Targeting IL-8 and Its Receptors in Prostate Cancer: Inflammation, Stress Response, and Treatment Resistance
Abstract
Simple Summary
Abstract
1. Introduction
2. Role and Regulation of IL-8 and CXCR1/2 in Prostate Cancer
2.1. IL-8: Role and Regulation
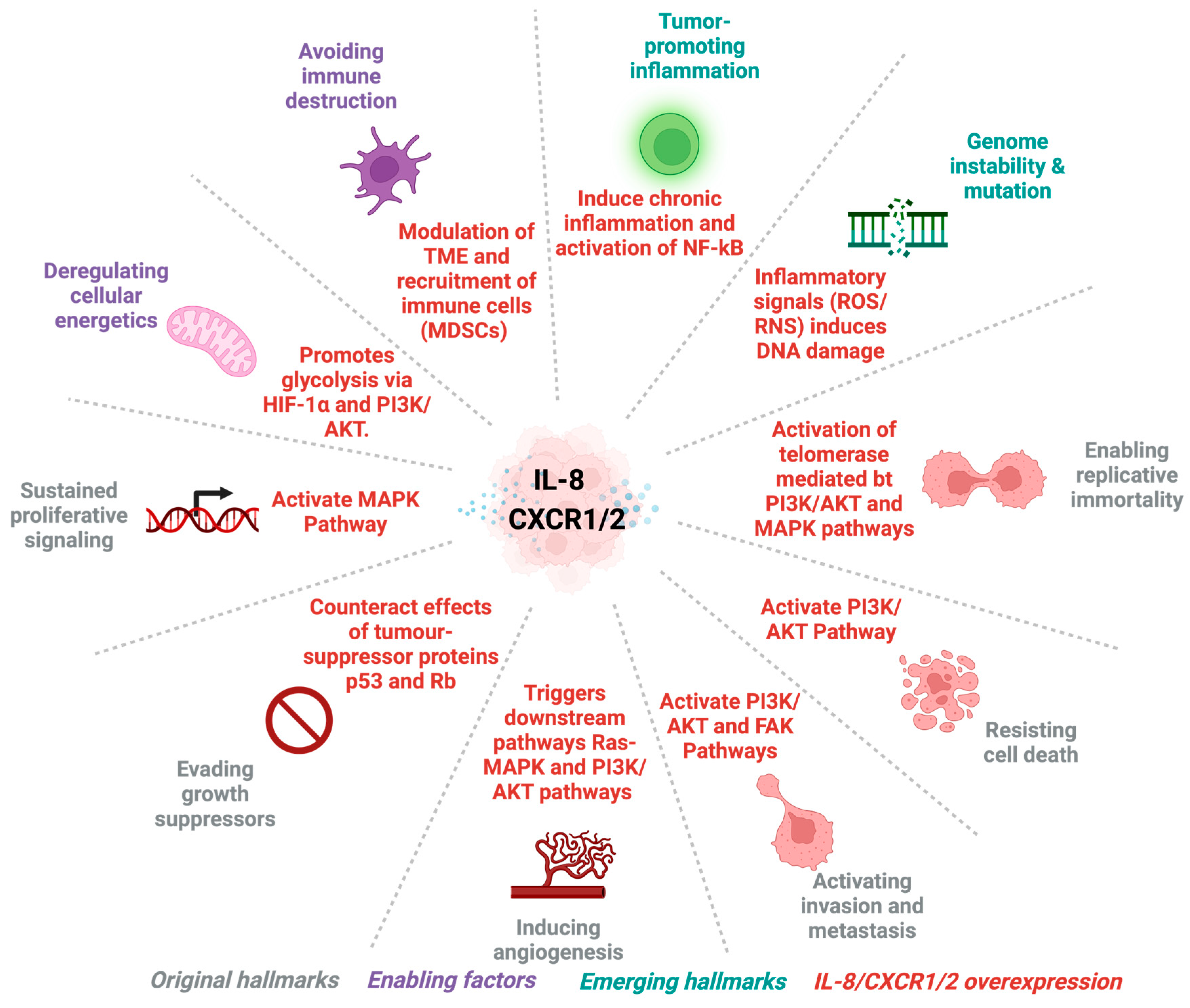
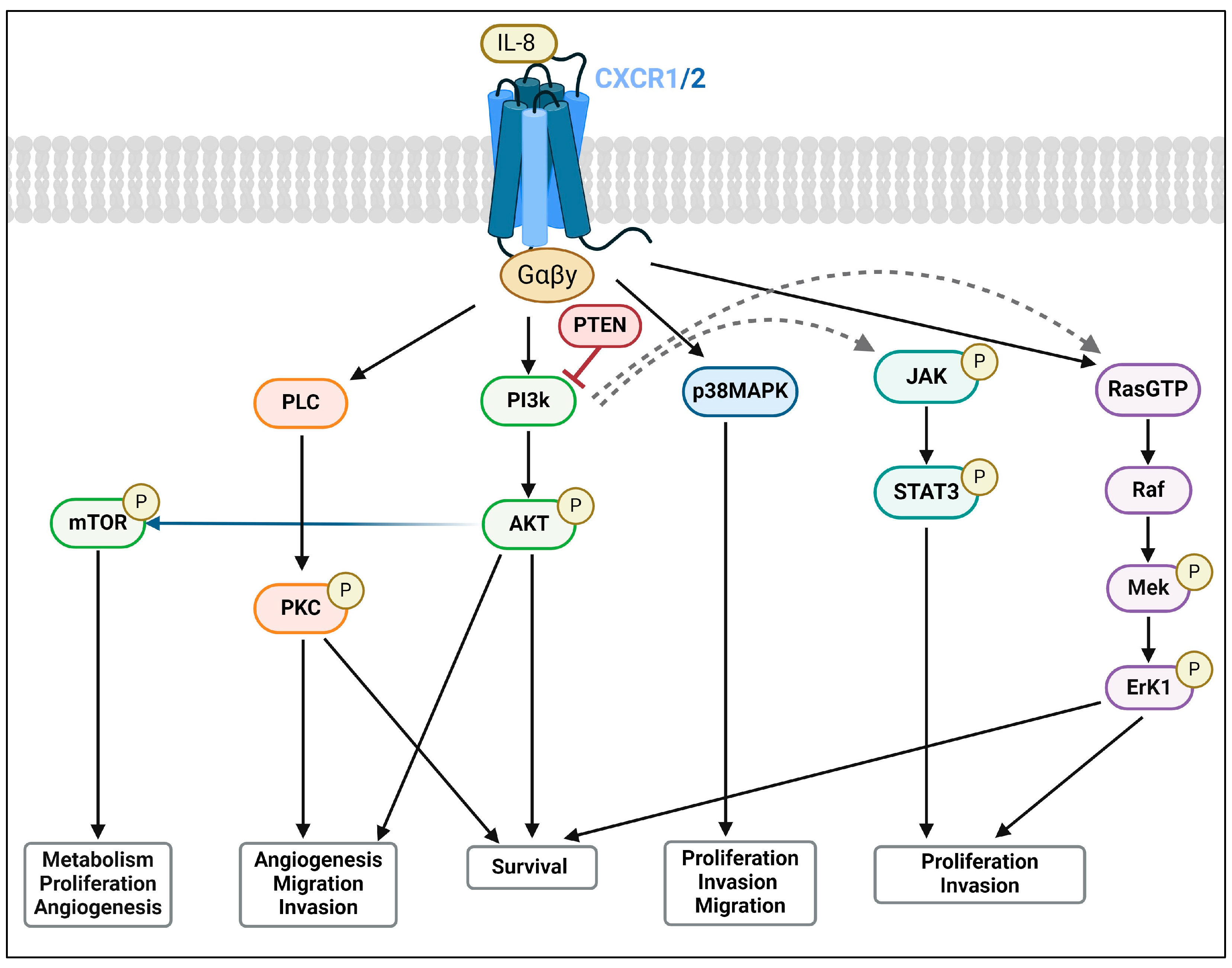
2.2. CXCR1 and CXCR2: Expression and Signalling Pathways
3. Impact of IL-8/CXCR1/2 on the Tumour Microenvironment
3.1. Vascular Dynamics and Cancer Cell Communication
Cancer Stem Cell-Mediated Tumour Progression and Treatment Resistance
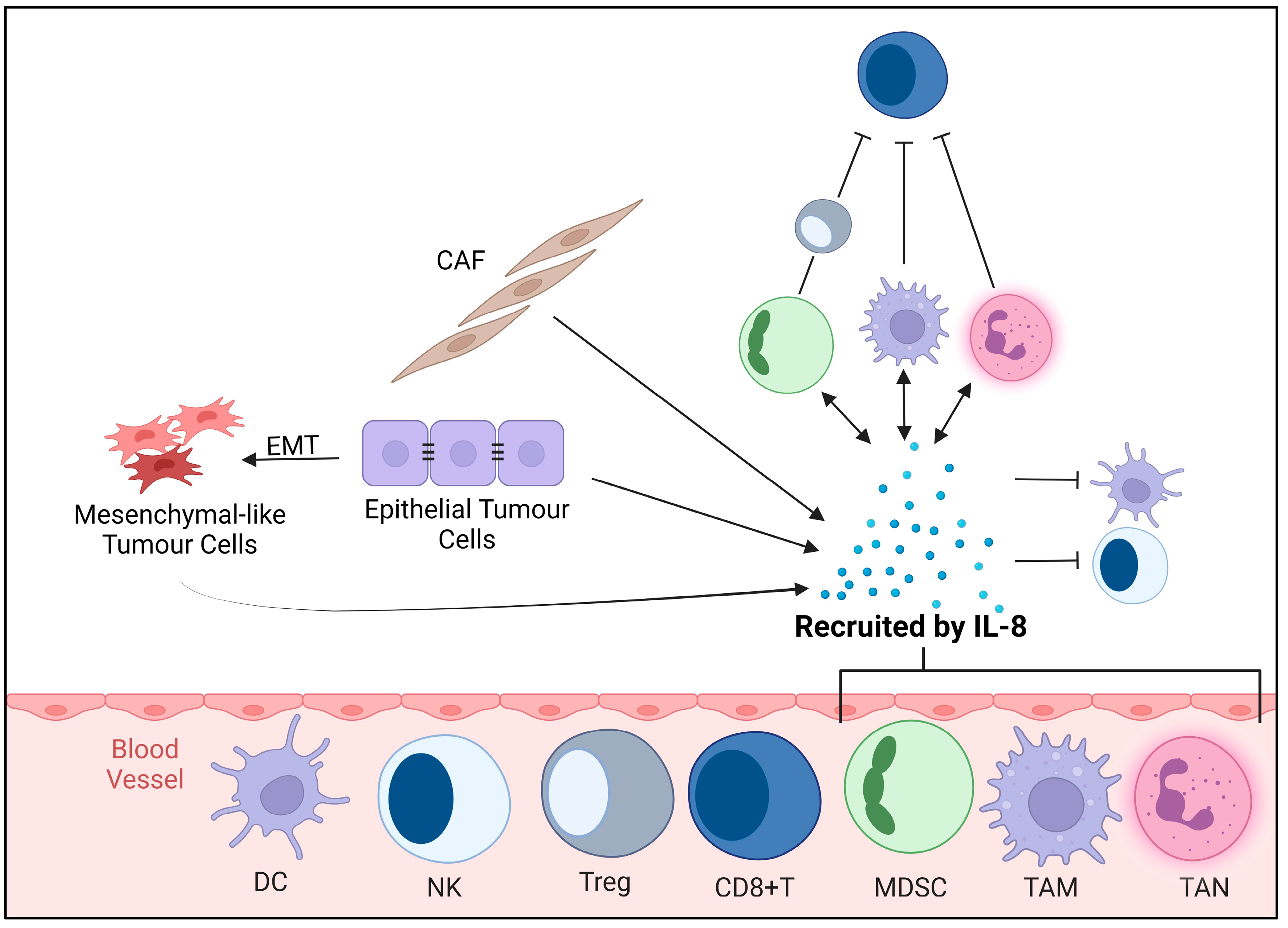
3.2. Immunosuppressive TME
3.3. Modulation of Immune Response and Inflammation
3.4. Cancer Cell Plasticity: Induction of Epithelial–Mesenchymal Transition (EMT) and Metastasis
4. TME and Its Significance in Cancer
4.1. ECM
4.2. Heterogeneity
4.3. Inflammation
4.4. Immune Response
5. Therapeutic Implications and Challenges
5.1. Therapeutic Implications
5.2. Therapeutic Inhibition of CXCL8/CXCR1/CXCR2: Small-Molecule Inhibitors, Antagonists, and Monoclonal Antibodies
5.3. Combinational Therapies and Emerging Treatment Modalities
5.4. Clinical Trials
6. Challenges in Implementing Therapies Targeting IL-8-CXCR1/2 Axis for PCa Treatment
6.1. Development of Treatment Resistance
6.2. Specificity and Efficacy of CXCR1/2 Targeting
6.3. Side Effects
7. Discussion and Conclusions
8. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Proof of Concept Phase I/II Trial of the CXCR2 Antagonist AZD5069, Administered in Combination with enzalutamide, in Patients with Metastatic Castration-Resistant Prostate Cancer |
| ADT | Androgen deprivation therapy |
| AKT | Protein kinase B |
| AR | Androgen receptor |
| ASIs | Androgen-signalling inhibitors |
| BSP | Bone sialoprotein |
| CAFs | Cancer-associated fibroblasts |
| CD3+ T-cells, CD20+ B-cells, NK cells | Immune cells including T-cells, B-cells, and natural killer cells |
| CRPC | Castration-resistant prostate cancer |
| CSCs | Cancer stem cells |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| CTLA-5 | Cytotoxic T-Lymphocyte Antigen 5 |
| CXCL8 | Chemokine (C-X-C motif) Ligand 8 (IL-8) |
| CXCR1 | C-X-C chemokine receptor type 1 |
| CXCR2 | C-X-C chemokine receptor type 2 |
| ECM | Extracellular matrix |
| ECs | Endothelial cells |
| EGF | Epidermal growth factor |
| EMT | Epithelial–mesenchymal transition |
| ENA-78 | Epithelial-derived neutrophil-activating peptide 78 |
| FDA | U.S. Food and Drug Administration |
| FGF | Fibroblast growth factor |
| GEMMs | Genetically engineered mouse model |
| Gleason score | A grading system for the histological patterns of prostate cancer |
| GPCR | G protein-coupled receptor |
| HCC | Hepatocellular carcinoma |
| hCXCR1 K1 | Human CXCR1 knock-in |
| IGF | Insulin-like growth factor |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IR | Ionizing radiation |
| JAK2 | Janus kinase |
| KDM5C | Lysine (K)-specific Demethylase 5C |
| MAPK | Mitogen-activated protein kinase |
| mCRPC | Metastatic castration-resistant prostate cancer |
| MDSCs | Myeloid-derived suppressor cells |
| MMPs | Matrix metalloproteinases |
| MSCs | Mesenchymal stem cells |
| mTOR | Mammalian Target of Rapamycin |
| NEPC | Neuroendocrine prostate cancer |
| NF-kB | Nuclear Factor Kappa B |
| NK Cells | Natural killer cells |
| NLR | Neutrophil-to-lymphocyte ratio |
| P53 | Tumour protein p53 |
| PARP | Polyadenosine-diphosphate-ribose polymerase |
| PCa | Prostate cancer |
| PD-1 | Programmed cell death protein 1 |
| PDGF | Platelet-derived growth factor |
| PD-L1 | Programmed death-ligand 1 |
| PDXs | Patient-derived xenografts |
| PI3K/AKT | Phosphoinositide 3-kinase/protein kinase B |
| PKC | Protein kinase C |
| PMN | Polymorphonuclear |
| PSA | Prostate-specific antigen |
| PSMA | Prostate-specific membrane antigen |
| PTEN | Phosphatase and Tensin Homolog |
| RNA | Ribonucleic acid |
| RNT | Radionuclide therapy |
| STAT3 | Single Transducer and Activation of Transcription |
| TAMs | Tumour-associated macrophages |
| TGF-β | Transforming growth factor-beta |
| TILs | Tumour-infiltrating lymphocytes |
| TME | Tumour microenvironment |
| TMPRSS-ERG | Transmembrane Protease, Serine 2—Erythroblast Transformation-Specific (ETS)-Related Gene |
| TNF | Tumour necrosis factor |
| TNFRSF14 | Tumour necrosis factor Receptor Superfamily Member 14 |
| TNF-α | Tumour necrosis factor-alpha |
| UBE2R2-AS1 | Ubiquitin-Conjugating Enzyme E2 R2 Antisense RNA 1 |
| VEGF | Vascular Endothelial Growth Factor |
| VEGF-A | Vascular Endothelial Growth Factor A |
References
- American Cancer Society. Key Statistics for Prostate Cancer. 4 August 2023. Available online: https://www.cancer.org/cancer/types/prostate-cancer/about/key-statistics.html#:~:text=stage%20prostate%20cancer.-,Risk%20of%20prostate%20cancer,rare%20in%20men%20under%2040 (accessed on 17 January 2024).
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef]
