Surface Markers and Chemokines/Cytokines of Tumor-Associated Macrophages in Osteosarcoma and Other Carcinoma Microenviornments—Contradictions and Comparisons
Abstract
Simple Summary
Abstract
1. Introduction
2. Tumor Microenvironment (TME) of OS
M1/M2 Macrophages
3. Tumor-Associated Macrophages (TAMs)
3.1. M1/M2-Related TAM Markers
3.1.1. Iba-1
3.1.2. iNOS
3.1.3. CD80/CD86
3.1.4. CD68
3.1.5. CD163
3.1.6. CD204 (MSR1)
3.1.7. CD206 or C-Type Mannose Receptor 1 (MRC1)
3.1.8. Dendritic Cell-Specific C-Type Lectin (DC-SIGN) or CD209
3.2. Are M1/M2 TAM Markers Reliable Indicators of OS Prognosis?
4. TAM-Related Biomarkers in the TME
4.1. Chemokines
4.1.1. CCL2/Monocyte Chemoattractant Protein-1 (MCP-1)
4.1.2. CCL5 (RANTES)
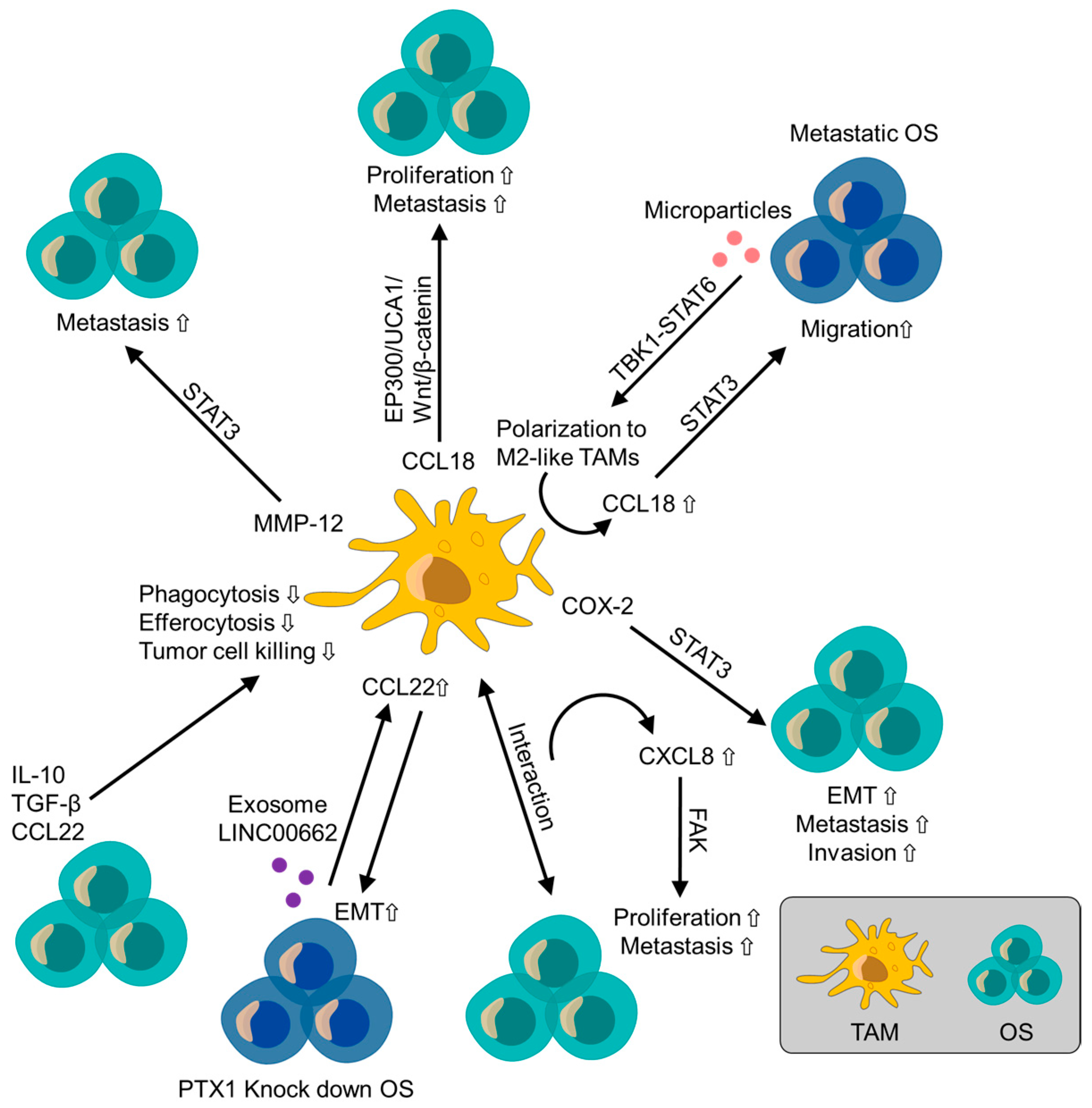
4.1.3. CCL18
4.1.4. CCL22
4.1.5. CXCL1
4.1.6. CXCL5
4.1.7. CXCL6/Granulocyte Chemotaxis Protein 2 (GCP-2)
4.1.8. CXCL8 (IL-8)
4.2. Cytokines and Enzymes
4.2.1. IL-6
4.2.2. Cyclooxygenase-2 (COX-2)
4.2.3. Matrix Metalloproteinase-12 (MMP-12)
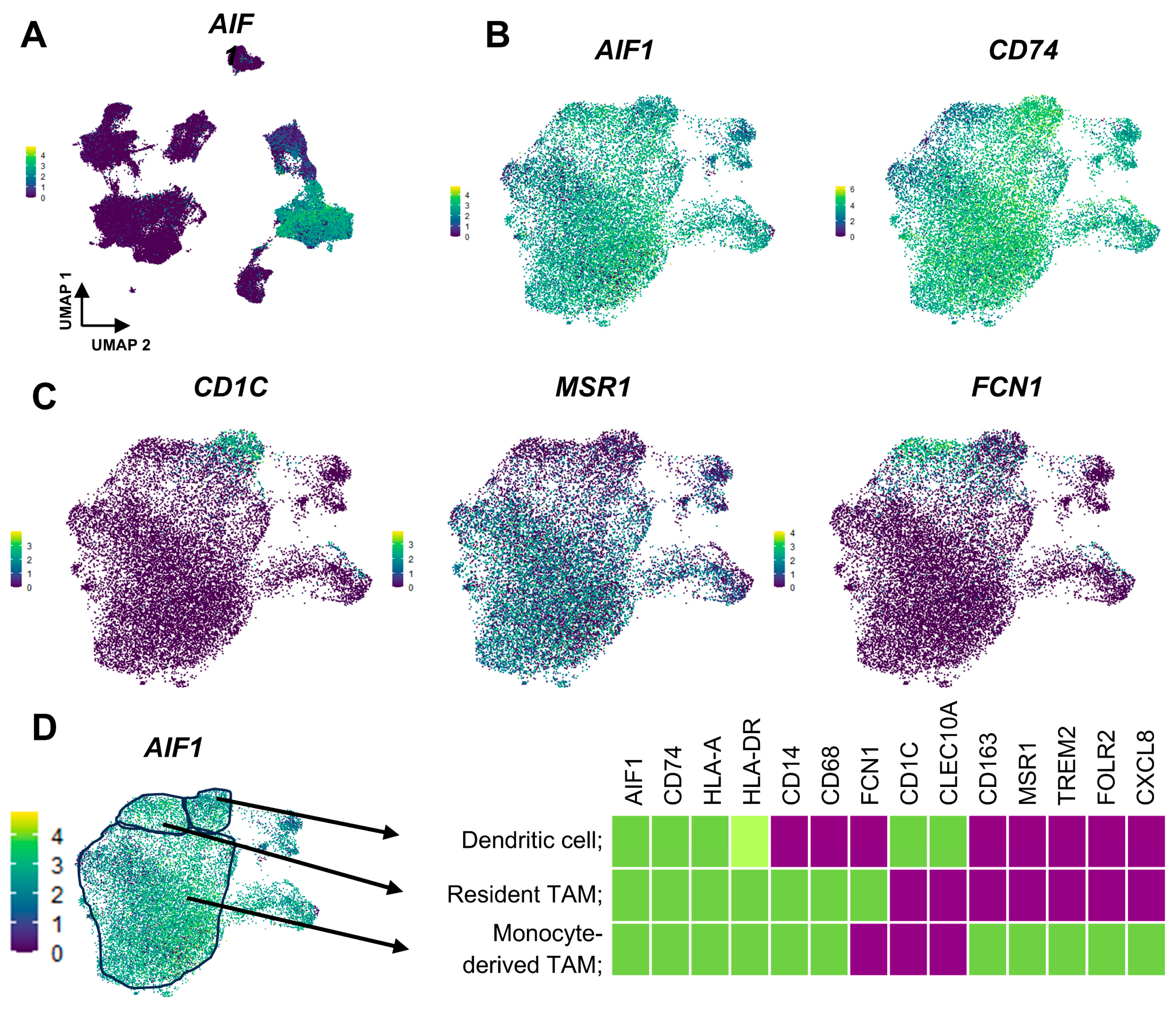
5. Origin of TAMs; Aspect from Single-Cell RNA-Sequence Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allison, D.C.; Carney, S.C.; Ahlmann, E.R.; Hendifar, A.; Chawla, S.; Fedenko, A.; Angeles, C.; Menendez, L.R. A Meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012, 2012, 704872. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Scanagatta, P. Metastatic osteosarcoma: A challenging multidisciplinary treatment. Expert Rev. Anticancer. Ther. 2016, 16, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Smeland, S.; Bruland, Ø.S.; Hjorth, L.; Brosjö, O.; Bjerkehagen, B.; Österlundh, G.; Jakobson, Å.; Hall, K.S.; Monge, O.R.; Björk, O.; et al. Results of the Scandinavian Sarcoma Group XIV protocol for classical osteosarcoma: 63 patients with a minimum follow-up of 4 years. Acta Orthop. 2011, 82, 211–216. [Google Scholar] [CrossRef]
- Inagaki, Y.; Hookway, E.; Williams, K.A.; Hassan, A.B.; Oppermann, U.; Tanaka, Y.; Soilleux, E.; Athanasou, N.A. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin. Sarcoma Res. 2016, 6, 13. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Gao, Q.; Huang, L.; Wang, L.; Wang, J.; Wang, S.; Zhang, B.; Zhang, Y. CD163+CD14+ macrophages, a potential immune biomarker for malignant pleural effusion. Cancer Immunol. Immunother. 2015, 64, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Tang, X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013, 332, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Wen, X.-Y.; Yang, H.K.; Kim, W.H.; Kang, G.H. Prognostic Implication of M2 Macrophages Are Determined by the Proportional Balance of Tumor Associated Macrophages and Tumor Infiltrating Lymphocytes in Microsatellite-Unstable Gastric Carcinoma. PLoS ONE 2015, 10, e0144192. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, M.; Urakawa, N.; Nakamura, T.; Nishio, M.; Watajima, T.; Kuroda, D.; Komori, T.; Kakeji, Y.; Semba, S.; Yokozaki, H. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013, 104, 1112–1119. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Yang, Q.; Lv, X.; Huang, W.; Zhou, Z.; Wang, Y.; Zhang, Z.; Yuan, T.; Ding, X.; et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020, 11, 6322. [Google Scholar] [CrossRef]
- Ichikawa, J.; Ando, T.; Kawasaki, T.; Sasaki, T.; Shirai, T.; Tsukiji, N.; Kimura, Y.; Aoki, K.; Hayakawa, K.; Suzuki-Inoue, K.; et al. Role of Platelet C-Type Lectin-Like Receptor 2 in Promoting Lung Metastasis in Osteosarcoma. J. Bone Miner. Res. 2020, 35, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Ichikawa, J.; Ando, T.; Schoenecker, J.G.; Ohba, T.; Koyama, K.; Suzuki-Inoue, K.; Haro, H. Platelet-Derived TGF-β Induces Tissue Factor Expression via the Smad3 Pathway in Osteosarcoma Cells. J. Bone Miner. Res. 2018, 33, 2048–2058. [Google Scholar] [CrossRef]
- Tan, S.; Chao, R. An Exploration of Osteosarcoma Metastasis Diagnostic Markers Based on Tumor-Associated Neutrophils. Discov. Med. 2023, 35, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Han, X.-G.; Tu, B.; Wang, M.-Q.; Qiao, H.; Zhang, S.-H.; Fan, Q.-M.; Tang, T.-T. CXCR1/Akt signaling activation induced by mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell anoikis resistance and pulmonary metastasis. Cell Death Dis. 2018, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Tanaka, K.; Itonaga, I.; Iwasaki, T.; Tsumura, H. Interaction between human osteosarcoma and mesenchymal stem cells via an interleukin-8 signaling loop in the tumor microenvironment. Cell Commun. Signal. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Komohara, Y.; Oshiumi, H. The role of macrophages in anti-tumor immune responses: Pathological significance and potential as therapeutic targets. Hum. Cell 2021, 34, 1031–1039. [Google Scholar] [CrossRef]
