Pre-emptive Laparoscopic Colostomy Creation in Obstructing Locally Advanced Rectal and Anal Cancer Does Not Delay the Starting of Oncological Treatments
Abstract
Simple Summary
Abstract
1. Introduction
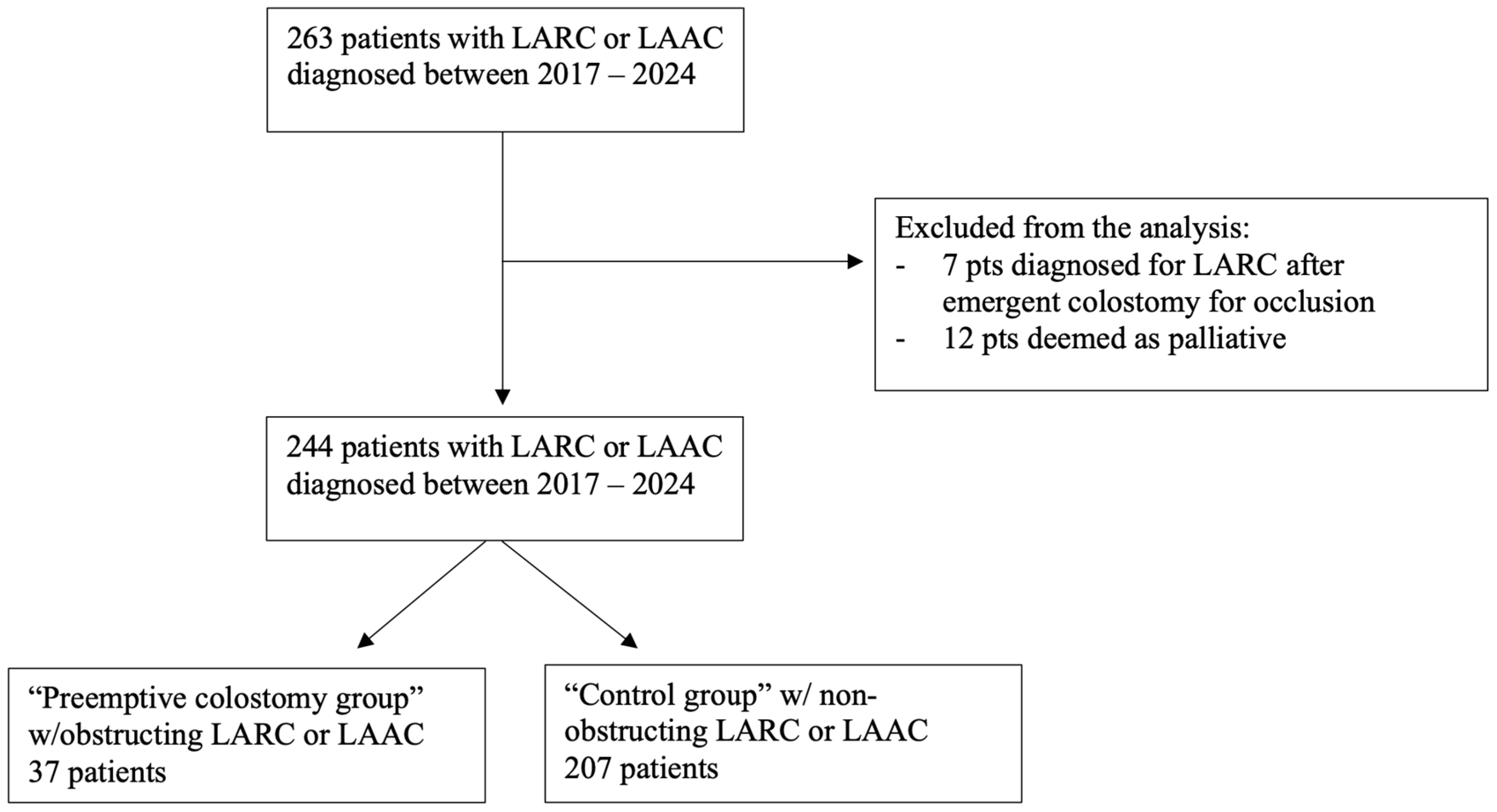
2. Methods
Statistical Analysis
3. Results
4. Discussion and Conclusions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birgisson, H.; Påhlman, L.; Gunnarsson, U.; Glimelius, B.; Swedish Rectal Cancer Trial Group. Adverse effects of preoperative radiation therapy for rectal cancer: Long-term follow-up of the Swedish Rectal Cancer Trial. J. Clin. Oncol. 2005, 23, 8697–8705. [Google Scholar] [CrossRef]
- Glasbey, J.; FOxTROT Collaborating Group. Risk of Bowel Obstruction in Patients Undergoing Neoadjuvant Chemotherapy for High-risk Colon Cancer: A Nested Case-control Matched Analysis of an International, Multi-centre, Randomised Controlled Trial (FOxTROT). Ann. Surg. 2023, 280, 283–293. [Google Scholar] [CrossRef]
- Morton, A.J.; Rashid, A.; Shim, J.S.C.; West, J.; Humes, D.J.; Grainge, M.J. Long-term adverse effects and healthcare burden of rectal cancer radiotherapy: Systematic review and meta-analysis. ANZ J. Surg. 2023, 93, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, T.A.; Orsini, R.G.; Nieuwenhuijzen, G.A.P.; Rutten, H.J.T.; Daams, F. Stoma placement in obstructive rectal cancer prior to neo-adjuvant treatment and definitive surgery: A practical guideline. Eur. J. Surg. Oncol. 2016, 42, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, L.F.; Nahas, C.S.R.; Marques, C.F.S.; Sobrado, C.W.; Nahas, S.C. Pretreatment colostomy in patients with anal squamous cell carcinoma: Risk factors for a permanent stoma. J. Surg. Oncol. 