Assessing the Adequacy of Traditional Vertebral Landmarks as Upper Border of Whole Pelvic Radiotherapy Field for Stage IB2-IIB Cervical Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Comparison Group, Endpoints, and Statistical Methods
3. Results
3.1. Patient Characteristics
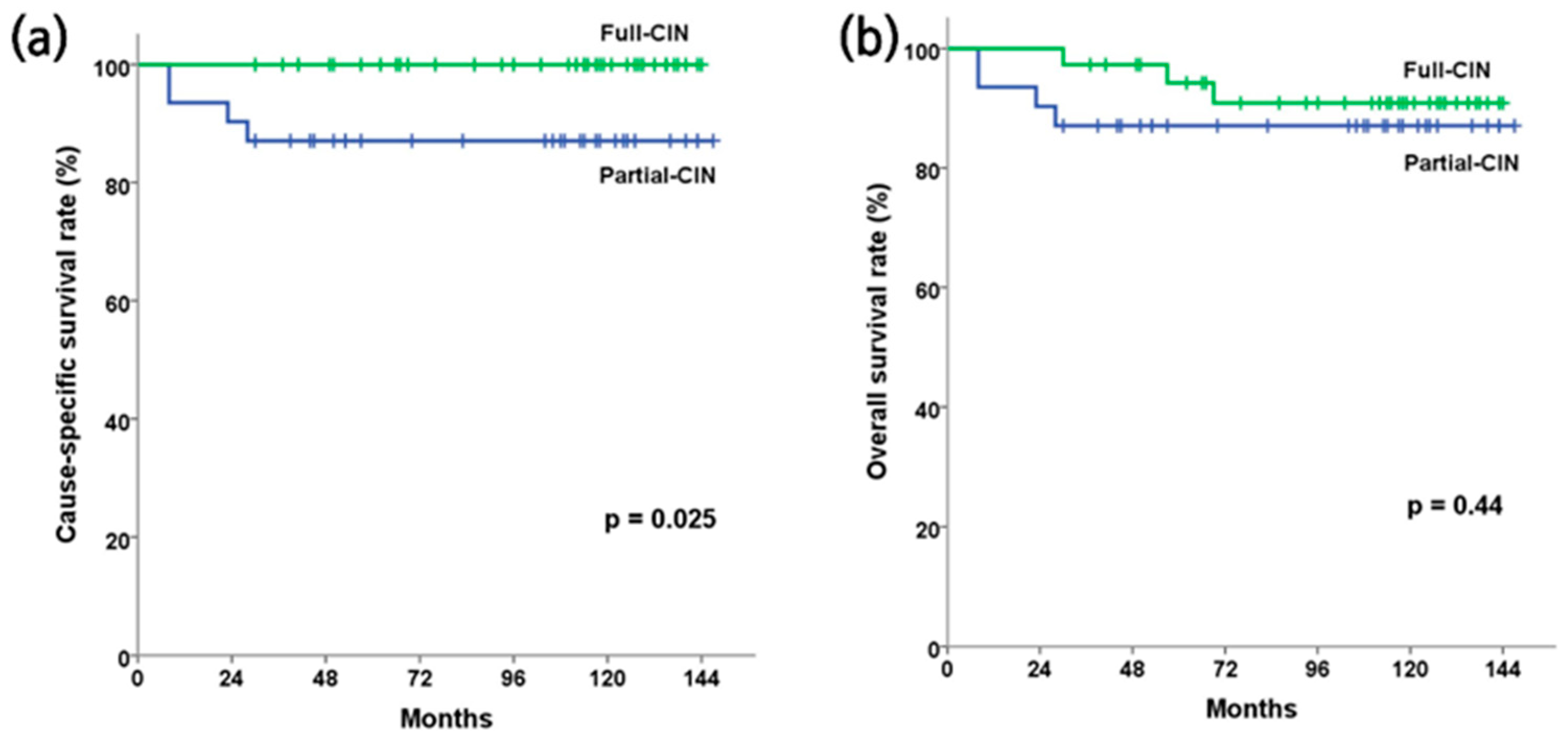
3.2. Clinical and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Eifel, P.J.; Lu, J.; Grigsby, P.W.; Levenback, C.; Stevens, R.E.; Rotman, M.; Gershenson, D.M.; Mutch, D.G. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N. Engl. J. Med. 1999, 340, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C., Jr.; Clarke-Pearson, D.L.; Liao, S.-Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin. Oncol. 1999, 17, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.A., 3rd; Liu, P.Y.; Barrett, R.J.; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Eifel, P.J.; Winter, K.; Morris, M.; Levenback, C.; Grigsby, P.W.; Cooper, J.; Rotman, M.; Gershenson, D.; Mutch, D.G. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J. Clin. Oncol. 2004, 22, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E. The lymphatic spread of carcinoma of the cervix and of the body of the uterus; a study of 420 necropsies. Obstet. Gynecol. 1949, 58, 924–942. [Google Scholar]
- McMahon, C.J.; Rofsky, N.M.; Pedrosa, I. Lymphatic metastases from pelvic tumors: Anatomic classification, characterization, and staging. Radiology 2010, 254, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Olthof, E.P.; Van der Aa, M.A.; Adam, J.A.; Stalpers, L.J.A.; Wenzel, H.H.B.; Van der Velden, J.; Mom, C.H. The role of lymph nodes in cervical cancer: Incidence and identification of lymph node metastases-a literature review. Int. J. Clin. Oncol. 2021, 26, 1600–1610. [Google Scholar] [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie-Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Virchows Arch. 2018, 472, 919–936. [Google Scholar] [CrossRef]