- Chen, J.J.; Yao, P.-L.; Yuan, A.; Hong, T.-M.; Shun, C.-T.; Kuo, M.-L.; Lee, Y.-C.; Yang, P.-C. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: Its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin. Cancer Res. 2003, 9, 729–737. [Google Scholar] [PubMed]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Heideveld, E.; Hampton-O’Neil, L.A.; Cross, S.J.; van Alphen, F.P.J.; van den Biggelaar, M.; Toye, A.M.; van den Akker, E. Glucocorticoids induce differentiation of monocytes towards macrophages that share functional and phenotypical aspects with erythroblastic island macrophages. Haematologica 2018, 103, 395–405. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Takeya, M.; Komohara, Y. Role of tumor-associated macrophages in human malignancies: Friend or foe? Pathol. Int. 2016, 66, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Shinchi, Y.; Ishizuka, S.; Komohara, Y.; Matsubara, E.; Mito, R.; Pan, C.; Yoshii, D.; Yonemitsu, K.; Fujiwara, Y.; Ikeda, K.; et al. The expression of PD-1 ligand 1 on macrophages and its clinical impacts and mechanisms in lung adenocarcinoma. Cancer Immunol. Immunother. 2022, 71, 2645–2661. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.H.M.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed]
- Ambarus, C.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.; Reedquist, K.; Tak, P.; Baeten, D. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef]
- Stöger, J.L.; Gijbels, M.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.; Daemen, M.J.; Lutgens, E.; de Winther, M.P. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef]
- Ohri, C.M.; Shikotra, A.; Green, R.H.; Waller, D.A.; Bradding, P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur. Respir. J. 2009, 33, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Durafourt, B.A.; Moore, C.S.; Zammit, D.A.; Johnson, T.A.; Zaguia, F.; Guiot, M.; Bar-Or, A.; Antel, J.P. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 2012, 60, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, R.; Ichikawa, J.; Komohara, Y.; Pan, C.; Kawasaki, T.; Enomoto, A.; Aoki, K.; Hayakawa, K.; Iwata, S.; Jubashi, T.; et al. Pivotal role of IL-8 derived from the interaction between osteosarcoma and tumor-associated macrophages in osteosarcoma growth and metastasis via the FAK pathway. Cell Death Dis. 2024, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Dumars, C.; Ngyuen, J.-M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.-F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, H.; Chen, Y.; Chen, H.; Yuan, W.; Wang, X. The Heterogeneity of Infiltrating Macrophages in Metastatic Osteosarcoma and Its Correlation with Immunotherapy. J. Oncol. 2021, 2021, 4836292. [Google Scholar] [CrossRef] [PubMed]
- Dhupkar, P.; Gordon, N.; Stewart, J.; Kleinerman, E.S. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. 2018, 7, 2654–2664. [Google Scholar] [CrossRef]
- Li, J.; Su, L.; Xiao, X.; Wu, F.; Du, G.; Guo, X.; Kong, F.; Yao, J.; Zhu, H. Development and Validation of Novel Prognostic Models for Immune-Related Genes in Osteosarcoma. Front. Mol. Biosci. 2022, 9, 828886. [Google Scholar] [CrossRef]
- Song, Y.-J.; Xu, Y.; Zhu, X.; Fu, J.; Deng, C.; Chen, H.; Xu, H.; Song, G.; Lu, J.; Tang, Q.; et al. Immune Landscape of the Tumor Microenvironment Identifies Prognostic Gene Signature CD4/CD68/CSF1R in Osteosarcoma. Front. Oncol. 2020, 10, 1198. [Google Scholar] [CrossRef]
- Han, Q.; Shi, H.; Liu, F. CD163 + M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int. Immunopharmacol. 2016, 34, 101–106. [Google Scholar] [CrossRef]
- Zheng, S.; Peng, J.; Jia, J.; Wu, T.; Cheng, X. Revealing the link between macrophage in microenvironment of osteosarcoma and poor prognosis by utilizing the Integrated analysis. J. Musculoskelet. Neuronal Interact. 2021, 21, 130–137. [Google Scholar] [PubMed]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.-M.; Marie, B.; Larousserie, F.; Entz-Werlé, N.; de Pinieux, G.; et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. OncoImmunology 2017, 6, e1331193. [Google Scholar] [CrossRef]
- Han, Z.-P.; Liu, D.-B.; Wu, L.-Q.; Li, Q.; Wang, Z.-G.; Zang, X.-F. IL-1β secreted by macrophage M2 promotes metastasis of osteosarcoma via NF-κB/miR-181α-5p/RASSF1A/Wnt pathway. Transl. Cancer Res. 2020, 9, 2721–2733. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ding, G.; Jiang, W.; Fan, X.; Zhu, L. Effect of tumor-associated macrophages on lncRNA PURPL/miR-363/PDZD2 axis in osteosarcoma cells. Cell Death Discov. 2021, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, H.; Wang, Y.; Zhan, F.; Quan, Z. Identification of Immune-Related Genes MSR1 and TLR7 in Relation to Macrophage and Type-2 T-Helper Cells in Osteosarcoma Tumor Micro-Environments as Anti-metastasis Signatures. Front. Mol. Biosci. 2020, 7, 576298. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Bellini, G.; Tortora, C.; Di Pinto, D.; Argenziano, M.; Pota, E.; Di Paola, A.; Di Martino, M.; Rossi, F. Mifamurtide and TAM-like macrophages: Effect on proliferation, migration and differentiation of osteosarcoma cells. Oncotarget 2020, 11, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.-J.; Xiang, S.-F.; Chen, Y.-Q.; Zhang, N.; Cao, J.; Zhu, H.; Yang, B.; Zhou, Q.; Ying, M.-D.; He, Q.-J. Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharmacol. Sin. 2019, 40, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Motoshima, T.; Miura, Y.; Wakigami, N.; Kusada, N.; Takano, T.; Inoshita, N.; Okaneya, T.; Sugiyama, Y.; Kamba, T.; Takeya, M.; et al. Phenotypical change of tumor-associated macrophages in metastatic lesions of clear cell renal cell carcinoma. Med. Mol. Morphol. 2018, 51, 57–63. [Google Scholar] [CrossRef]