- Hoffman, K.E.; Voong, K.R.; Levy, L.B.; Allen, P.K.; Choi, S.; Schlembach, P.J.; Lee, A.K.; McGuire, S.E.; Nguyen, Q.; Pugh, T.J.; et al. Randomized Trial of Hypofractionated, Dose-Escalated, Intensity-Modulated Radiation Therapy (IMRT) versus Conventionally Fractionated IMRT for Localized Prostate Cancer. J. Clin. Oncol. 2018, 36, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Dignam, J.J.; Amin, M.B.; Bruner, D.W.; Low, D.; Swanson, G.P.; Shah, A.B.; D’Souza, D.P.; Michalski, J.M.; Dayes, I.S.; et al. Randomized Phase III Noninferiority Study Comparing Two Radiotherapy Fractionation Schedules in Patients with Low-Risk Prostate Cancer. J. Clin. Oncol. 2016, 34, 2325–2332. [Google Scholar] [CrossRef]
- Sandhu, S.; Guo, C.; Hofman, M.S. Radionuclide Therapy in Prostate Cancer: From standalone to combination PSMA theranostics. J. Nucl. Med. 2021, 62, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef]
- Pezaro, C. PARP inhibitor combinations in prostate cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835919897537. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Philippou, Y.A.; Kuntz, G.M.; Konety, B.R.; Gupta, S.; Lamb, A.D.; Dahm, P. Taxane-based chemohormonal therapy for metastatic hormone-sensitive prostate cancer: A Cochrane Review. BJU Int. 2019, 124, 370–372. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Amit, M.; Baruch, E.; Nagarajan, P.; Gleber-Netto, F.; Rao, X.; Xie, T.; Akhter, S.; Adewale, A.; Islam, S.; Mattson, B.; et al. Inflammation induced by tumor-associated nerves promotes resistance to anti-PD-1 therapy in cancer patients and is targetable by IL-6 blockade. Res. Sq. 2023; preprints. [Google Scholar]
- Armstrong, C.W.D.; Coulter, J.A.; Ong, C.W.; Maxwell, P.J.; Walker, S.; Butterworth, K.T.; Lyubomska, O.; Berlingeri, S.; Gallagher, R.; O’Sullivan, J.M.; et al. Clinical and functional characterization of CXCR1/CXCR2 biology in the relapse and radiotherapy resistance of primary PTEN-deficient prostate carcinoma. NAR Cancer 2020, 2, zcaa012. [Google Scholar] [CrossRef]
- Korbecki, J.; Bosiacki, M.; Chlubek, D.; Baranowska-Bosiacka, I. Bioinformatic Analysis of the CXCR2 Ligands in Cancer Processes. Int. J. Mol. Sci. 2023, 24, 13287. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Nair, M.P.; Sufrin, G.; Mahajan, S.D.; Chadha, K.C.; Chawda, R.P.; Schwartz, S.A. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004, 64, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, F.; Kou, H.; Zheng, Y.; Yang, J.; Xu, Z.; Fang, Y.; Sun, W.; Zhu, S.; Jiang, Q.; et al. Stromal cell-derived small extracellular vesicles enhance radioresistance of prostate cancer cells via interleukin-8-induced autophagy. J. Extracell. Vesicles 2023, 12, e12342. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, H.A.G.; Zapata-Copete, J.A.; Sanchez, A. Molecular alterations associated with prostate cancer. Cent. Eur. J. Urol. 2018, 71, 168–176. [Google Scholar]
- Holmes, W.E.; Lee, J.; Kuang, W.J.; Rice, G.C.; Wood, W.I. Structure and functional expression of a human interleukin-8 receptor. Science 1991, 253, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.M.; Tiffany, H.L. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science 1991, 253, 1280–1283. [Google Scholar] [CrossRef]
- Chen, L.; Fan, J.; Chen, H.; Meng, Z.; Chen, Z.; Wang, P.; Liu, L. The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci. Rep. 2014, 4, 5911. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Simões, B.M.; Howell, S.J.; Farnie, G.; Clarke, R.B. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, N.; Kaira, K.; Tomizawa, Y.; Shimizu, K.; Imai, H.; Takahashi, G.; Kakegawa, S.; Ohtaki, Y.; Nagashima, T.; Kasahara, N.; et al. Clinicopathological and prognostic significance of interleukin-8 expression and its relationship to KRAS mutation in lung adenocarcinoma. Br. J. Cancer 2014, 110, 2047–2053. [Google Scholar] [CrossRef]
- Dahal, S.; Chaudhary, P.; Jung, Y.S.; Kim, J.A. Megakaryocyte-Derived IL-8 Acts as a Paracrine Factor for Prostate Cancer Aggressiveness through CXCR2 Activation and Antagonistic AR Downregulation. Biomol. Ther. 2023, 31, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, X.L.; Wei, Y.Q.; Wei, X.W. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; Scullin, P.; Maxwell, P.J.; Wilson, C.; Pettigrew, J.; Gallagher, R.; O’Sullivan, J.M.; Johnston, P.G.; Waugh, D.J. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis 2008, 29, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Maxwell, P.J.; Longley, D.B.; Wilson, R.H.; Johnston, P.G.; Waugh, D.J. Constitutive and treatment-induced CXCL8-signalling selectively modulates the efficacy of anti-metabolite therapeutics in metastatic prostate cancer. PLoS ONE 2012, 7, e36545. [Google Scholar] [CrossRef]
- Asokan, S.; Bandapalli, O.R. CXCL8 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2021, 1302, 25–39. [Google Scholar]
- Saxena, S.; Singh, R.K. Chemokines orchestrate tumor cells and the microenvironment to achieve metastatic heterogeneity. Cancer Metastasis Rev. 2021, 40, 447–476. [Google Scholar] [CrossRef]
