kanmura, S.; et al. Next-generation sequencing analysis of pancreatic cancer using residual liquid cytology specimens from endoscopic ultrasound-guided fine-needle biopsy: A prospective comparative study with tissue specimens. Diagnostics 2023, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Okuno, N.; Hara, K.; Mizuno, N.; Haba, S.; Kuwahara, T.; Kuraishi, Y.; Fumihara, D.; Yanaidani, T. Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of pancreatic cancer. Clin. Endosc. 2023, 56, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Kuwatani, M.; Suda, G.; Ishikawa, M.; Sugiura, R.; Kato, S.; Kawakubo, K.; Sakamoto, N. A novel approach for the genetic analysis of biliary tract cancer specimens obtained through endoscopic ultrasound-guided fine needle aspiration using targeted amplicon sequencing. Clin. Transl. Gastroenterol. 2019, 10, e00022. [Google Scholar] [CrossRef] [PubMed]
- Yanaidani, T.; Hara, K.; Okuno, N.; Haba, S.; Kuwahara, T.; Kuraishi, Y.; Mizuno, N.; Ishikawa, S.; Yamada, M.; Yasuda, T. Clinical utility of endoscopic ultrasound-guided tissue acquisition for comprehensive genomic profiling of patients with biliary tract cancer, especially with intrahepatic cholangiocarcinoma. Clin. Endosc. 2024, 57, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Crino, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Sunami, K.; Ichikawa, H.; Kubo, T.; Kato, M.; Fujiwara, Y.; Shimomura, A.; Koyama, T.; Kakishima, H.; Kitami, M.; Matsushita, H.; et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 2019, 110, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Nakamura, H.; Nagai, M.; Kubo, T.; Elzawahry, A.; Totoki, Y.; Tanabe, Y.; Furukawa, E.; Miyamoto, J.; Sakamoto, H.; et al. A computational tool to detect DNA alterations tailored to formalin-fixed paraffin-embedded samples in cancer clinical sequencing. Genome Med. 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- FoundationOneMedicine. FoundationOne CDx [Internet]; FoundationOne Medicine: Cambridge, MA, USA, 2024; Available online: https://www.foundationmedicine.com/test/foundationone-cdx (accessed on 1 May 2024).