- Yonemitsu, K.; Pan, C.; Fujiwara, Y.; Miyasato, Y.; Shiota, T.; Yano, H.; Hosaka, S.; Tamada, K.; Yamamoto, Y.; Komohara, Y. GM-CSF derived from the inflammatory microenvironment potentially enhanced PD-L1 expression on tumor-associated macrophages in human breast cancer. Sci. Rep. 2022, 12, 12007. [Google Scholar] [CrossRef]
- Tartey, S.; Neale, G.; Vogel, P.; Malireddi, R.S.; Kanneganti, T.-D. A MyD88/IL1R Axis Regulates PD-1 Expression on Tumor-Associated Macrophages and Sustains Their Immunosuppressive Function in Melanoma. Cancer Res. 2021, 81, 2358–2372. [Google Scholar] [CrossRef]
- Komohara, Y.; Takeya, H.; Wakigami, N.; Kusada, N.; Bekki, H.; Ishihara, S.; Takeya, M.; Nakashima, Y.; Oda, Y. Positive correlation between the density of macrophages and T-cells in undifferentiated sarcoma. Med. Mol. Morphol. 2019, 52, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, D.; Fujiwara, Y.; Horlad, H.; Saito, Y.; Iriki, T.; Tsuboki, J.; Cheng, P.; Nakagata, N.; Mizuta, H.; Bekki, H.; et al. CD163 Is Required for Protumoral Activation of Macrophages in Human and Murine Sarcoma. Cancer Res. 2018, 78, 3255–3266. [Google Scholar] [CrossRef]
- Kashfi, K. Nitric oxide in cancer and beyond. Biochem. Pharmacol. 2020, 176, 114006. [Google Scholar] [CrossRef]
- Chan, E.D.; Riches, D.W.H. IFN-γ + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 mapk in a mouse macrophage cell line. Am. J. Physiol. Cell. Physiol. 2001, 280, C441–C450. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Q.; Su, S.; Dong, W.; Zong, S.; Ma, Q.; Yang, X.; Zuo, D.; Zheng, S.; Meng, X.; et al. Interleukin 37 Suppresses M1 Macrophage Polarization Through Inhibition of the Notch1 and Nuclear Factor Kappa B Pathways. Front. Cell Dev. Biol. 2020, 8, 56. [Google Scholar] [CrossRef]
- Wang, X.; Yuwen, T.-J.; Zhong, Y.; Li, Z.-G.; Wang, X.-Y. A new method for predicting the prognosis of colorectal cancer patients through a combination of multiple tumor-associated macrophage markers at the invasive front. Heliyon 2023, 9, e13211. [Google Scholar] [CrossRef]
- Peach, R.J.; Bajorath, J.; Naemura, J.; Leytze, G.; Greene, J.; Aruffo, A.; Linsley, P.S. Both Extracellular Immunoglobin-like domains of CD80 contain residues critical for binding T cell surface receptors CTLA-4 and CD28. J. Biol. Chem. 1995, 270, 21181–21187. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Fushida, S.; Yamamoto, Y.; Tsukada, T.; Kinoshita, J.; Oyama, K.; Miyashita, T.; Tajima, H.; Ninomiya, I.; Munesue, S.; et al. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer 2016, 19, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tian, Z.; Du, Z.; Wu, K.; Xu, G.; Dai, M.; Wang, Y.; Xiao, M. M1-like tumor-associated macrophages cascade a mesenchymal/stem-like phenotype of oral squamous cell carcinoma via the IL6/Stat3/THBS1 feedback loop. J. Exp. Clin. Cancer Res. 2022, 41, 10. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Liu, F.; Yang, K. Role of CD68 in tumor immunity and prognosis prediction in pan-cancer. Sci. Rep. 2022, 12, 7844. [Google Scholar] [CrossRef]
- A Pulford, K.; Rigney, E.M.; Micklem, K.J.; Jones, M.; Stross, W.P.; Gatter, K.C.; Mason, D.Y. KP1: A new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J. Clin. Pathol. 1989, 42, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Rashed, R.; Zaki, M.; Mohamed, N.; Mansou, O.; Refaey, F. Prognostic Value of Tumor Associated Macrophage Markers CD163 and CD68 Immunohistochemistry in Classical Hodgkin Lymphoma. Clin. Lab. 2021, 67, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Dong, Y.; Zhang, X.; Ding, Z.; Song, Y.; Huang, X.; Chen, S.; Wang, Z.; Ni, Y.; Ding, L. Lipid Droplet-Related PLIN2 in CD68+ Tumor-Associated Macrophage of Oral Squamous Cell Carcinoma: Implications for Cancer Prognosis and Immunotherapy. Front. Oncol. 2022, 12, 824235. [Google Scholar] [CrossRef]
- Kayal, S.; Mathur, S.; Karak, A.K.; Kumar, L.; Sharma, A.; Bakhshi, S.; Raina, V. CD68 tumor-associated macrophage marker is not prognostic of clinical outcome in classical Hodgkin lymphoma. Leuk. Lymphoma 2014, 55, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Buddingh, E.P.; Kuijjer, M.L.; Duim, R.A.; Bürger, H.; Agelopoulos, K.; Myklebost, O.; Serra, M.; Mertens, F.; Hogendoorn, P.C.; Lankester, A.C.; et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin. Cancer Res. 2011, 17, 2110–2119. [Google Scholar] [CrossRef]
- Gomez-Brouchet, A.; Gilhodes, J.; Van Acker, N.; Brion, R.; Bouvier, C.; Assemat, P.; Gaspar, N.; Aubert, S.; Guinebretiere, J.-M.; Marie, B.; et al. Characterization of Macrophages and Osteoclasts in the Osteosarcoma Tumor Microenvironment at Diagnosis: New Perspective for Osteosarcoma Treatment? Cancers 2021, 13, 423. [Google Scholar] [CrossRef]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef]