- Singh, A.J.; Gray, J.W. Chemokine signaling in cancer-stroma communications. J. Cell Commun. Signal. 2021, 15, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bujanda, Z.A.; Haffner, M.C.; Chaimowitz, M.G.; Chowdhury, N.; Venturini, N.J.; Patel, R.A.; Obradovic, A.; Hansen, C.S.; Jacków, J.; Maynard, J.P.; et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat. Cancer 2021, 2, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.W.; Coulter, J.A.; Ong, C.W.; Maxwell, P.J.; Walker, S.; Butterworth, K.T.; Lyubomska, O.; Berlingeri, S.; Gallagher, R.; O’Sullivan, J.M.; et al. Targeting CXCR1 and CXCR2 to overcome radiotherapy resistance in PTEN-deficient prostate carcinoma. bioRxiv 2020. [Google Scholar] [CrossRef]
- Madan, R.A.; Palena, C. Behind the IL-8 ball in prostate cancer. Nat. Cancer 2021, 2, 775–776. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Dell’Endice, S.; Jaschke, N.; Pählig, S.; Shahid, A.; Hofbauer, L.C.; Rachner, T.D. The Role of Inflammation in Breast and Prostate Cancer Metastasis to Bone. Int. J. Mol. Sci. 2021, 22, 5078. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ye, Y.L.; Li, M.X.; Ye, S.B.; Huang, W.R.; Cai, T.T.; He, J.; Peng, J.Y.; Duan, T.H.; Cui, J.; et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 2017, 36, 2095–2104. [Google Scholar] [CrossRef]
- Xia, M.; Hyman, B.T. GROalpha/KC, a chemokine receptor CXCR2 ligand, can be a potent trigger for neuronal ERK1/2 and PI-3 kinase pathways and for tau hyperphosphorylation-a role in Alzheimer’s disease? J. Neuroimmunol. 2002, 122, 55–64. [Google Scholar] [CrossRef]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). Int. J. Oncol. 2016, 48, 5–12. [Google Scholar] [CrossRef]
- Burger, M.; Hartmann, T.; Burger, J.A.; Schraufstatter, I. KSHV-GPCR and CXCR2 transforming capacity and angiogenic responses are mediated through a JAK2-STAT3-dependent pathway. Oncogene 2005, 24, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.; Gearing, K.; Rees, S. Target validation of G-protein coupled receptors. Drug Discov. Today 2002, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.K.; Murphy, P.M. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996, 271, 20545–20550. [Google Scholar] [CrossRef] [PubMed]
- Bozic, C.R.; Gerard, N.P.; von Uexkull-Guldenband, C.; Kolakowski, L.F., Jr.; Conklyn, M.J.; Breslow, R.; Showell, H.J.; Gerard, C. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J. Biol. Chem. 1994, 269, 29355–29358. [Google Scholar] [CrossRef] [PubMed]
- Rovai, L.E.; Herschman, H.R.; Smith, J.B. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J. Leukoc. Biol. 1998, 64, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Asfaha, S.; Dubeykovskiy, A.N.; Tomita, H.; Yang, X.; Stokes, S.; Shibata, W.; Friedman, R.A.; Ariyama, H.; Dubeykovskaya, Z.A.; Muthupalani, S.; et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology 2013, 144, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, C.; Beccari, A.R.; Bertini, R.; Cavicchia, M.R.; Giorgini, S.; Allegretti, M. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol. Ther. 2006, 112, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, F.; Alam, M.J.; Ang, C.; Adhyatma, G.P.; Xie, L.; Mackay, C.R.; Robert, R. Human CXCR1 knock-in mice infer functional expression of a murine ortholog. J. Leukoc. Biol. 2023, 114, 373–380. [Google Scholar] [CrossRef]
- Centenera, M.M.; Vincent, A.D.; Moldovan, M.; Lin, H.M.; Lynn, D.J.; Horvath, L.G.; Butler, L.M. Harnessing the Heterogeneity of Prostate Cancer for Target Discovery Using Patient-Derived Explants. Cancers 2022, 14, 1708. [Google Scholar] [CrossRef]
- Karkampouna, S.; La Manna, F.; Benjak, A.; Kiener, M.; De Menna, M.; Zoni, E.; Grosjean, J.; Klima, I.; Garofoli, A.; Bolis, M.; et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nat. Commun. 2021, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, N.; Yang, J.; Shepherd, P.D.A.; Li-Ning-Tapia, E.M.; Labanca, E.; Manyam, G.C.; Ravoori, M.K.; Kundra, V.; Araujo, J.C.; Efstathiou, E.; et al. The MD Anderson Prostate Cancer Patient-derived Xenograft Series (MDA PCa PDX) Captures the Molecular Landscape of Prostate Cancer and Facilitates Marker-driven Therapy Development. Clin. Cancer Res. 2020, 26, 4933–4946. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Wang, B.D. Organoids: An Emerging Precision Medicine Model for Prostate Cancer Research. Int. J. Mol. Sci. 2024, 25, 1093. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Brockman, A.A.; Cross, C.E.; Hayes, M.J.; Mobley, B.C.; Mistry, A.M.; Chotai, S.; Weaver, K.D.; Thompson, R.C.; Chambless, L.B.; et al. IL-8 Instructs Macrophage Identity in Lateral Ventricle Contacting Glioblastoma. bioRxiv 2024. [Google Scholar] [CrossRef]