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Kapacee, Z.; Breeze, M.; Bell, C.; Belcher, D.; Staiger, H.; Taylor, C.; McNamara, M.G.; Hubner, R.A.; Valle, J.W. Molecular profiling in daily clinical practice: Practicalities in advanced cholangiocarcinoma and other biliary tract cancers. J. Clin. Med. 2020, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Kuraishi, Y.; Hara, K.; Haba, S.; Kuwahara, T.; Okuno, N.; Yanaidani, T.; Ishikawa, S.; Yasuda, T.; Yamada, M.; Fukui, T.; et al. Diagnostic performance and safety of endoscopic ultrasound-guided fine-needle aspiration/biopsy for gallbladder lesions. Dig. Endosc. 2024, 36, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Wardell, C.P.; Fujita, M.; Yamada, T.; Simbolo, M.; Fassan, M.; Karlic, R.; Polak, P.; Kim, J.; Hatanaka, Y.; Maejima, K.; et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J. Hepatol. 2018, 68, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Tamada, K.; Satoh, Y.; Tomiyama, T.; Ohashi, A.; Wada, S.; Ido, K.; Sugano, K. Multiple bile duct biopsies using a sheath with a side port: Usefulness of intraductal sonography. Am. J. Roentgenol. 2001, 176, 797–802. [Google Scholar] [CrossRef]
- Ogawa, T.; Ito, K.; Koshita, S.; Kanno, Y.; Masu, K.; Kusunose, H.; Sakai, T.; Murabayashi, T.; Hasegawa, S.; Noda, Y. Usefulness of cholangioscopic-guided mapping biopsy using SpyGlass DS for preoperative evaluation of extrahepatic cholangiocarcinoma: A pilot study. Endosc. Int. Open 2018, 6, E199–E204. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Hirose, Y.; Ueno, S.; Okuda, A.; Nishioka, N.; Miyano, A.; Yamamoto, Y.; Ueshima, K.; Higuchi, K. Prospective registration study of diagnostic yield and sample size in forceps biopsy using a novel device under digital cholangioscopy guidance with macroscopic on-site evaluation. J. Hepato-Biliary-Pancreat. Sci. 2023, 30, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Mohamadnejad, M.; Islami, F.; Keshtkar, A.; Biglari, M.; Malekzadeh, R.; Eloubeidi, M.A. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: A systematic review and meta-analysis. Gastrointest. Endosc. 2016, 83, 290–298.e1. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Agarwal, R.; Anikhindi, S.A.; Puri, P.; Kumar, M.; Ranjan, P.; Kumar, A.; Sharma, P.; Bansal, N.; Bakshi, P.; et al. Role of EUS-FNA for gallbladder mass lesions with biliary obstruction: A large single-center experience. Endosc. Int. Open 2019, 7, E1403–E1409. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients, n = 78 | TPB Group, n = 35 | EUS-TA Group, n = 43 | p-Value |
|---|---|---|---|---|
| Age, years, median (IQR) | 71 (64–79) | 72 (66–80) | 71 (61–77) | 0.393 |
| Sex, Male, n (%) | 50 (64.1) | 27 (77.1) | 23 (53.5) | 0.035 |
| Primary tumor, n (%) | ||||
| pCCA | 27 (34.6) | 20 (57.1) | 7 (16.3) | <0.001 |
| iCCA | 20 (25.6) | 1 (2.9) | 19 (44.2) | <0.001 |