- Medrek, C.; Pontén, F.; Jirström, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.-J.; Law, S.K.A.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Komohara, Y.; Jinushi, M.; Takeya, M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014, 105, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Yuan, T.; Wang, L.; Zang, L.; Liu, Q.; Wang, L.; Huo, X.; Huo, B.; Tang, Y.; et al. Tumor-associated macrophages and PD-L1 in prostate cancer: A possible key to unlocking immunotherapy efficacy. Aging 2024, 16, 445–465. [Google Scholar] [CrossRef]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Xie, T.; Fu, D.-J.; Li, Z.-M.; Lv, D.-J.; Song, X.-L.; Yu, Y.-Z.; Wang, C.; Li, K.-J.; Zhai, B.; Wu, J.; et al. Correction: CircSMARCC1 facilitates tumor progression by disrupting the crosstalk between prostate cancer cells and tumor-associated macrophages via miR-1322/CCL20/CCR6 signaling. Mol. Cancer 2023, 22, 173. [Google Scholar] [CrossRef]
- Bao, D.; Zhao, J.; Zhou, X.; Yang, Q.; Chen, Y.; Zhu, J.; Yuan, P.; Yang, J.; Qin, T.; Wan, S.; et al. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene 2019, 38, 5007–5020. [Google Scholar] [CrossRef]
- Yoshida, C.; Kadota, K.; Yamada, K.; Fujimoto, S.; Ibuki, E.; Ishikawa, R.; Haba, R.; Yokomise, H. Tumor-associated CD163+ macrophage as a predictor of tumor spread through air spaces and with CD25+ lymphocyte as a prognostic factor in resected stage I lung adenocarcinoma. Lung Cancer 2022, 167, 34–40. [Google Scholar] [CrossRef]
- Guo, F.; Feng, Y.-C.; Zhao, G.; Zhang, R.; Cheng, Z.-Z.; Kong, W.-N.; Wu, H.-L.; Xu, B.; Lv, X.; Ma, X.-M. Tumor-Associated CD163+ M2 Macrophage Infiltration is Highly Associated with PD-L1 Expression in Cervical Cancer. Cancer Manag. Res. 2020, 12, 5831–5843. [Google Scholar] [CrossRef]
- Ramos, R.N.; Rodriguez, C.; Hubert, M.; Ardin, M.; Treilleux, I.; Ries, C.H.; Lavergne, E.; Chabaud, S.; Colombe, A.; Trédan, O.; et al. CD163+ tumor-associated macrophage accumulation in breast cancer patients reflects both local differentiation signals and systemic skewing of monocytes. Clin. Transl. Immunol. 2020, 9, e1108. [Google Scholar] [CrossRef]
- Liang, Y.; Lei, Y.; Liang, M.; Du, M.; Liu, Z.; Li, X.; Meng, X.; Zhou, B.; Gao, Y. GBE1 Is an Independent Prognostic Marker and Associated With CD163+ Tumor-Associated Macrophage Infiltration in Lung Adenocarcinoma. Front. Oncol. 2021, 11, 781344. [Google Scholar] [CrossRef]
- Zhu, D.; Shi, X.; Tian, Y.; Li, H.; Tang, B.; Zhang, Z.; Zhang, Z.; Zuo, L. Combining expression of RNF43 and infiltration level of CD163+ tumor associated macrophage predicts prognosis of clear cell renal cell carcinoma. Cancer Med. 2023, 12, 3962–3971. [Google Scholar] [CrossRef]
- Edin, S.; Wikberg, M.L.; Dahlin, A.M.; Rutegård, J.; Öberg, Å.; Oldenborg, P.-A.; Palmqvist, R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS ONE 2012, 7, e47045. [Google Scholar] [CrossRef]
- Nyström, H.; Jönsson, M.; Nilbert, M.; Carneiro, A. Immune-cell infiltration in high-grade soft tissue sarcomas; prognostic implications of tumor-associated macrophages and B-cells. Acta Oncol. 2023, 62, 33–39. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, S. Tumor-Associated CD204-Positive Macrophage Is a Prognostic Marker in Clinical Stage I Lung Adenocarcinoma. BioMed Res. Int. 2018, 2018, 8459193. [Google Scholar] [CrossRef]
- He, Y.; Zhou, S.; Deng, F.; Zhao, S.; Chen, W.; Wang, D.; Chen, X.; Hou, J.; Zhang, J.; Zhang, W.; et al. Clinical and transcriptional signatures of human CD204 reveal an applicable marker for the protumor phenotype of tumor-associated macrophages in breast cancer. Aging 2019, 11, 10883–10901. [Google Scholar] [CrossRef]
- Miyasato, Y.; Shiota, T.; Ohnishi, K.; Pan, C.; Yano, H.; Horlad, H.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Iwase, H.; Takeya, M.; et al. High density of CD204-positive macrophages predicts worse clinical prognosis in patients with breast cancer. Cancer Sci. 2017, 108, 1693–1700. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, G.; Xie, P.; Zhao, X.; Chen, C.; Li, X.; Zhang, Y.; Wang, B.; Luo, Y. High CD204+ tumor-associated macrophage density predicts a poor prognosis in patients with clear cell renal cell carcinoma. J. Cancer 2024, 15, 1511–1522. [Google Scholar] [CrossRef]
- Li, Z.; Maeda, D.; Yoshida, M.; Umakoshi, M.; Nanjo, H.; Shiraishi, K.; Saito, M.; Kohno, T.; Konno, H.; Saito, H.; et al. The intratumoral distribution influences the prognostic impact of CD68- and CD204-positive macrophages in non-small cell lung cancer. Lung Cancer 2018, 123, 127–135. [Google Scholar] [CrossRef]
- Ikarashi, D.; Kitano, S.; Tsuyukubo, T.; Takenouchi, K.; Nakayama, T.; Onagi, H.; Sakaguchi, A.; Yamashita, M.; Mizugaki, H.; Maekawa, S.; et al. Pretreatment tumour immune microenvironment predicts clinical response and prognosis of muscle-invasive bladder cancer in the neoadjuvant chemotherapy setting. Br. J. Cancer 2022, 126, 606–614. [Google Scholar] [CrossRef]
- Ichimura, T.; Morikawa, T.; Kawai, T.; Nakagawa, T.; Matsushita, H.; Kakimi, K.; Kume, H.; Ishikawa, S.; Homma, Y.; Fukayama, M. Prognostic significance of CD204-positive macrophages in upper urinary tract cancer. Ann. Surg. Oncol. 2014, 21, 2105–2112. [Google Scholar] [CrossRef]