2022, 126, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.A.; Fleshman, J.W.; Hunt, S.R.; Safar, B.; Birnbaum, E.H.; Lin, A.Y.; Mutch, M.G. Is an elective diverting colostomy warranted in patients with an endoscopically obstructing rectal cancer before neoadjuvant chemotherapy? Dis. Colon Rectum 2012, 55, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Hill, E.G.; Sweeney, R.E.; Wahlquist, A.E.; Marshall, D.T.; Staveley O’Carroll, K.F.; Cole, D.J.; Camp, E.R. The impact of surgical diversion before neoadjuvant therapy for rectal cancer. Am. Surg. 2015, 81, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Sandén, G.; Svensson, J.; Ljuslinder, I.; Rutegård, M. Defunctioning stoma before neoadjuvant treatment or resection of endoscopically obstructing rectal cancer. Int. J. Color. Dis. 2023, 38, 24. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, E.T.; Galanis, I.N.; Pavlidis, T.E. Management of obstructed colorectal carcinoma in an emergency setting: An update. World J. Gastrointest. Oncol. 2024, 16, 598–613. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M.; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.A.; Gollub, M.; Eng, C.; Brierley, J.; Palefsky, J.; Gress, D.; Williams, A.; Goldberg, R.; Washington, M.K. AJCC Cancer Staging System: Anus: Version 9 of the AJCC Cancer Staging System; American College of Surgeon (ACS): Chicago, IL, USA, 2023. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.A. The comprehensive complication index: A novel continuous scale to measure surgical morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Ding, J. Information criteria for model selection. WIREs Comput. Stat. 2023, 15, e1607. [Google Scholar] [CrossRef]