- Chino, J.; Annunziata, C.M.; Beriwal, S.; Bradfield, L.; Erickson, B.A.; Fields, E.C.; Fitch, K.; Harkenrider, M.M.; Holschneider, C.H.; Kamrava, M.; et al. Radiotherapy for cervical cancer: Executive summary of an ASTRO clinical practice guideline. Pract. Radiat. Oncol. 2020, 10, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Small, W.; Bosch, W.R.; Harkenrider, M.M.; Strauss, J.B.; Abu-Rustum, N.; Albuquerque, K.V.; Beriwal, S.; Creutzberg, C.L.; Eifel, P.J.; Erickson, B.A.; et al. NRG OncologyRTOG Consensus Guidelines for Delineation of Clinical Target Volume for Intensity Modulated Pelvic Radiation Therapy in Postoperative Treatment of Endometrial and Cervical Cancer: An Update. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Park, W. Patterns of definitive radiotherapy practice for cervical cancer in South Korea: A survey endorsed by the Korean Radiation Oncology Group (KROG 20-06). J. Gynecol. Oncol. 2021, 32, e43. [Google Scholar] [CrossRef] [PubMed]
- Greer, B.; Koh, W.-J.; Figge, D.; Russell, A.; Cain, J.; Tamimi, H. Gynecologic radiotherapy fields defined by intraoperative measurements. Gynecol. Oncol. 1990, 38, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Chithriki, M.; Jaibaji, M.; Steele, R.D. The anatomical relationship of the aortic bifurcation to the lumbar vertebrae: A MRI study. Surg. Radiol. Anat. 2002, 24, 308–312. [Google Scholar] [PubMed]
- Lee, C.H.; Seo, B.K.; Choi, Y.C.; Shin, H.J.; Park, J.H.; Jeon, H.J.; Kim, K.A.; Park, C.M.; Kim, B.H. Using MRI to evaluate anatomic significance of aortic bifurcation, right renal artery, and conus medullaris when locating lumbar vertebral segments. AJR Am. J. Roentgenol. 2004, 182, 1295–1300. [Google Scholar] [CrossRef]
- McAlpine, J.; Schlaerth, J.; Lim, P.; Chen, D.; Eisenkop, S.; Spirtos, N. Radiation fields in gynecologic oncology: Correlation of soft tissue (surgical) to radiologic landmarks. Gynecol. Oncol. 