- Qi, Y.-Q.; Xiong, F.; Chen, Y.-J. The Correlation between tumor-associated macrophages and the prognosis of east asian hepatocellular carcinoma patients: A systematic review and meta-analysis. Pathol. Res. Pr. 2023, 252, 154919. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a dendritic cell–specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, Z.; Zeng, H.; Qi, Y.; Chen, Y.; Wang, T.; Wang, J.; Chang, Y.; Bai, Q.; Xia, Y.; et al. Blockade of DC-SIGN+ Tumor-Associated Macrophages Reactivates Antitumor Immunity and Improves Immunotherapy in Muscle-Invasive Bladder Cancer. Cancer Res. 2020, 80, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Ohnishi, K.; Pan, C.; Yano, H.; Fujiwara, Y.; Shiota, T.; Mikami, Y.; Komohara, Y. Expression of macrophage/dendritic cell–related molecules in lymph node sinus macrophages. Microbiol. Immunol. 2023, 67, 490–500. [Google Scholar] [CrossRef]
- He, M.; Jiang, X.; Miao, J.; Feng, W.; Xie, T.; Liao, S.; Qin, Z.; Tang, H.; Lin, C.; Li, B.; et al. A new insight of immunosuppressive microenvironment in osteosarcoma lung metastasis. Exp. Biol. Med. 2023, 248, 1056–1073. [Google Scholar] [CrossRef]
- Graziano, F.; Vicenzi, E.; Poli, G. Plastic restriction of HIV-1 replication in human macrophages derived from M1/M2 polarized monocytes. J. Leukoc. Biol. 2016, 100, 1147–1153. [Google Scholar] [CrossRef]
- Ugel, S.; De Sanctis, F.; Mandruzzato, S.; Bronte, V. Tumor-induced myeloid deviation: When myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Investig. 2015, 125, 3365–3376. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The Chemokine Superfamily Revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Cho, H.R.; Kumari, N.; Vu, H.T.; Kim, H.; Park, C.-K.; Choi, S.H. Increased Antiangiogenic Effect by Blocking CCL2-dependent Macrophages in a Rodent Glioblastoma Model: Correlation Study with Dynamic Susceptibility Contrast Perfusion MRI. Sci. Rep. 2019, 9, 11085. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z.; Wei, S.; Liu, Z.; Chen, G. Epigenetic silencing of chemokine CCL2 represses macrophage infiltration to potentiate tumor development in small cell lung cancer. Cancer Lett. 2021, 499, 148–163. [Google Scholar] [CrossRef]
- Cranford, T.L.; Velázquez, K.T.; Enos, R.T.; Bader, J.E.; Carson, M.S.; Chatzistamou, I.; Nagarkatti, M.; Murphy, E.A. Loss of monocyte chemoattractant protein-1 expression delays mammary tumorigenesis and reduces localized inflammation in the C3(1)/SV40Tag triple negative breast cancer model. Cancer Biol. Ther. 2017, 18, 85–93. [Google Scholar] [CrossRef]
- Rogic, A.; Pant, I.; Grumolato, L.; Fernandez-Rodriguez, R.; Edwards, A.; Das, S.; Sun, A.; Yao, S.; Qiao, R.; Jaffer, S.; et al. High endogenous CCL2 expression promotes the aggressive phenotype of human inflammatory breast cancer. Nat. Commun. 2021, 12, 6889. [Google Scholar] [CrossRef]
- Moisan, F.; Francisco, E.B.; Brozovic, A.; Duran, G.E.; Wang, Y.C.; Chaturvedi, S.; Seetharam, S.; Snyder, L.A.; Doshi, P.; Sikic, B.I. Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol. Oncol. 2014, 8, 1231–1239. [Google Scholar] [CrossRef]
- Liu, J.-F.; Chen, P.-C.; Chang, T.-M.; Hou, C.-H. Monocyte Chemoattractant Protein-1 promotes cancer cell migration via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. J. Exp. Clin. Cancer Res. 2020, 39, 254. [Google Scholar] [CrossRef]
- Ohba, T.; A Cole, H.; Cates, J.M.; A Slosky, D.; Haro, H.; Ando, T.; Schwartz, H.S.; Schoenecker, J.G. Bisphosphonates inhibit osteosarcoma-mediated osteolysis via attenuation of tumor expression of MCP-1 and RANKL. J. Bone Miner. Res. 2014, 29, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, J.; Li, J.; Zhang, Q.; Zuo, Q. TIPE1 suppresses osteosarcoma tumor growth by regulating macrophage infiltration. Clin. Transl. Oncol. 2019, 21, 334–341. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2022, 9, 12–27. [Google Scholar] [CrossRef]
- Huang, R.; Wang, S.; Wang, N.; Zheng, Y.; Zhou, J.; Yang, B.; Wang, X.; Zhang, J.; Guo, L.; Wang, S.; et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Yonemitsu, K.; Miyasato, Y.; Shiota, T.; Shinchi, Y.; Fujiwara, Y.; Hosaka, S.; Yamamoto, Y.; Komohara, Y. Soluble Factors Involved in Cancer Cell–Macrophage Interaction Promote Breast Cancer Growth. Anticancer. Res. 2021, 41, 4249–4258. [Google Scholar] [CrossRef]
- Nie, Y.; Huang, H.; Guo, M.; Chen, J.; Wu, W.; Li, W.; Xu, X.; Lin, X.; Fu, W.; Yao, Y.-D.; et al. Breast Phyllodes Tumors Recruit and Repolarize Tumor-Associated Macrophages via Secreting CCL5 to Promote Malignant Progression, Which Can Be Inhibited by CCR5 Inhibition Therapy. Clin. Cancer Res. 2019, 25, 3873–3886. [Google Scholar] [CrossRef] [PubMed]
- Terashima, Y.; Toda, E.; Itakura, M.; Otsuji, M.; Yoshinaga, S.; Okumura, K.; Shand, F.H.W.; Komohara, Y.; Takeda, M.; Kokubo, K.; et al. Targeting FROUNT with disulfiram suppresses macrophage accumulation and its tumor-promoting properties. Nat. Commun. 2020, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shayiti, F.; Ma, J.; Wei, M.; Hua, T.; Zhang, R.; Su, J.; Chen, P. Tumor-associated macrophage-derived CCL5 promotes chemotherapy resistance and metastasis in prostatic cancer. Cell Biol. Int. 2021, 45, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-W.; Wu, H.-H.; Liu, S.-C.; Wang, P.-C.; Ou, W.-C.; Chou, W.-Y.; Shen, Y.-S.; Tang, C.-H. CCL5 and CCR5 Interaction Promotes Cell Motility in Human Osteosarcoma. PLoS ONE 2012, 7, e35101. [Google Scholar] [CrossRef]