- Lin, W.; Li, C.; Clement, E.A.; Brown, C.J.; Raval, M.J.; Karimuddin, A.A.; Ghuman, A.; Phang, P.T. Surgical Outcomes in Total Neoadjuvant Therapy for Rectal Cancer Versus Standard Long-course Chemoradiation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2024, 279, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Koneru, S.; Patton, V.; Ng, K.-S. Quality of life in permanent ostomates—What really matters to them? ANZ J. Surg. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Koneru, S.; Builth-Snoad, L.; Rickard, M.J.F.X.; Keshava, A.; Chapuis, P.H.; Ng, K.-S. Major low anterior resection syndrome has equivalent health-related quality of life implications as having a permanent colostomy. Tech. Coloproctol. 2023, 28, 17. [Google Scholar] [CrossRef] [PubMed]
- Wiltink, L.M.; Nout, R.A.; van der Voort van Zyp, J.R.; Ceha, H.M.; Fiocco, M.; Meershoek-Klein Kranenbarg, E.; Marinelli, A.W.; van de Velde, C.J.; Marijnen, C.A. Long-Term Health-Related Quality of Life in Patients With Rectal Cancer After Preoperative Short-Course and Long-Course (Chemo) Radiotherapy. Clin. Color. Cancer 2016, 15, e93–e99. [Google Scholar] [CrossRef][Green Version]
- Garcia-Aguilar, J.; Renfro, L.A.; Chow, O.S.; Shi, Q.; Carrero, X.W.; Lynn, P.B.; Thomas, C.H., Jr.; Chan, E.; Cataldo, P.A.; Marcet, J.E.; et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): Results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015, 16, 1537–1546. [Google Scholar] [CrossRef]
- Montroni, I.; Ugolini, G.; Saur, N.M.; Rostoft, S.; Spinelli, A.; Van Leeuwen, B.L.; De Liguori Carino, N.; Ghignone, F.; Jaklitsch, M.T.; Kenig, J.; et al. Predicting Functional Recovery and Quality of Life in Older Patients Undergoing Colorectal Cancer Surgery: Real-World Data From the International GOSAFE Study. J. Clin. Oncol. 