2004, 92, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.; Joiner, A.J.v.d.K. Fractionation: The linear-quadratic approach. In Basic Clinical Radiobiology, 5th ed.; Michael, C., Joiner, S.M.B., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Benedetti-Panici, P.; Maneschi, F.; Scambia, G.; Greggi, S.; Cutillo, G.; D’Andrea, G.; Rabitti, C.; Coronetta, F.; Capelli, A.; Mancuso, S. Lymphatic spread of cervical cancer: An anatomical and pathological study based on 225 radical hysterectomies with systematic pelvic and aortic lymphadenectomy. Gynecol. Oncol. 1996, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, G.; Toita, T.; Furutani, K.; Kodaira, T.; Ohno, T.; Kaneyasu, Y.; Yoshimura, R.; Uno, T.; Yogi, A.; Ishikura, S.; et al. Distribution patterns of metastatic pelvic lymph nodes assessed by CT/MRI in patients with uterine cervical cancer. Radiat. Oncol. 2013, 8, 139. [Google Scholar] [CrossRef]
- Russell, A.H.; Walter, J.P.; Anderson, M.W.; Zukowski, C.L. Sagittal magnetic resonance imaging in the design of lateral radiation treatment portals for patients with locally advanced squamous cancer of the cervix. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 449–455. [Google Scholar] [CrossRef]
- Pendlebury, S.C.; Cahill, S.; Crandon, A.J.; Bull, C.A. Role of bipedal lymphangiogram in radiation treatment planning for cervix cancer. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; McGinnis, L.; Spencer, S.A.; Meredith, R.F.; Jennelle, R.L.; Salter, M.M. Conventional four-field pelvic radiotherapy technique without computed tomography-treatment planning in cancer of the cervix: Potential geographic miss and its impact on pelvic control. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 109–112. [Google Scholar] [CrossRef]
- Bonin, S.R.; Lanciano, R.M.; Corn, B.W.; Hogan, W.M.; Hartz, W.H.; Hanks, G.E. Bony landmarks are not an adequate substitute for lymphangiography in defining pelvic lymph node location for the treatment of cervical cancer with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.; Rosato, O.; Lucino, S.; Jauregui, E.; Rossi, L.; Venencia, D. Anatomic study of the pelvis in carcinoma of the uterine cervix as related to the box technique. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Finlay, M.H.; Ackerman, I.; Tirona, R.G.; Hamilton, P.; Barbera, L.; Thomas, G. Use of CT simulation for treatment of cervical cancer to assess the adequacy of lymph node coverage of conventional pelvic fields based on bony landmarks. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Köhler, C.; Schneider, A.; Seiler, F.; Hinkelbein, W. Interindividual variability of lymph drainages in patients with cervical cancer. Implication on irradiation planning. Strahlenther. Onkol. 2006, 182, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Gulia, A.; Patel, F.; Rai, B.; Sharma, S.C.; Bansal, A. Conventional four field radiotherapy versus computed tomography-based treatment planning in cancer cervix: A dosimetric study. South. Asian J. Cancer 2013, 2, 132–135. [Google Scholar] [CrossRef]
- Uno, T.; Isobe, K.; Ueno, N.; Kobayashi, H.; Sanayama, Y.; Mitsuhashi, A.; Shozu, M.; Ito, H. Vessel-contouring-based pelvic radiotherapy in patients with uterine cervical cancer. Jpn. J. Clin. Oncol. 2009, 39, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, H. Evaluation of pelvic lymph node coverage of conventional radiotherapy fields based on bony landmarks in Chinese cervical cancer patients using CT simulation. J. Zhejiang Univ. Sci. B 2009, 10, 683–688. [Google Scholar] [CrossRef]
- Beadle, B.M.; Jhingran, A.; Yom, S.S.; Ramirez, P.T.; Eifel, P.J. Patterns of regional recurrence after definitive radiotherapy for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1396–1403. [Google Scholar] [CrossRef]
- Rai, B.; Bansal, A.; Patel, F.; Gulia, A.; Kapoor, R.; Sharma, S.C. Pelvic nodal CTV from L4-L5 or aortic bifurcation? An audit of the patterns of regional failures in cervical cancer patients treated with pelvic radiotherapy. Jpn. J. Clin. Oncol. 2014, 44, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef] [PubMed]
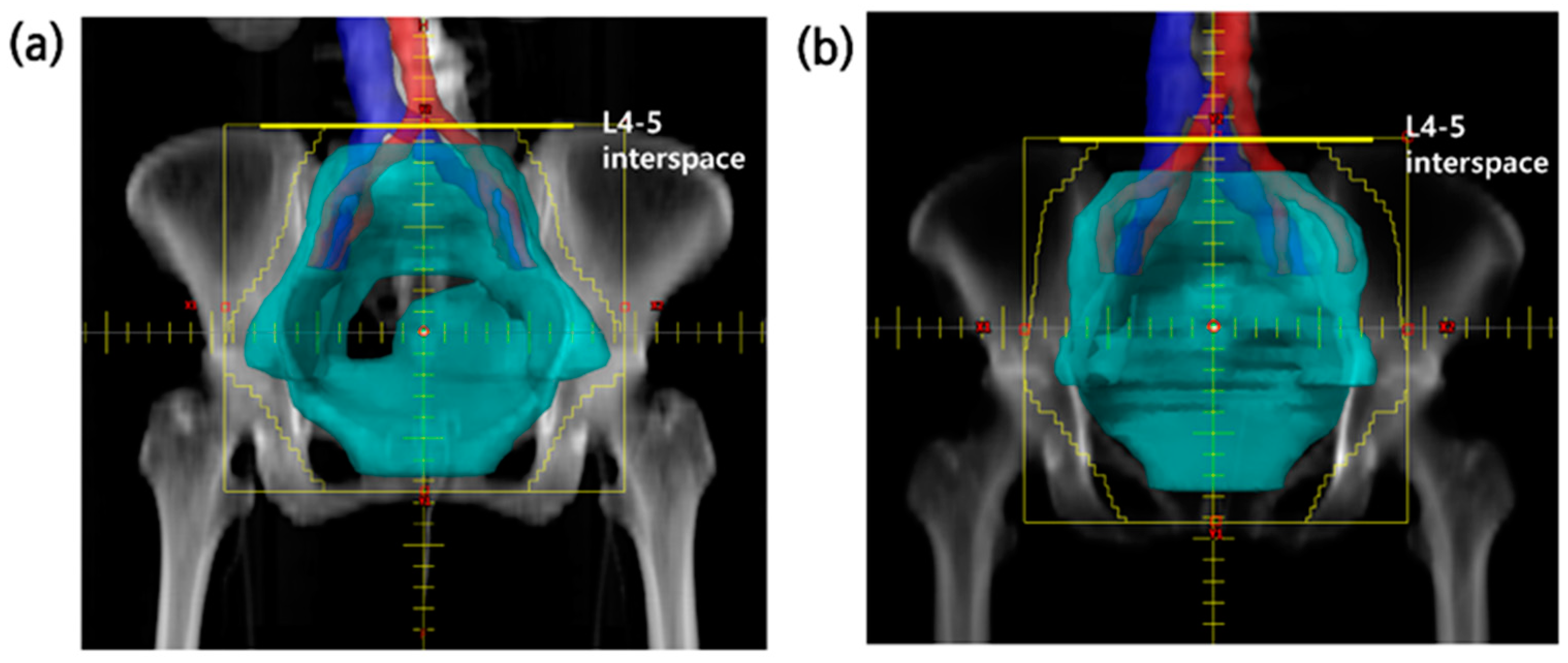
| Variables | Full-CIN Group | Partial-CIN Group | p Value |
|---|---|---|---|
| N = 37 | n = 31 | ||
| Upper border of RT (vertebral landmark) | 0.011 | ||
| L3-4 interspace | 7 | 0 | |
| L4-5 interspace | 30 | 31 | |
| Age (years, mean ± SD) | 54.8 ± 12.5 | 53.2 ± 11.8 | 0.589 |