- Wang, S.-W.; Liu, S.-C.; Sun, H.-L.; Huang, T.-Y.; Chan, C.-H.; Yang, C.-Y.; Yeh, H.-I.; Huang, Y.-L.; Chou, W.-Y.; Lin, Y.-M.; et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 2015, 36, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.A.; Ling, M.F.; Leung, J.; Shreffler, W.G.; Luster, A.D. Identification of human CCR8 as a CCL18 receptor. J. Exp. Med. 2013, 210, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Chen, C.; Zhou, X.; Wen, X.; Shi, C.; Chen, G.; Liu, J.; He, Z.; Yao, Y.; Li, Y.; et al. Integrative analysis of bulk and single-cell gene expression profiles to identify tumor-associated macrophage-derived CCL18 as a therapeutic target of esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2023, 42, 51. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, Y.-S.; Yao, Y.-D.; Chen, J.-Q.; Chen, J.-N.; Huang, S.-Y.; Zeng, Y.-J.; Yao, H.-R.; Zeng, S.-H.; Fu, Y.-S.; et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget 2015, 6, 34758–34773. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, Y.; Gong, C.; Yu, F.; Su, S.; Chen, J.; Liu, B.; Deng, H.; Wang, F.; Lin, L.; et al. CCL18 from Tumor-Associated Macrophages Promotes Breast Cancer Metastasis via PITPNM3. Cancer Cell 2011, 19, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhou, Y.; Sun, Y.-J.; Wang, Y.-L.; Yin, J.-Y.; Huang, Y.-J.; Zhang, J.-J.; He, A.-N.; Han, K.; Zhang, H.-Z.; et al. Macrophage-derived CCL18 promotes osteosarcoma proliferation and migration by upregulating the expression of UCA1. J. Mol. Med. 2019, 97, 49–61. [Google Scholar] [CrossRef]
- Li, C.; Xiang, F.; Gong, Y.; Fu, Y.; Chen, G.; Wang, Z.; Li, Z.; Wei, D. Tumor-derived microparticles promoted M2-like macrophages polarization to stimulate osteosarcoma progression. Int. J. Biochem. Cell Biol. 2024, 166, 106494. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, D.; Zhang, L.; Zhang, J.; Xiao, Y.; Wu, Q.; Wang, Y.; Zhan, Q. Tumor-associated macrophage (TAM)-derived CCL22 induces FAK addiction in esophageal squamous cell carcinoma (ESCC). Cell. Mol. Immunol. 2022, 19, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Yaguchi, T.; Ohmura, G.; Ohta, S.; Kobayashi, A.; Kawamura, N.; Fujita, T.; Nakano, H.; Shimada, T.; Takahashi, T.; et al. Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma. Int. J. Cancer 2013, 132, 2755–2766. [Google Scholar] [CrossRef]
- Wolf-Dennen, K.; Gordon, N.; Kleinerman, E.S. Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. OncoImmunology 2020, 9, 1747677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Chen, C.; Guo, H.; Zhou, C.; Wang, H.; Liu, Z. PITX1 suppresses osteosarcoma metastasis through exosomal LINC00662-mediated M2 macrophage polarization. Clin. Exp. Metastasis 2023, 40, 79–93. [Google Scholar] [CrossRef]
- Korbecki, J.; Bosiacki, M.; Szatkowska, I.; Kupnicka, P.; Chlubek, D.; Baranowska-Bosiacka, I. The Clinical Significance and Involvement in Molecular Cancer Processes of Chemokine CXCL1 in Selected Tumors. Int. J. Mol. Sci. 2024, 25, 4365. [Google Scholar] [CrossRef]
- Miyake, M.; Hori, S.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Anai, S.; Torimoto, K.; Aoki, K.; Tanaka, N.; Shimada, K.; et al. Mediated Interaction of Cancer Cells with Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia 2016, 18, 636–646, Erratum in Neoplasia 2016, 18, 250–251. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, S.; Wang, L.; Zhao, L.; Li, T.; Zhao, X.; Zhang, L. M2 macrophages promote PD-L1 expression in triple-negative breast cancer via secreting CXCL1. Pathol. Res. Pr. 2024, 260, 155458. [Google Scholar] [CrossRef]
- Lee, C.-W.; Chiang, Y.-C.; Yu, P.-A.; Peng, K.-T.; Chi, M.-C.; Lee, M.-H.; Fang, M.-L.; Lee, K.-H.; Hsu, L.-F.; Liu, J.-F. A Role of CXCL1 Drives Osteosarcoma Lung Metastasis via VCAM-1 Production. Front. Oncol. 2021, 11, 735277. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-C.; Lee, C.-W.; Chang, T.-M.; Chen, P.-C.; Liu, J.-F. CXCL1/CXCR2 Paracrine Axis Contributes to Lung Metastasis in Osteosarcoma. Cancers 2020, 12, 459. [Google Scholar] [CrossRef]
- Chang, M.S.; McNinch, J.; Basu, R.; Simonet, S. Cloning and characterization of the human neutrophil-activating peptide (ENA-78) gene. J. Biol. Chem. 1994, 269, 25277–25282. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Jiang, L.; Zhang, Y.; Yu, T.; Kang, W.; Liu, Y.; Yu, J. Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/mTOR pathway in gastric cancer. Cancer Cell Int. 2022, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Wu, W.; Wang, B.; Cui, C.; Niu, J.; Chen, J.; Chen, Z.; Liu, Y. CXCL5 Plays a Promoting Role in Osteosarcoma Cell Migration and Invasion in Autocrine- and Paracrine-Dependent Manners. Oncol. Res. 2017, 25, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Wuyts, A.; Conings, R.; Lenaerts, J.P.; Billiau, A.; Opdenakker, G.; Van Damme, J. Human and bovine granulocyte chemotactic protein-2: Complete amino acid sequence and functional characterization as chemokines. Biochemistry 1993, 32, 10170–10177. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-L.; Tsai, Y.-C.; Pikatan, N.W.; Yeh, C.-T.; Yadav, V.K.; Chen, M.-Y.; Tsai, J.-T. Tumor-Associated Macrophages Affect the Tumor Microenvironment and Radioresistance via the Upregulation of CXCL6/CXCR2 in Hepatocellular Carcinoma. Biomedicines 2023, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; An, L.; Zhang, H.; Du, P.; Sheng, Y. Activation of CXCL6/CXCR1/2 Axis Promotes the Growth and Metastasis of Osteosarcoma Cells in vitro and in vivo. Front. Pharmacol. 2019, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Knall, C.; Worthen, G.S.; Johnson, G.L. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc. Natl. Acad. Sci. USA 1997, 94, 3052–3057. [Google Scholar] [CrossRef]