2023, 41, 5247–5262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Lin, K.-H.; Kang, J.-C.; Hu, J.-M.; Chen, C.-Y.; Pu, T.-W. Benefits of laparoscopy-assisted ileostomy in colorectal cancer patients with bowel obstruction. World J. Clin. Cases 2023, 11, 5660–5665. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J.A.; Fox, J.P.; Dharmarajan, S.; Silviera, M.L.; Hunt, S.R.; Wise, P.E.; Mutch, M.G. Acute health care resource utilization for ileostomy patients is higher than expected. Dis. Colon Rectum 2014, 57, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Fish, D.R.; Mancuso, C.A.; Garcia-Aguilar, J.E.; Lee, S.W.; Nash, G.M.; Sonoda, T.; Charlson, M.E.; Temple, L.K. Readmission After Ileostomy Creation: Retrospective Review of a Common and Significant Event. Ann. Surg. 2017, 265, 379–387. [Google Scholar] [CrossRef]
- Dylen, M.Y.C.; Lee, J.W.K.; Ting, L.Y.; Ragupathi, T.; Yu, N.J.; Lim, F.; Farouk, R.; Seng, C.C. Transverse Colostomy Differs in Outcomes Compared to Sigmoid Colostomy: A Cohort Analysis. J. Investig. Surg. 2022, 35, 783–787. [Google Scholar] [CrossRef]
| Characteristics | N(%) or Mean (SD) |
|---|---|
| Sex | |
| Male | 21 (56.8) |
| Female | 16 (43.2) |
| Age | 68.9 ± 2.2 |
| BMI (kg/m2) | 23.5 ± 0.6 |
| CACI | 6 ± 0.5 |
| ASA | |
| 1 | 2 (5.4) |
| 2 | 16 (43.2) |
| 3 | 17 (45.9) |
| 4 | 2 (5.4) |
| Type of cancer | |
| Rectal cancer (Adenocarcinoma) | 29 (78.4) |
| Anal cancer (Squamous cell carcinoma) | 8 (21.6) |
| Distance from AV (cm) | 7.2 ± 0.8 |
| ECOG-PS | |
| 0–1 | 35 (94.6) |
| 2–3 | 2 (5.4) |
| NRS | |
| 0–1 | 22 (59.5) |
| 2–3 | 15 (40.5) |
| Therapy after colostomy | |
| LC-RT | 10 (27.0) |