- Harshman, L.C.; Wang, V.X.; Hamid, A.A.; Santone, G.; Drake, C.G.; Carducci, M.A.; DiPaola, R.S.; Fichorova, R.N.; Sweeney, C.J. Impact of baseline serum IL-8 on metastatic hormone-sensitive prostate cancer outcomes in the Phase 3 CHAARTED trial (E3805). Prostate 2020, 80, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.M.; Li, J. A small-molecule antagonist of CXCR1 and CXCR2 inhibits cell proliferation, migration and invasion in melanoma via PI3K/AKT pathway. Med. Clin. 2019, 152, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sharp, A.; Gurel, B.; Crespo, M.; Figueiredo, I.; Jain, S.; Vogl, U.; Rekowski, J.; Rouhifard, M.; Gallagher, L.; et al. Targeting myeloid chemotaxis to reverse prostate cancer therapy resistance. Nature 2023, 623, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Diao, J.; Li, L.; Kondo, H.; Li, L.; Hirono, I. Molecular characterization and expression analysis of Japanese flounder (Paralichthys olivaceus) chemokine receptor CXCR2 in comparison with CXCR1. Dev. Comp. Immunol. 2021, 120, 104047. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Dasgupta, P.; Murphy, J.J. Prostate Cancer: The Role of Inflammation and Chemokines. Am. J. Pathol. 2019, 189, 2119–2137. [Google Scholar] [CrossRef]
- Wei, F.; Wang, D.; Wei, J.; Tang, N.; Tang, L.; Xiong, F.; Guo, C.; Zhou, M.; Li, X.; Li, G.; et al. Metabolic crosstalk in the tumor microenvironment regulates antitumor immunosuppression and immunotherapy resisitance. Cell. Mol. Life Sci. 2021, 78, 173–193. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; McGurk, M.; Pettigrew, J.; Santinelli, A.; Mazzucchelli, R.; Johnston, P.G.; Montironi, R.; Waugh, D.J. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin. Cancer Res. 2005, 11, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Flesken-Nikitin, A.; Corney, D.C.; Wang, W.; Goodrich, D.W.; Roy-Burman, P.; Nikitin, A.Y. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006, 66, 7889–7898. [Google Scholar] [CrossRef] [PubMed]
- Torres-Estay, V.; Carreño, D.V.; Francisco, I.F.S.; Sotomayor, P.; Godoy, A.S.; Smith, G.J. Androgen receptor in human endothelial cells. J. Endocrinol. 2015, 224, R131–R137. [Google Scholar] [CrossRef]
- Fang, B.; Lu, Y.; Li, X.; Wei, Y.; Ye, D.; Wei, G.; Zhu, Y. Targeting the tumor microenvironment, a new therapeutic approach for prostate cancer. Prostate Cancer Prostatic Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Lu, Y.; Roca, H.; Keller, J.M.; Zhang, J.; McCauley, L.K.; Keller, E.T. Immune mediators in the tumor microenvironment of prostate cancer. Chin. J. Cancer 2017, 36, 29. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Archer, M.; Dogra, N.; Kyprianou, N. Inflammation as a Driver of Prostate Cancer Metastasis and Therapeutic Resistance. Cancers 2020, 12, 2984. [Google Scholar] [CrossRef] [PubMed]
- Infanger, D.W.; Cho, Y.; Lopez, B.S.; Mohanan, S.; Liu, S.C.; Gursel, D.; Boockvar, J.A.; Fischbach, C. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013, 73, 7079–7089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, Z.; Li, G.; Zhang, Y.; Liu, X.; Li, B.; Wang, J.; Li, X. Cancer stem cells and their niche in cancer progression and therapy. Cancer Cell Int. 2023, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Germann, M.; Wetterwald, A.; Guzmán-Ramirez, N.; van der Pluijm, G.; Culig, Z.; Cecchini, M.G.; Williams, E.D.; Thalmann, G.N. Stem-like cells with luminal progenitor phenotype survive castration in human prostate cancer. Stem Cells 2012, 30, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Z.; Sarkar, F.H.; Wei, W. Targeting prostate cancer stem cells for cancer therapy. Discov. Med. 2012, 13, 135–142. [Google Scholar] [PubMed]
- Du, J.; He, Y.; Li, P.; Wu, W.; Chen, Y.; Ruan, H. IL-8 regulates the doxorubicin resistance of colorectal cancer cells via modulation of multidrug resistance 1 (MDR1). Cancer Chemother. Pharmacol. 2018, 81, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, T.; Yamauchi, S.; Fukunaga, T.; Orita, H.; Kato, K. Novel subset of granulocytic MDSCs as immunosuppressive regulators and therapeutic targets in gastric cancer. Cancer Res. 2023, 83 (Suppl. 7), 2889. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Garcia, A.J.; Ruscetti, M.; Arenzana, T.L.; Tran, L.M.; Bianci-Frias, D.; Sybert, E.; Priceman, S.J.; Wu, L.; Nelson, P.S.; Smale, S.T.; et al. Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol. Cell. Biol. 2014, 34, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, R.; Lilljebjörn, L.; Johansson, M.; Leandersson, K.; Bjartell, A. The STAT3 inhibitor galiellalactone inhibits the generation of MDSC-like monocytes by prostate cancer cells and decreases immunosuppressive and tumorigenic factors. Prostate 2019, 79, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.J.; McKechnie, M.; Armstrong, C.W.; Manley, J.M.; Ong, C.W.; Worthington, J.; Mills, I.G.; Longley, D.B.; Quigley, J.P.; Zoubeidi, A.; et al. Attenuating Adaptive VEGF-A and IL8 Signaling Restores Durable Tumor Control in AR Antagonist-Treated Prostate Cancers. Mol. Cancer Res. 2022, 20, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Vourda, A.; Syggelos, S.; Gyftopoulos, K. Cell Plasticity and Prostate Cancer: The Role of Epithelial-Mesenchymal Transition in Tumor Progression, Invasion, Metastasis and Cancer Therapy Resistance. Cancers 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Valerie Odero-Marah, O.H.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal Transition (EMT) and Prostate Cancer. Adv. Exp. Med. Biol. 2018, 1095, 101–110. [Google Scholar]