| GBC | 17 (21.8) | 5 (14.3) | 12 (27.9) | 0.177 |
| dCCA | 7 (9.0) | 4 (11.4) | 3 (7.0) | 0.694 |
| AC | 7 (9.0) | 5 (14.3) | 2 (4.7) | 0.233 |
| T category, n (%) | ||||
| T4 | 33 (42.3) | 14 (40.0) | 19 (44.2) | 0.819 |
| T3 | 40 (51.3) | 17 (48.6) | 23 (53.5) | 0.820 |
| T2 | 5 (6.4) | 4 (11.4) | 1 (2.3) | 0.168 |
| Form of primary tumor, n (%) | ||||
| Mass lesion | 56 (71.8) | 24 (68.6) | 32 (74.4) | 0.619 |
| Thickened wall | 22 (28.2) | 11 (31.4) | 11 (25.6) | - |
| Reason for unresectable, n (%) | ||||
| Metastasis | 51 (65.4) | 18 (51.4) | 33 (76.7) | 0.031 |
| Locally advanced | 21 (26.9) | 13 (37.1) | 8 (18.6) | 0.078 |
| Poor performance status | 6 (7.7) | 4 (11.4) | 2 (4.7) | 0.400 |
| Purpose of tissue acquisition, n (%) | ||||
| Histological diagnosis | 74 (94.9) | 35 (100) | 39 (90.7) | 0.123 |
| CGP | 4 (5.1) | 0 (0) | 4 (8.7) | - |
| Timing of tissue acquisition, n (%) | ||||
| Before chemotherapy | 70 (89.7) | 34 (97.1) | 36 (83.7) | 0.068 |
| During chemotherapy | 8 (10.3) | 1 (2.9) | 7 (15.2) | - |
| Procedural Details | All Patients, n = 78 | TPB Group, n = 35 | EUS-TA Group, n = 43 | p-Value |
|---|---|---|---|---|
| Type of biopsy forceps, n (%) | ||||
| POCS-compatible biopsy forceps | 20 (25.6) | 20 (57.1) | - | - |
| Standard biopsy forceps | 9 (11.5) | 9 (25.7) | - | - |
| One-sided opening-cup biopsy forceps | 6 (7.7) | 6 (17.1) | - | - |
| Peroral cholangioscopy, n (%) | 4 (5.1) | 4 (11.4) | - | - |
| Needle type, n (%) | ||||
| FNB | 32 (41.0) | - | 32 (74.4) | - |
| FNA | 11 (14.1) | - | 11 (25.6) | - |
| Needle size, n (%) | ||||
| 22-gauge | 35 (44.9) | - | 35 (81.4) | - |
| 19-gauge | 8 (10.3) | - | 8 (18.6) | - |
| Puncture route, n (%) | ||||
| Transduodenal | 25 (32.1) | - | 25 (58.1) | - |
| Transgastric | 18 (23.1) | - | 18 (41.9) | - |
| Suction method, n (%) | ||||
| Slow pull | 30 (38.5) | - | 30 (69.8) | - |
| Suction (20 mL) | 13 (16.7) | - | 13 (30.2) | - |
| Target size, mm, median (IQR) | ||||
| Mass lesion | 28.4 (19.8–42.6) | 24.5 (18.9–30.4) | 34.3 (20.0–46.9) | 0.122 |
| Thickened wall | 7.0 (5.3–8.2) | 6.0 (4.3–8.0) | 7.3 (7.0–9.4) | 0.263 |
| Number of biopsies, median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.742 |
| Adverse events, n (%) | 7 (9.0) | 6 (17.1) | 1 (2.3) | 0.041 |
| CGP | Variables | All Patients, n = 78 | TPB Group, n = 35 | EUS-TA Group, n = 43 | p-Value |
|---|---|---|---|---|---|
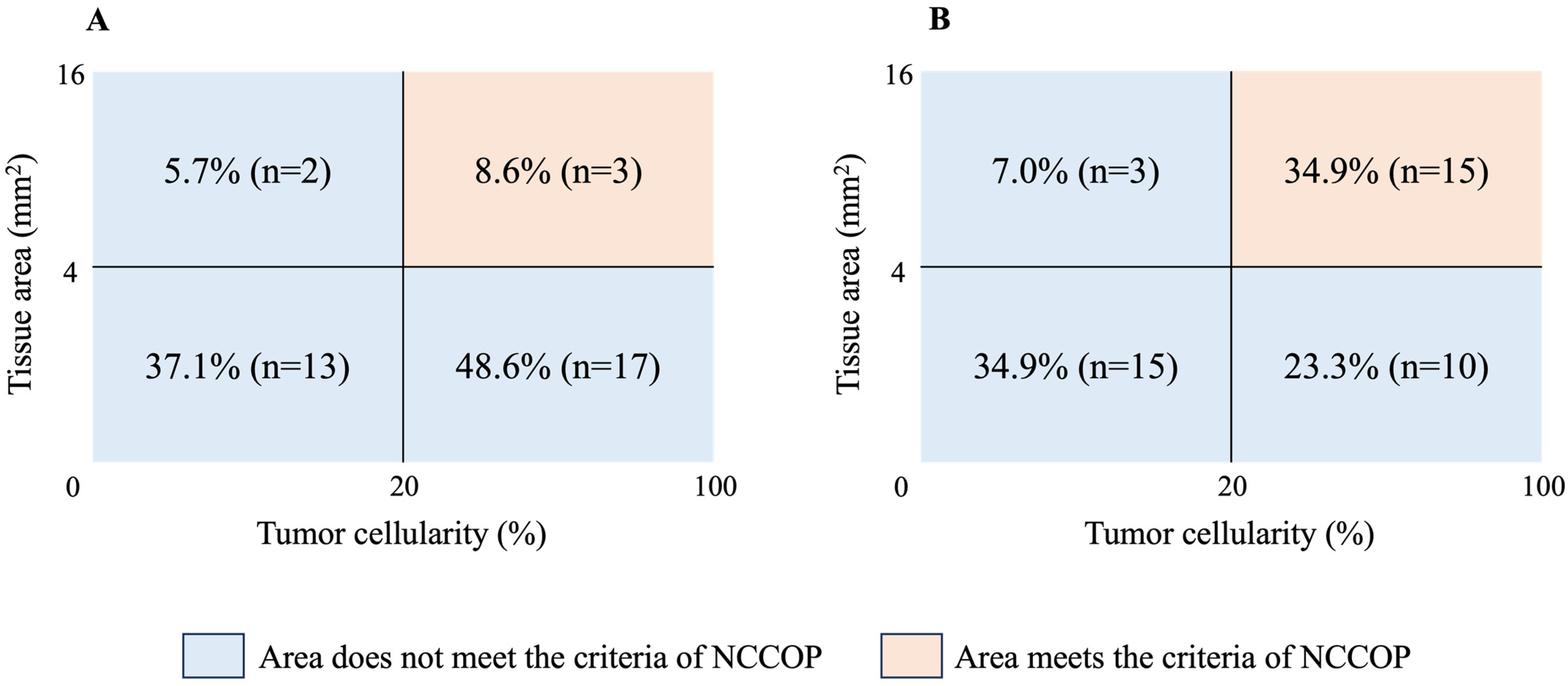