| TNT | 15 (40.5) |
| First-line chemotherapy | 12 (32.5) |
| Stage according to AJCC | |
| 2 | 4 (10.8) |
| 3 | 21 (56.8) |
| 4 | 12 (32.4) |
| Time from diagnosis to colostomy placement (days) | 16.7 ± 11.1 |
| Starting therapy from colostomy (days) | 23.4 ± 1.8 |
| Starting therapy from diagnosis (days) | 38.3 ± 2.3 |
| Laparoscopic approach | 37 (100) |
| Any postoperative complications | 14 (37.8) |
| Clavien Dindo | |
| 0 | 23 (62.2) |
| 1 | 3 (8.1) |
| 2 | 10 (27.0) |
| 3 | 1 (2.7) |
| CCI (%) | 9.0 ± 2.3 |
| Length of stay (days) | 4.1 ± 0.8 |
| Readmission | 2 (5.4) |
| Reoperation | 0 (0) |
| Radical resection rate after therapy or resection not needed *? | 21/34 * (61.7) |
| Characteristics | Diverting Colostomy Group | Non-Obstructing Group | p Value |
|---|---|---|---|
| Sex | |||
| Male | 21 (56.8) | 108 (52.2) | 0.607 |
| Female | 16 (43.2) | 99 (47.8) | |
| Age (mean ± SD) | 68.9 ± 2.2 | 67.7 ± 0.8 | 0.579 |
| ECOG-PS | |||
| 0–1 | 35 (94.6) | 189 (91.3) | 0.502 |
| 2–3 | 2 (5.4) | 18 (8.7) | |
| NRS | |||
| 0–1 | 22 (59.5) | 170 (82.1) | 0.002 |
| 2–3 | 15 (40.5) | 37 (17.9) | |
| Type of cancer | |||
| Rectal cancer (Adenocarcinoma) | 29 (78.4) | 153 (73.9) | 0.566 |
| Anal cancer (Squamous cell carcinoma) | 8 (21.6) | 54 (26.1) | |
| Distance from AV (cm; mean ± SD) | 7.2 ± 0.8 | 7.7 ± 1.8 | 0.169 |
| Stage according to AJCC 8th edition | |||
| 2 | 4 (10.8) | 91 (43.9) | <0.001 |
| 3 | 21 (56.8) | 108 (52.2) | |
| 4 | 12 (32.4) | 8 (3.9) | |
| Type of Therapy | |||
| LC-RT | 10 (27.0) | 131 (63.3) | 0.016 |
| TNT | 15 (40.5) | 66 (31.9) | |
| First line | 12 (32.5) | 10 (4.8) | |
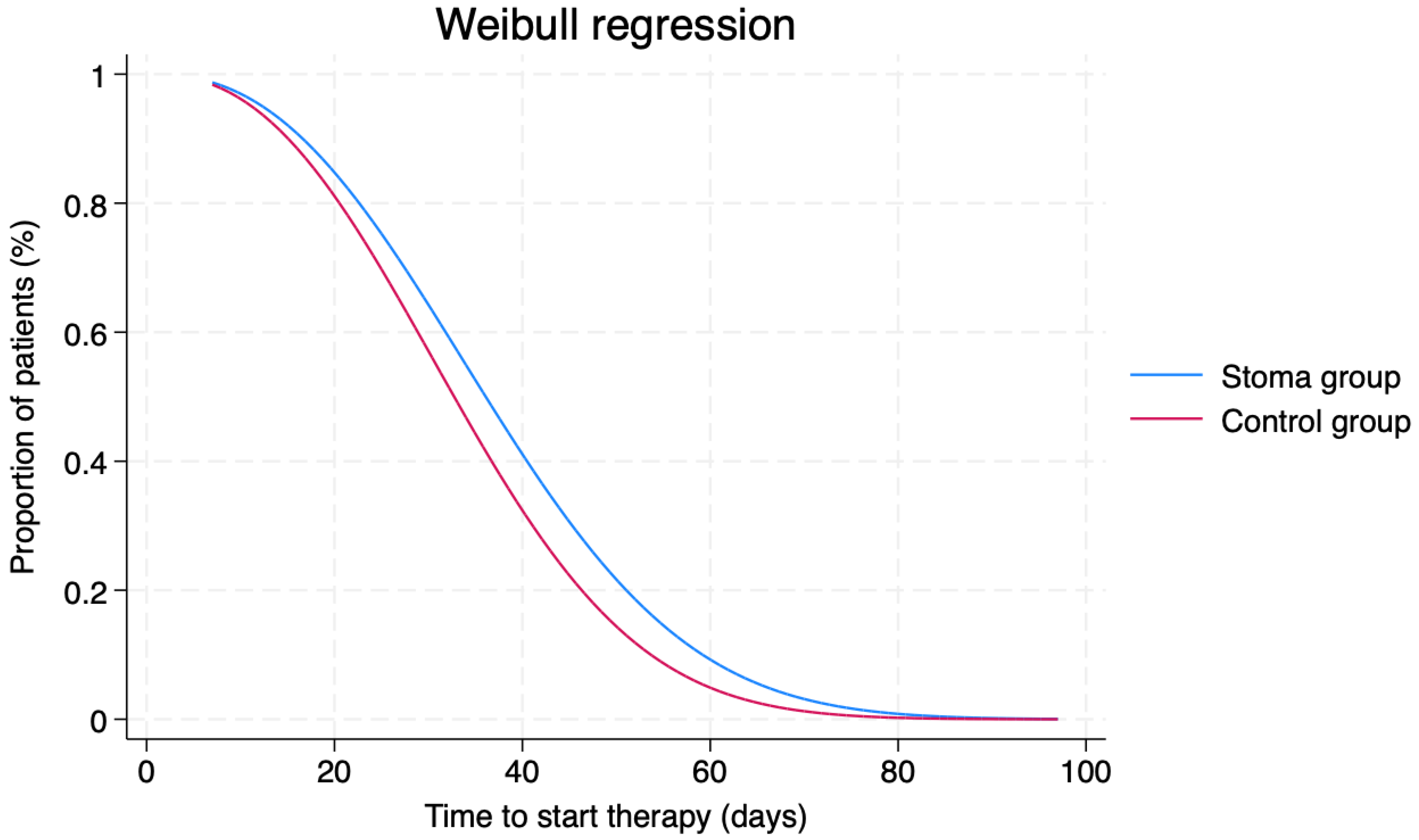
| Starting therapy from diagnosis (days; mean ± SD) | 38.3 ± 14.3 | 33.5 ± 14.9 | 0.083 |