- Cheaito, K.A.; Bahmad, H.F.; Hadadeh, O.; Saleh, E.; Dagher, C.; Hammoud, M.S.; Shahait, M.; Mrad, Z.A.; Nassif, S.; Tawil, A.; et al. EMT Markers in Locally-Advanced Prostate Cancer: Predicting Recurrence? Front. Oncol. 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, M.; Jiang, Y.; Xia, S.; Sun, D.; Zhou, D.; Dong, Z. LncRNA UBE2R2-AS1, as prognostic marker, promotes cell proliferation and EMT in prostate cancer. Histol. Histopathol. 2023, 38, 637–645. [Google Scholar] [PubMed]
- Lemster, A.L.; Sievers, E.; Pasternack, H.; Lazar-Karsten, P.; Klümper, N.; Sailer, V.; Offermann, A.; Brägelmann, J.; Perner, S.; Kirfel, J. Histone Demethylase KDM5C Drives Prostate Cancer Progression by Promoting EMT. Cancers 2022, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Dominguez, C.; Hamilton, D.H.; Palena, C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22. [Google Scholar] [CrossRef]
- Torrealba, N.; Rodríguez-Berriguete, G.; Fraile, B.; Olmedilla, G.; Martínez-Onsurbe, P.; Guil-Cid, M.; Paniagua, R.; Royuela, M. Expression of several cytokines in prostate cancer: Correlation with clinical variables of patients. Relationship with biochemical progression of the malignance. Cytokine 2017, 89, 105–115. [Google Scholar] [CrossRef]
- McShane, R.; Arya, S.; Stewart, A.J.; Caie, P.D.; Bates, M. Prognostic features of the tumour microenvironment in oesophageal adenocarcinoma. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188598. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, N.A.; Krchniakova, M.; Stacy, A.E.; Skoda, J.; Jansson, P.J. Tumour Microenvironment Stress Promotes the Development of Drug Resistance. Antioxidants 2021, 10, 1801. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Panadero, R.; Lucantoni, F.; Gamero-Sandemetrio, E.; Cruz-Merino, L.; Álvaro, T.; Noguera, R. The tumour microenvironment as an integrated framework to understand cancer biology. Cancer Lett. 2019, 461, 112–122. [Google Scholar] [CrossRef]
- Kiefer, J.A.; Farach-Carson, M.C. Type I collagen-mediated proliferation of PC3 prostate carcinoma cell line: Implications for enhanced growth in the bone microenvironment. Matrix Biol. 2001, 20, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019, 38, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Hirz, T.; Mei, S.; Sarkar, H.; Kfoury, Y.; Wu, S.; Verhoeven, B.M.; Subtelny, A.O.; Zlatev, D.V.; Wszolek, M.W.; Salari, K.; et al. Dissecting the immune suppressive human prostate tumor microenvironment via integrated single-cell and spatial transcriptomic analyses. Nat. Commun. 2023, 14, 663. [Google Scholar] [CrossRef]
- Shackleton, E.G.; Ali, H.Y.; Khan, M.; Pockley, G.A.; McArdle, S.E. Novel Combinatorial Approaches to Tackle the Immunosuppressive Microenvironment of Prostate Cancer. Cancers 2021, 13, 1145. [Google Scholar] [CrossRef]
- Tonry, C.; Finn, S.; Armstrong, J.; Pennington, S.R. Clinical proteomics for prostate cancer: Understanding prostate cancer pathology and protein biomarkers for improved disease management. Clin. Proteom. 2020, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Rycaj, K.; Cho, E.J.; Liu, X.; Chao, H.P.; Liu, B.; Li, Q.; Devkota, A.K.; Zhang, D.; Chen, X.; Moore, J.; et al. Longitudinal tracking of subpopulation dynamics and molecular changes during LNCaP cell castration and identification of inhibitors that could target the PSA-/lo castration-resistant cells. Oncotarget 2016, 7, 14220–14240. [Google Scholar] [CrossRef]
- Karavitakis, M.; Ahmed, H.U.; Abel, P.D.; Hazell, S.; Winkler, M.H. Tumor focality in prostate cancer: Implications for focal therapy. Nat. Rev. Clin. Oncol. 2011, 8, 48–55. [Google Scholar] [CrossRef]
- Baca, S.C.; Prandi, D.; Lawrence, M.S.; Mosquera, J.M.; Romanel, A.; Drier, Y.; Park, K.; Kitabayashi, N.; MacDonald, T.Y.; Ghandi, M.; et al. Punctuated evolution of prostate cancer genomes. Cell 2013, 153, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Cotter, K.; Rubin, M.A. The evolving landscape of prostate cancer somatic mutations. Prostate 2022, 82 (Suppl. 1), S13–S24. [Google Scholar] [CrossRef]
- Yoosuf, N.; Navarro, J.F.; Salmén, F.; Ståhl, P.L.; Daub, C.O. Identification and transfer of spatial transcriptomics signatures for cancer diagnosis. Breast Cancer Res. 2020, 22, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Ruzzo, W.L. Spatial modeling of prostate cancer metabolic gene expression reveals extensive heterogeneity and selective vulnerabilities. Sci. Rep. 2020, 10, 3490. [Google Scholar] [CrossRef] [PubMed]
- Siewe, N.; Friedman, A. Combination therapy for mCRPC with immune checkpoint inhibitors, ADT and vaccine: A mathematical model. PLoS ONE 2022, 17, e0262453. [Google Scholar] [CrossRef]