| Cases with tumor cellularity ≥ 20%, n (%) | 45 (57.7) | 20 (57.1) | 25 (58.1) | 1.000 | |
| Cases with tissue area ≥ 4 mm2, n (%) | 23 (29.5) | 5 (14.3) | 18 (41.9) | 0.012 | |
| Cases with tissue area ≥ 25 mm2, n (%) | 0 (0) | 0 (0) | 0 (0) | - | |
| NCCOP criteria | Cases with tumor cellularity ≥20% and tissue area ≥ 4 mm2, n (%) | 18 (23.1) | 3 (8.6) | 15 (34.9) | 0.007 |
| F1CDx criteria | Cases with tumor cellularity ≥ 20% and tissue area ≥ 25 mm2, n (%) | 0 (0) | 0 (0) | 0 (0) | - |
| Factors | All Patients (n = 78) | ||||
|---|---|---|---|---|---|
| Adequate, % (n) | Univariate | Multivariate | |||
| OR (95% CI) | p-Value | aOR (95% CI) | p-Value | ||
| Method of tissue acquisition | |||||
| EUS-TA | 34.9 (15/43) | 5.60 (1.38–33.3) | 0.007 | 5.32 (1.31–21.6) | 0.019 |
| TPB | 8.6 (3/35) | - | - | ||
| Primary tumor site | |||||
| pCCA | 7.4 (2/27) | 0.18 (0.04–0.83) | 0.023 | 0.33 (0.06–1.79) | 0.200 |
| iCCA | 35.0 (7/20) | 2.30 (0.74–7.11) | 0.216 | ||
| Others (reference) | 29.0 (9/31) | 1.0 (reference) | - | ||
| T category | |||||
| T4 | 18.2 (6/33) | 0.61 (0.17–2.06) | 0.427 | - | - |
| T2 or T3 | 26.7 (12/45) | - | - | ||
| Form of primary tumor | |||||
| Mass lesion | 28.6 (16/56) | 3.94 (0.80–38.7) | 0.080 | 3.62 (0.71–18.4) | 0.121 |
| Thickened wall | 9.1 (2/23) | - | - | ||
| Unresectable status | |||||
| Metastatic | 27.5 (14/51) | 2.16 (0.58–10.1) | 0.266 | - | - |
| Non-metastatic | 14.8 (4/27) | - | - | ||
| Purpose of tissue acquisition | |||||
| Histological diagnosis | 23.0 (17/74) | - | 1.000 | - | - |
| CGP | 25.0 (1/4) | 1.11 (0.02–15.0) | - | ||
| Timing of tissue acquisition | |||||
| Before chemotherapy | 22.9 (16/70) | - | 1.000 | - | - |
| During chemotherapy | 25.0 (2/8) | 1.12 (0.10–7.12) | - | ||
| Number of biopsies | |||||
| ≥3 | 26.7 (12/45) | 1.63 (0.49–6.02) | 0.427 | - | - |
| <3 | 18.2 (6/33) | - | - | ||
| Factors | TPB Group (n = 35) | |
|---|---|---|
| Adequate, % (n) | Univariate | |
| p-Value | ||
| Primary tumor site | ||
| pCCA/dCCA | 12.5 (3/24) | 0.536 |
| Others | 0 (0/11) | - |
| T category | ||
| T4 | 0 (0/14) | 0.259 |
| T2 or T3 | 14.3 (3/21) | - |
| Form of primary tumor | ||
| Mass lesion | 8.3 (2/24) | 1.000 |
| Thickened wall | 9.1 (1/11) | - |
| Macroscopic type (exc. iCCA) | ||
| Papillary type | 66.7 (2/3) | 0.016 |
| Nodular or flat type | 3.2 (1/31) | - |
| Number of biopsies | ||
| ≥3 | 5.0 (1/20) | 0.565 |
| <3 | 13.3 (2/15) | - |
| Type of biopsy forceps | ||
| POCS compatible | 15.0 (3/20) | 0.244 |
| Others | 0 (0/15) | - |