| Radical resection or resection not needed *? (%) | 21/34 * (61.7) | 196/202 * (97) | 0.021 |
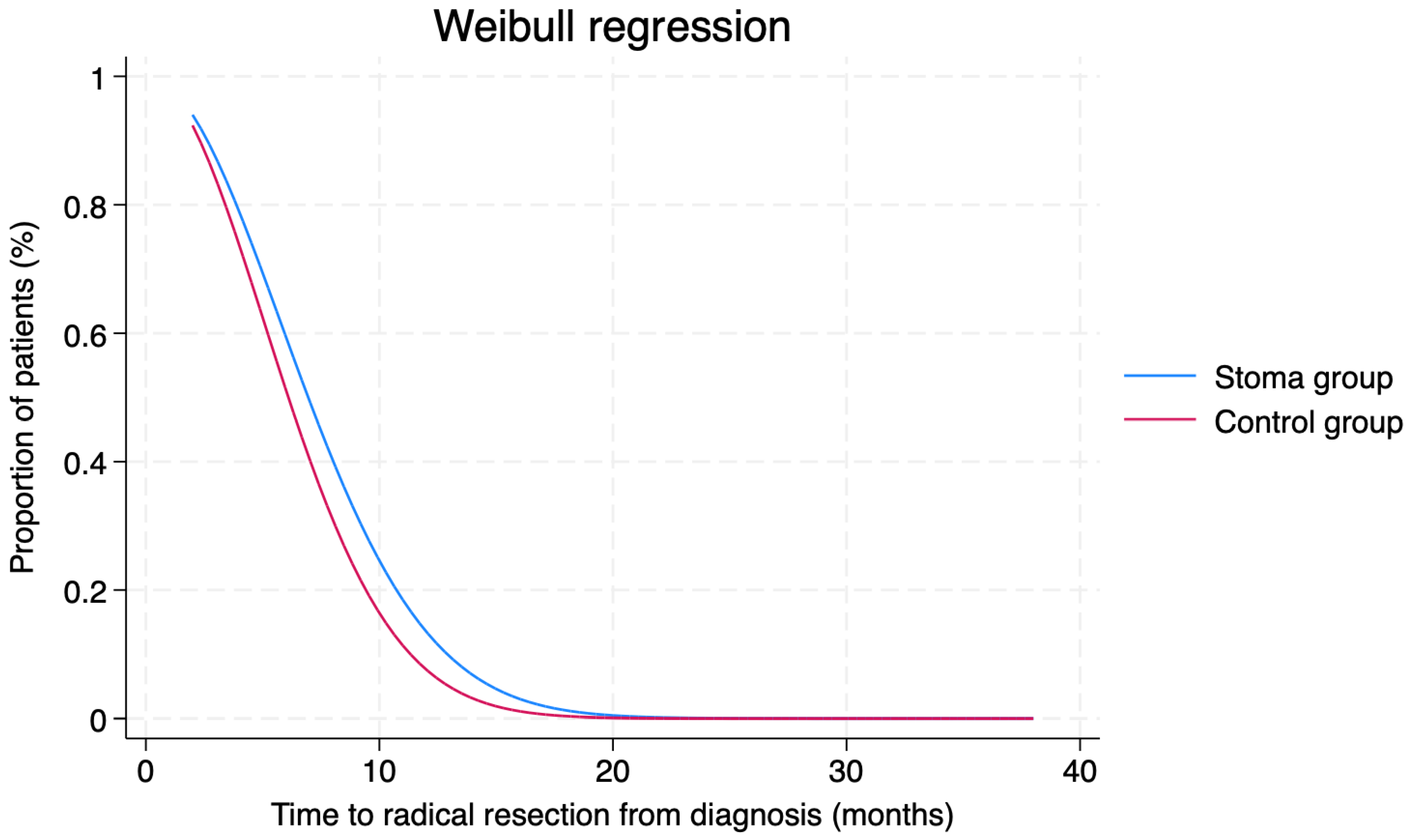
| Time to radical resection (months; mean ± SD) | 7.8 ± 0.8 | 6.5 ± 0.3 | 0.187 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taffurelli, G.; Montroni, I.; Dileo, C.; Boccaccino, A.; Ghignone, F.; Zattoni, D.; Frascaroli, G.; Ugolini, G. Pre-emptive Laparoscopic Colostomy Creation in Obstructing Locally Advanced Rectal and Anal Cancer Does Not Delay the Starting of Oncological Treatments. Cancers 2024, 16, 2799. https://doi.org/10.3390/cancers16162799
Taffurelli G, Montroni I, Dileo C, Boccaccino A, Ghignone F, Zattoni D, Frascaroli G, Ugolini G. Pre-emptive Laparoscopic Colostomy Creation in Obstructing Locally Advanced Rectal and Anal Cancer Does Not Delay the Starting of Oncological Treatments. Cancers. 2024; 16(16):2799. https://doi.org/10.3390/cancers16162799
Chicago/Turabian StyleTaffurelli, Giovanni, Isacco Montroni, Claudia Dileo, Alessandra Boccaccino, Federico Ghignone, Davide Zattoni, Giacomo Frascaroli, and Giampaolo Ugolini. 2024. "Pre-emptive Laparoscopic Colostomy Creation in Obstructing Locally Advanced Rectal and Anal Cancer Does Not Delay the Starting of Oncological Treatments" Cancers 16, no. 16: 2799. https://doi.org/10.3390/cancers16162799
APA StyleTaffurelli, G., Montroni, I., Dileo, C., Boccaccino, A., Ghignone, F., Zattoni, D., Frascaroli, G., & Ugolini, G. (2024). Pre-emptive Laparoscopic Colostomy Creation in Obstructing Locally Advanced Rectal and Anal Cancer Does Not Delay the Starting of Oncological Treatments. Cancers, 16(16), 2799. https://doi.org/10.3390/cancers16162799