- Al-Akhras, A.; Chehade, C.H.; Narang, A.; Swami, U. PARP Inhibitors in Metastatic Castration-Resistant Prostate Cancer: Unraveling the Therapeutic Landscape. Life 2024, 14, 198. [Google Scholar] [CrossRef]
- Turnham, D.J.; Bullock, N.; Dass, M.S.; Staffurth, J.N.; Pearson, H.B. The PTEN Conundrum: How to Target PTEN-Deficient Prostate Cancer. Cells 2020, 9, 2342. [Google Scholar] [CrossRef]
- van Dessel, L.F.; van Riet, J.; Smits, M.; Zhu, Y.; Hamberg, P.; van der Heijden, M.S.; Bergman, A.M.; van Oort, I.M.; de Wit, R.; Voest, E.E.; et al. The genomic landscape of metastatic castration-resistant prostate cancers reveals multiple distinct genotypes with potential clinical impact. Nat. Commun. 2019, 10, 5251. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; De Marzo, A.M. Prostate cancer and inflammation: The evidence. Histopathology 2012, 60, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.E.; Thorek, D.L.J.; Meeker, A.K.; Isaacs, J.T.; Brennen, W.N. Tumor-infiltrating mesenchymal stem cells: Drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate 2019, 79, 320–330. [Google Scholar] [CrossRef]
- de Bono, J.S.; Guo, C.; Gurel, B.; De Marzo, A.M.; Sfanos, K.S.; Mani, R.S.; Gil, J.; Drake, C.G.; Alimonti, A. Prostate carcinogenesis: Inflammatory storms. Nat. Rev. Cancer 2020, 20, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, X.; Xu, W.; Chen, Y.; Mu, C.; Zhao, X.; Yang, T.; Wang, G.; Wei, L.; Ma, B. Prostate cancer cells synergistically defend against CD8(+) T cells by secreting exosomal PD-L1. Cancer Med. 2023, 12, 16405–16415. [Google Scholar] [CrossRef] [PubMed]
- Willsmore, Z.N.; Coumbe, B.G.T.; Crescioli, S.; Reci, S.; Gupta, A.; Harris, R.J.; Chenoweth, A.; Chauhan, J.; Bax, H.J.; McCraw, A.; et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur. J. Immunol. 2021, 51, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Chon, H.J.; Kim, C. Combination Immunotherapies to Overcome Intrinsic Resistance to Checkpoint Blockade in Microsatellite Stable Colorectal Cancer. Cancers 2021, 13, 4906. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Denmeade, S.R.; Antonarakis, E.S. Targeting the spectrum of immune checkpoints in prostate cancer. Expert. Rev. Clin. Pharmacol. 2021, 14, 1253–1266. [Google Scholar] [CrossRef]
- Yu, S.; Xiong, G.; Zhao, S.; Tang, Y.; Tang, H.; Wang, K.; Liu, H.; Lan, K.; Bi, X.; Duan, S. Nanobodies targeting immune checkpoint molecules for tumor immunotherapy and immunoimaging (Review). Int. J. Mol. Med. 2021, 47, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; den Eertwegh, A.; Krainer, M.; Houede, N.; et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors. Eur. Urol. 2020, 78, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Aubert, N.; Brunel, S.; Olive, D.; Marodon, G. Blockade of HVEM for Prostate Cancer Immunotherapy in Humanized Mice. Cancers 2021, 13, 3009. [Google Scholar] [CrossRef] [PubMed]
- Czernin, J.; Current, K.; Mona, C.E.; Nyiranshuti, L.; Hikmat, F.; Radu, C.G.; Lückerath, K. Immune-Checkpoint Blockade Enhances (225)Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer. J. Nucl. Med. 2021, 62, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Porras, V.; Pardo, J.C.; Notario, L.; Etxaniz, O.; Font, A. Immune Checkpoint Inhibitors: A Promising Treatment Option for Metastatic Castration-Resistant Prostate Cancer? Int. J. Mol. Sci. 2021, 22, 4712. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, C.; Wang, Z.; Xu, Y.; Shao, S.; Shao, F.; Wang, H.; Liu, J. Neutrophils in cancer: Dual roles through intercellular interactions. Oncogene 2024, 43, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Jurcevic, S.; Humfrey, C.; Uddin, M.; Warrington, S.; Larsson, B.; Keen, C. The effect of a selective CXCR2 antagonist (AZD5069) on human blood neutrophil count and innate immune functions. Br. J. Clin. Pharmacol. 2015, 80, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; O’Malley, J.; Chaudhary, A.K.; Inigo, J.R.; Yadav, N.; Kumar, R.; Chandra, D. Hsp60 and IL-8 axis promotes apoptosis resistance in cancer. Br. J. Cancer 2019, 121, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, H. Doxorubicin-Induced Cancer Cell Senescence Shows a Time Delay Effect and Is Inhibited by Epithelial-Mesenchymal Transition (EMT). Med. Sci. Monit. 2019, 25, 3617–3623. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Q.L.; Meng, L.; Huang, H.; Liu, H.; Zhang, N.; Liu, N.; Yang, J.; Zhang, Y.Z.; Huang, Q. TAZ-regulated expression of IL-8 is involved in chemoresistance of hepatocellular carcinoma cells. Arch. Biochem. Biophys. 2020, 693, 108571. [Google Scholar] [CrossRef]
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marté, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2019, 7, 240. [Google Scholar] [CrossRef] [PubMed]