| POCS | ||
| Used | 50.0 (2/4) | 0.029 |
| Not used | 3.3 (1/31) | - |
| Factors | EUS-TA Group (n = 43) | |
|---|---|---|
| Adequate, % (n) | Univariate | |
| p-Value | ||
| Primary tumor site | ||
| pCCA | 0 (0/7) | 0.077 |
| Others | 41.7 (15/36) | - |
| T category | ||
| T4 | 31.6 (6/19) | 0.755 |
| T2 or T3 | 37.5 (9/24) | - |
| Form of primary tumor | ||
| Mass lesion | 43.8 (14/32) | 0.065 |
| Thickened wall | 9.1 (1/11) | - |
| Macroscopic type (exc. iCCA) | ||
| Papillary type | 100 (1/1) | 0.333 |
| Nodular or flat type | 30.4 (7/23) | - |
| Number of biopsies | ||
| ≥3 | 44.0 (11/25) | 0.199 |
| <3 | 22.2 (4/18) | - |
| Needle type | ||
| FNB | 37.5 (12/32) | 0.719 |
| FNA | 27.3 (3/11) | - |
| Needle size | ||
| 19-gauge | 37.5 (3/8) | 1.000 |
| 22-gauge | 34.3 (12/35) | - |
| Target size (only mass lesion) | ||
| ≥18.5 mm | 51.9 (14/27) | 0.053 |
| <18.5 mm | 0 (0/5) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, S.; Hijioka, S.; Nagashio, Y.; Yamashige, D.; Agarie, D.; Hagiwara, Y.; Okamoto, K.; Yagi, S.; Komori, Y.; Kuwada, M.; et al. Utility of Transpapillary Biopsy and Endoscopic Ultrasound-Guided Tissue Acquisition for Comprehensive Genome Profiling of Unresectable Biliary Tract Cancer. Cancers 2024, 16, 2819. https://doi.org/10.3390/cancers16162819
Fukuda S, Hijioka S, Nagashio Y, Yamashige D, Agarie D, Hagiwara Y, Okamoto K, Yagi S, Komori Y, Kuwada M, et al. Utility of Transpapillary Biopsy and Endoscopic Ultrasound-Guided Tissue Acquisition for Comprehensive Genome Profiling of Unresectable Biliary Tract Cancer. Cancers. 2024; 16(16):2819. https://doi.org/10.3390/cancers16162819
Chicago/Turabian StyleFukuda, Soma, Susumu Hijioka, Yoshikuni Nagashio, Daiki Yamashige, Daiki Agarie, Yuya Hagiwara, Kohei Okamoto, Shin Yagi, Yasuhiro Komori, Masaru Kuwada, and et al. 2024. "Utility of Transpapillary Biopsy and Endoscopic Ultrasound-Guided Tissue Acquisition for Comprehensive Genome Profiling of Unresectable Biliary Tract Cancer" Cancers 16, no. 16: 2819. https://doi.org/10.3390/cancers16162819
APA StyleFukuda, S., Hijioka, S., Nagashio, Y., Yamashige, D., Agarie, D., Hagiwara, Y., Okamoto, K., Yagi, S., Komori, Y., Kuwada, M., Maruki, Y., Morizane, C., Ueno, H., Hiraoka, N., Tsuchiya, K., & Okusaka, T. (2024). Utility of Transpapillary Biopsy and Endoscopic Ultrasound-Guided Tissue Acquisition for Comprehensive Genome Profiling of Unresectable Biliary Tract Cancer. Cancers, 16(16), 2819. https://doi.org/10.3390/cancers16162819