- Brat, D.J.; Bellail, A.C.; Van Meir, E.G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-Oncol. 2005, 7, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, X.; Han, D.; Cao, J.; Tian, J. Tumour-associated macrophages mediate the invasion and metastasis of bladder cancer cells through CXCL8. PeerJ 2020, 8, e8721. [Google Scholar] [CrossRef]
- Fang, W.; Ye, L.; Shen, L.; Cai, J.; Huang, F.; Wei, Q.; Fei, X.; Chen, X.; Guan, H.; Wang, W.; et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis 2014, 35, 1780–1787. [Google Scholar] [CrossRef]
- Piao, H.; Fu, L.; Wang, Y.; Liu, Y.; Wang, Y.; Meng, X.; Yang, D.; Xiao, X.; Zhang, J. A positive feedback loop between gastric cancer cells and tumor-associated macrophage induces malignancy progression. J. Exp. Clin. Cancer Res. 2022, 41, 174. [Google Scholar] [CrossRef] [PubMed]
- Hosono, M.; Koma, Y.-I.; Takase, N.; Urakawa, N.; Higashino, N.; Suemune, K.; Kodaira, H.; Nishio, M.; Shigeoka, M.; Kakeji, Y.; et al. CXCL8 derived from tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression by promoting migration and invasion of cancer cells. Oncotarget 2017, 8, 106071–106088. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Cao, X.; Mao, Y.; Lv, Z.; Lv, M.; Wang, Y.; Wang, H.; Liu, C. Tumor-associated macrophages-mediated CXCL8 infiltration enhances breast cancer metastasis: Suppression by Danirixin. Int. Immunopharmacol. 2021, 95, 107153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, X.; Miao, W.; Wang, B.; Qiu, Y. CXCL8 promotes the invasion of human osteosarcoma cells by regulation of PI3K/Akt signaling pathway. APMIS 2017, 125, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.-I.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Y.; Sun, J.; Chen, W.; Zhao, L.; Ma, C.; Wang, Q.; Sun, J.; Huang, B.; Zhang, Y.; et al. M2-like tumor-associated macrophages drive vasculogenic mimicry through amplification of IL-6 expression in glioma cells. Oncotarget 2017, 8, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Chang, Z.-L.; Liao, Y.-Y.; Chou, M.-C.; Tang, C.-H. IL-6 promotes ICAM-1 expression and cell motility in human osteosarcoma. Cancer Lett. 2013, 328, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Du, L.; Fan, Q.-M.; Tang, Z.; Tang, T.-T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012, 325, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef]
- Qin, Q.; Ji, H.; Li, D.; Zhang, H.; Zhang, Z.; Zhang, Q. Tumor-associated macrophages increase COX-2 expression promoting endocrine resistance in breast cancer via the PI3K/Akt/mTOR pathway. Neoplasma 2021, 68, 938–946. [Google Scholar] [CrossRef]
- Tsai, C.-S.; Chen, F.-H.; Wang, C.-C.; Huang, H.-L.; Jung, S.-M.; Wu, C.-J.; Lee, C.-C.; McBride, W.H.; Chiang, C.-S.; Hong, J.-H. Macrophages From Irradiated Tumors Express Higher Levels of iNOS, Arginase-I and COX-2, and Promote Tumor Growth. Int. J. Radiat. Oncol. 2007, 68, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019, 440–441, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, W.; Ali, M.A.M.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, J.; Koike, T.; Sun, H.; Ichikawa, T.; Kitajima, S.; Morimoto, M.; Shikama, H.; Watanabe, T.; Sasaguri, Y.; et al. Overexpression of human matrix metalloproteinase-12 enhances the development of inflammatory arthritis in transgenic rabbits. Am. J. Pathol. 2004, 165, 1375–1383. [Google Scholar] [CrossRef]
- Kerzeli, I.K.; Kostakis, A.; Türker, P.; Malmström, P.-U.; Hemdan, T.; Mezheyeuski, A.; Ward, D.G.; Bryan, R.T.; Segersten, U.; Lord, M.; et al. Elevated levels of MMP12 sourced from macrophages are associated with poor prognosis in urothelial bladder cancer. BMC Cancer 2023, 23, 605. [Google Scholar] [CrossRef]
- Zhou, Q.; Xian, M.; Xiang, S.; Xiang, D.; Shao, X.; Wang, J.; Cao, J.; Yang, X.; Yang, B.; Ying, M.; et al. All-Trans Retinoic Acid Prevents Osteosarcoma Metastasis by Inhibiting M2 Polarization of Tumor-Associated Macrophages. Cancer Immunol. Res. 2017, 5, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, M.; Koma, Y.-I.; Hosono, M.; Urakawa, N.; Tanigawa, K.; Shimizu, M.; Kodama, T.; Sakamoto, H.; Nishio, M.; Shigeoka, M.; et al. Chemokine (C-C Motif) Ligand 1 Derived from Tumor-Associated Macrophages Contributes to Esophageal Squamous Cell Carcinoma Progression via CCR8-Mediated Akt/Proline-Rich Akt Substrate of 40 kDa/Mammalian Target of Rapamycin Pathway. Am. J. Pathol. 2021, 191, 686–703. [Google Scholar] [CrossRef]