- Dhayni, K.; Zibara, K.; Issa, H.; Kamel, S.; Bennis, Y. Targeting CXCR1 and CXCR2 receptors in cardiovascular diseases. Pharmacol. Ther. 2022, 237, 108257. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, D.R.; Molczyk, C.; Purohit, A.; Saxena, S.; Sturgeon, R.; Dave, B.J.; Kumar, S.; Batra, S.K.; Singh, R.K. Small molecule antagonist of CXCR2 and CXCR1 inhibits tumor growth, angiogenesis, and metastasis in pancreatic cancer. Cancer Lett. 2023, 563, 216185. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Jackstadt, R.; van Hooff, S.R.; Leach, J.D.; Cortes-Lavaud, X.; Lohuis, J.O.; Ridgway, R.A.; Wouters, V.M.; Roper, J.; Kendall, T.J.; Roxburgh, C.S.; et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 2019, 36, 319–336.e7. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.; Mackey, J.B.G.; Jamieson, T.; Ramon-Gil, E.; Drake, T.M.; Fercoq, F.; Clark, W.; Gilroy, K.; Hedley, A.; Nixon, C.; et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut 2022, 71, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Adekoya, T.O.; Smith, N.; Kothari, P.; Richardson, R.M. Abstract PO-134: Differential effects of CXCR1 and CXCR2 receptors on prostate tumorigenesis. Cancer Epidemiol. Biomark. Prev. 2022, 31, PO-134. [Google Scholar] [CrossRef]
- Nanda, J.S.; Koganti, P.; Perri, G.; Ellis, L. Phenotypic Plasticity—Alternate Transcriptional Programs Driving Treatment Resistant Prostate Cancer. Crit. Rev. Oncog. 2022, 27, 45–60. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Caffo, O.; Wissing, M.; Bianchini, D.; Bergman, A.; Thomsen, F.B.; Schmid, S.; Yu, E.Y.; Bournakis, E.; Sella, A.; Zagonel, V.; et al. Survival Outcomes From a Cumulative Analysis of Worldwide Observational Studies on Sequential Use of New Agents in Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2020, 18, 69–76.e4. [Google Scholar] [CrossRef] [PubMed]
- Lombard, A.P.; Liu, L.; Cucchiara, V.; Liu, C.; Armstrong, C.M.; Zhao, R.; Yang, J.C.; Lou, W.; Evans, C.P.; Gao, A.C. Intra versus Inter Cross-resistance Determines Treatment Sequence between Taxane and AR-Targeting Therapies in Advanced Prostate Cancer. Mol. Cancer Ther. 2018, 17, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Trump, D. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Urol. Oncol. 2016, 34, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Y.; Butler, W.; Xu, L.; Chang, Y.; Lei, K.; Zhang, H.; Zhou, Y.; Gao, A.C.; Zhang, Q.; et al. Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Sci. Transl. Med. 2019, 11, eaax0428. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Puhr, M. Interleukin-6 and prostate cancer: Current developments and unsolved questions. Mol. Cell. Endocrinol. 2018, 462 Pt A, 25–30. [Google Scholar] [CrossRef]
- Culig, Z. Response to Androgens and Androgen Receptor Antagonists in the Presence of Cytokines in Prostate Cancer. Cancers 2021, 13, 2944. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef]
- Krause, W. Resistance to prostate cancer treatments. IUBMB Life 2023, 75, 390–410. [Google Scholar] [CrossRef]
- Debes, J.D.; Tindall, D.J. Mechanisms of androgen-refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1488–1490. [Google Scholar] [CrossRef]
- Cai, M.; Song, X.L.; Li, X.A.; Chen, M.; Guo, J.; Yang, D.H.; Chen, Z.; Zhao, S.C. Current therapy and drug resistance in metastatic castration-resistant prostate cancer. Drug Resist. Updat. 2023, 68, 100962. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.E.; Huang, H.; Tindall, D.J. Androgen receptor signaling in androgen-refractory prostate cancer. J. Natl. Cancer Inst. 2001, 93, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Wang, Z.; Montironi, R.; Jiang, Z.; Cheng, M.; Santoni, M.; Huang, K.; Massari, F.; Lu, X.; Cimadamore, A.; et al. Epigenetic modulations and lineage plasticity in advanced prostate cancer. Ann. Oncol. 2020, 31, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.C.; Liu, Y.N.; Yeh, H.L.; Chen, W.H.; Jiang, K.C.; Lin, S.R.; Huang, J.; Hsiao, M.; Chen, W.Y. TCF7L1 regulates cytokine response and neuroendocrine differentiation of prostate cancer. Oncogenesis 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.W.; Zhang, L.; Cai, X.R.; Wang, X.; She, F.F.; Chen, Y.L. Author Correction: IL-8 is a novel prometastatic chemokine in intrahepatic cholangiocarcinoma that induces CXCR2-PI3K/AKT signaling upon CD97 activation. Sci. Rep. 2023, 13, 18711. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Weng, Y.; Li, X.; Wang, T.; Fan, M.; Shi, Q. Overexpression of IL-8 promotes cell migration via PI3K-Akt signaling pathway and EMT in triple-negative breast cancer. Pathol. Res. Pract. 2021, 223, 152824. [Google Scholar] [CrossRef]
- Guo, Y.; Zang, Y.; Lv, L.; Cai, F.; Qian, T.; Zhang, G.; Feng, Q. IL-8 promotes proliferation and inhibition of apoptosis via STAT3/AKT/NF-κB pathway in prostate cancer. Mol. Med. Rep. 2017, 16, 9035–9042. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Purcell, C.; Seaton, A.; Oladipo, O.; Maxwell, P.J.; O’Sullivan, J.M.; Wilson, R.H.; Johnston, P.G.; Waugh, D.J. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J. Pharmacol. Exp. Ther. 2008, 327, 746–759. [Google Scholar] [CrossRef]