| Comorbidities | |||
| Hypertension | 10 | 3 | 0.070 |
| Diabetes mellitus | 2 | 0 | 0.189 |
| Histopathology | 0.742 | ||
| SCC | 30 | 27 | |
| Adenocarcinoma or ASC | 7 | 4 | |
| FIGO stage | 0.959 | ||
| IB2, IB3 | 13 | 12 | |
| IIA | 7 | 4 | |
| IIB | 17 | 15 | |
| Primary tumor size (mm, mean ± SD) | 3.63 ± 1.07 | 3.60 ± 1.03 | 0.918 |
| Primary tumor size, cm | 0.353 | ||
| <4 | 21 | 21 | |
| ≥4 | 16 | 10 | |
| SUVmax of PET in primary lesion (mean ± SD) | 9.78 ± 3.79 | 9.81 ± 3.74 | 0.971 |
| Pretreatment hematologic parameter | |||
| Hemoglobin (g/dL, mean ± SD) | 12.55 ± 1.50 | 11.90 ± 1.87 | 0.116 |
| SCC Ag. Level (ng/mL, mean ± SD) | 5.19 ± 4.86 | 7.27 ± 7.43 | 0.188 |
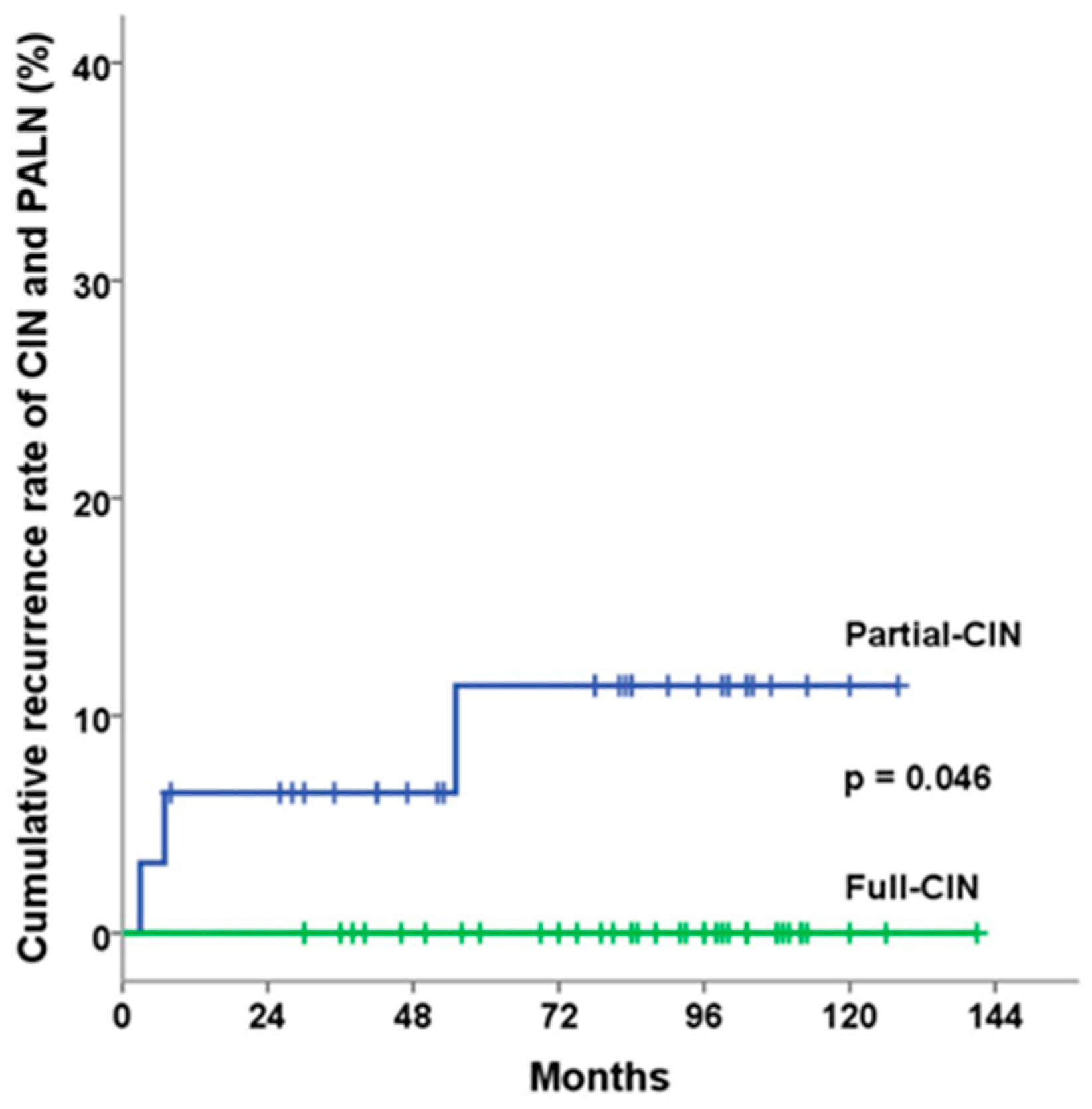
| Recurrence Pattern | Full-CIN Group (N = 37) | Partial-CIN Group (N = 31) | p Value |
|---|---|---|---|
| In-field pelvic recurrence | 1 | 2 | 0.588 |
| Out-of-field CIN and PAN recurrence | 0 | 3 | 0.090 |
| Distant recurrence | 2 | 2 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.H.; Lee, J.W.; Seol, K.H. Assessing the Adequacy of Traditional Vertebral Landmarks as Upper Border of Whole Pelvic Radiotherapy Field for Stage IB2-IIB Cervical Cancer. Cancers 2024, 16, 2743. https://doi.org/10.3390/cancers16152743
Jo JH, Lee JW, Seol KH. Assessing the Adequacy of Traditional Vertebral Landmarks as Upper Border of Whole Pelvic Radiotherapy Field for Stage IB2-IIB Cervical Cancer. Cancers. 2024; 16(15):2743. https://doi.org/10.3390/cancers16152743
Chicago/Turabian StyleJo, Ji Hwan, Jeong Won Lee, and Ki Ho Seol. 2024. "Assessing the Adequacy of Traditional Vertebral Landmarks as Upper Border of Whole Pelvic Radiotherapy Field for Stage IB2-IIB Cervical Cancer" Cancers 16, no. 15: 2743. https://doi.org/10.3390/cancers16152743
APA StyleJo, J. H., Lee, J. W., & Seol, K. H. (2024). Assessing the Adequacy of Traditional Vertebral Landmarks as Upper Border of Whole Pelvic Radiotherapy Field for Stage IB2-IIB Cervical Cancer. Cancers, 16(15), 2743. https://doi.org/10.3390/cancers16152743