- Liu, W.; Wang, W.; Wang, X.; Xu, C.; Zhang, N.; Di, W. Cisplatin-stimulated macrophages promote ovarian cancer migration via the CCL20-CCR6 axis. Cancer Lett. 2020, 472, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Kadomoto, S.; Izumi, K.; Hiratsuka, K.; Nakano, T.; Naito, R.; Makino, T.; Iwamoto, H.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Tumor-Associated Macrophages Induce Migration of Renal Cell Carcinoma Cells via Activation of the CCL20-CCR6 Axis. Cancers 2019, 12, 89. [Google Scholar] [CrossRef]
- Zhou, K.; Cheng, T.; Zhan, J.; Peng, X.; Zhang, Y.; Wen, J.; Chen, X.; Ying, M. Targeting tumor-associated macrophages in the tumor microenvironment. Oncol. Lett. 2020, 20, 234. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, R.; Xiang, W.; Kang, X.; Tang, B.; Li, C.; Gao, L.; Zhang, X.; Zhang, L.; Dai, R.; et al. The Agpat4/LPA axis in colorectal cancer cells regulates antitumor responses via p38/p65 signaling in macrophages. Signal Transduct. Target. Ther. 2020, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Peh, K.H.; Kolesar, J.M. Macrophage Repolarization as a Therapeutic Strategy for Osteosarcoma. Int. J. Mol. Sci. 2023, 24, 2858. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Li, F.; Zhao, Z.; Zhang, Z.; Hu, J.; Zhang, Y. Tumor-Associated Macrophage Promotes the Survival of Cancer Cells upon Docetaxel Chemotherapy via the CSF1/CSF1R–CXCL12/CXCR4 Axis in Castration-Resistant Prostate Cancer. Genes 2021, 12, 773. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Guo, W.; Ren, T.; Huang, Y.; Sun, K.; Zhang, H.; Yu, Y.; Wang, W.; Niu, J. Macrophages reduce the sensitivity of osteosarcoma to neoadjuvant chemotherapy drugs by secreting Interleukin-1 beta. Cancer Lett. 2020, 480, 4–14. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Antitumor and antimetastatic actions of dihydroxycoumarins (esculetin or fraxetin) through the inhibition of M2 macrophage differentiation in tumor-associated macrophages and/or G1 arrest in tumor cells. Eur. J. Pharmacol. 2015, 746, 115–125. [Google Scholar] [CrossRef]

| TAM Marker | Function | Outcome | References |
|---|---|---|---|
| Iba-1 | Pan TAM marker | Iba-1+ TAM primary site < metastases | [34] |
| iNOS | Anti-tumor | Negative correlation between expression and lung metastasis. Predictor of non-metastasis | [35] |
| CD80 | Anti-tumor | CD80+ TAM primary site > metastases Decreased M1 TAM is associated with osteosarcoma metastasis | [36] |
| CD86 | Anti-tumor | Anti-PD-1 treatment causes repolarization from M2 to M1 TAMs and suppresses pulmonary metastasis of osteosarcoma | [37] |
| CD86 | Anti-tumor | Positive correlation between expression and prognosis Mainly expressed in M2 TAMs | [38] |
| CD68 | Anti-tumor | Positive correlation between CD68+ TAM infiltration and prognosis | [39] |
| CD68 | - | The amount of CD68+ TAM is the same with and without metastasis | [35] |
| CD163 | Pro-tumor | Enhances T-cell suppression | [40] |
| CD163 | Pro-tumor | High CD163 expression correlates with poor OS prognosis | [41] |
| CD163 | Anti-tumor | Positive correlation with improved overall survival and prolonged metastasis-free survival | [42] |
| CD204 | Pro-tumor | Promotes OS cell progression and metastasis | [43] |
| CD68 CD163 CD204 | Pro-tumor | TAMs prepared by stimulating CD14+ PBMCs with M-CSF and 50% OS conditioned medium promote migration of OS cells | [44] |
| CD204 | Anti-tumor | Positive correlation of expression with resistance to metastasis and prolonged overall survival | [45] |
| iNOS CD206 | - | Macrophages activated by mifamurtide have increased levels of both iNOS and CD206, switch to an M1/M2 phenotype, inhibit cell proliferation, and induce tumor cell differentiation | [46] |
| CD209 | Pro-tumor | Promotes OS progression by activating cancer stem cells | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsuno, R.; Komohara, Y.; Pan, C.; Kawasaki, T.; Enomoto, A.; Jubashi, T.; Kono, H.; Wako, M.; Ashizawa, T.; Haro, H.; et al. Surface Markers and Chemokines/Cytokines of Tumor-Associated Macrophages in Osteosarcoma and Other Carcinoma Microenviornments—Contradictions and Comparisons. Cancers 2024, 16, 2801. https://doi.org/10.3390/cancers16162801
Tatsuno R, Komohara Y, Pan C, Kawasaki T, Enomoto A, Jubashi T, Kono H, Wako M, Ashizawa T, Haro H, et al. Surface Markers and Chemokines/Cytokines of Tumor-Associated Macrophages in Osteosarcoma and Other Carcinoma Microenviornments—Contradictions and Comparisons. Cancers. 2024; 16(16):2801. https://doi.org/10.3390/cancers16162801
Chicago/Turabian StyleTatsuno, Rikito, Yoshihiro Komohara, Cheng Pan, Tomonori Kawasaki, Atsushi Enomoto, Takahiro Jubashi, Hiroyuki Kono, Masanori Wako, Tomoyuki Ashizawa, Hirotaka Haro, and et al. 2024. "Surface Markers and Chemokines/Cytokines of Tumor-Associated Macrophages in Osteosarcoma and Other Carcinoma Microenviornments—Contradictions and Comparisons" Cancers 16, no. 16: 2801. https://doi.org/10.3390/cancers16162801
APA StyleTatsuno, R., Komohara, Y., Pan, C., Kawasaki, T., Enomoto, A., Jubashi, T., Kono, H., Wako, M., Ashizawa, T., Haro, H., & Ichikawa, J. (2024). Surface Markers and Chemokines/Cytokines of Tumor-Associated Macrophages in Osteosarcoma and Other Carcinoma Microenviornments—Contradictions and Comparisons. Cancers, 16(16), 2801. https://doi.org/10.3390/cancers16162801