- Park, G.Y.; Pathak, H.B.; Godwin, A.K.; Kwon, Y. Epithelial-stromal communication via CXCL1-CXCR2 interaction stimulates growth of ovarian cancer cells through p38 activation. Cell. Oncol. 2021, 44, 77–92. [Google Scholar] [CrossRef]
- Sitaru, S.; Budke, A.; Bertini, R.; Sperandio, M. Therapeutic inhibition of CXCR1/2: Where do we stand? Intern. Emerg. Med. 2023, 18, 1647–1664. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Westra, J.; Rutgers, A.; der Meer, B.D.-V.; Huitema, M.G.; Stegeman, C.A.; Abdulahad, W.H.; Satchell, S.C.; Mathieson, P.W.; Heeringa, P.; et al. Decreased CXCR1 and CXCR2 expression on neutrophils in anti-neutrophil cytoplasmic autoantibody-associated vasculitides potentially increases neutrophil adhesion and impairs migration. Arthritis Res. Ther. 2011, 13, R201. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zong, S.; Wang, J.; Feng, M.; Wang, J.; Zhang, H.; Xiong, L. Role of neutrophils on cancer cells and other immune cells in the tumor microenvironment. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119493. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Geva, R.; Chung, H.C.; Lemech, C.; Miller, W.H., Jr.; Hansen, A.R.; Lee, J.S.; Tsai, F.; Solomon, B.J.; Kim, T.M.; et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: A phase 2 randomized trial. Investig. New Drugs 2024, 42, 145–159. [Google Scholar] [CrossRef]
| Species | IL-8 Ligands | CXCR1/CXCR2 Ligands | CXCR1 Expression | CXCR2 Expression |
|---|---|---|---|---|
| Human | IL-8 (CXCL8) | CXCR1: CXCL6, CXCL8 CXCR2: CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, CXCL8 | Yes | Yes |
| Mouse | KC (CXCL1), MIP-2 (CXCL2), Gro-alpha (CXCL1) | CXCR1: CXCL6 CXCR2: CXCL1, CXCL2, CXCL3, CXCL5, CXCL6 | No | Yes |
| Inhibitor | Clinical Development | CXCR1 | CXCR2 | IL-8 | |
|---|---|---|---|---|---|
| Navarixin (SCH-527123) | Phase 2 | COPD Asthma Psoriasis Solid tumours | |||
| Reparixin | Phase 3 Pneumonia | Pneumonia Acute respiratory distress syndrome Diabetes mellitus Solid tumours | |||
| SB225002 | |||||
| SB265610 | |||||
| SX-682 | Phase 1 and 2 | Myelodysplastic syndromes Solid tumours | |||
| AZD5069 | Phase I2 Asthma Phase 1 and 2 mCRPC | Asthma Brochiectasis Solid tumours Pancreatic cancer HCC HNSCC | |||
| Danirixin (GSK1325756) | Phase 1/2 | COPD Viral disease | |||
| Benzoylaconitine | |||||
| Pectolinarin | |||||
| Auraotene | |||||
| CXCR2 antagonist 4 | |||||
| DF2726A | |||||
| BMS-986253 (HuMax-IL8) | Phase 1/2 | MDS Solid tumours | |||
| Drug Name | Trial Name | Target | Type | Last Reported Status | Summary | NCT Number | Results |
|---|---|---|---|---|---|---|---|
| AZD5069 (Astrazeneca) | ACE | CXCR2 | Small-molecule inhibitor | Phase 1 and 2: completed | AZD5069 in combination with enzalutamide. | NCT03177187 | Results described in Section 4.3 |
| Navarixin (Merck) | CXCR1/2 | Small-molecule inhibitor | Phase 1 and 2: completed | Navarixin (MK-7123) in combination with pembrolizumab (MK-3475) in adults with selected advanced/metastatic solid tumours. | NCT03473925 | Results posted on clinical trials.gov | |
| BMS-986253 (Previously Humax IL-8) (Bristol-Myers Squibb) | MAGIC-8 | IL-8 | mAb | Phase 1b/2: active, not recruiting | Nivolumab (anti-PD-1) or nivolumab plus BMS-986253 in combination with ADT using degarelix (LHRH antagonist) for men with hormone-sensitive prostate cancer and a rising prostate-specific antigen (PSA). | NCT03689699 | No results posted |
| Burixafor Hydrobromide | CXCR4 | Pilot study: completed | Investigate single-agent burixafor hydrobromide, docetaxel, and G-CSF. Burixafor hydrobromide, alone or in combination with G-CSF, is currently in Phase 2 testing for use as a hematopoietic stem cell (HSC) mobilization agent. | NCT02478125 | No results posted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClelland, S.; Maxwell, P.J.; Branco, C.; Barry, S.T.; Eberlein, C.; LaBonte, M.J. Targeting IL-8 and Its Receptors in Prostate Cancer: Inflammation, Stress Response, and Treatment Resistance. Cancers 2024, 16, 2797. https://doi.org/10.3390/cancers16162797
McClelland S, Maxwell PJ, Branco C, Barry ST, Eberlein C, LaBonte MJ. Targeting IL-8 and Its Receptors in Prostate Cancer: Inflammation, Stress Response, and Treatment Resistance. Cancers. 2024; 16(16):2797. https://doi.org/10.3390/cancers16162797
Chicago/Turabian StyleMcClelland, Shauna, Pamela J. Maxwell, Cristina Branco, Simon T. Barry, Cath Eberlein, and Melissa J. LaBonte. 2024. "Targeting IL-8 and Its Receptors in Prostate Cancer: Inflammation, Stress Response, and Treatment Resistance" Cancers 16, no. 16: 2797. https://doi.org/10.3390/cancers16162797
APA StyleMcClelland, S., Maxwell, P. J., Branco, C., Barry, S. T., Eberlein, C., & LaBonte, M. J. (2024). Targeting IL-8 and Its Receptors in Prostate Cancer: Inflammation, Stress Response, and Treatment Resistance. Cancers, 16(16), 2797. https://doi.org/10.3390/cancers16162797







