Molecular Factors Predicting Ovarian Chemotoxicity in Fertile Women: A Systematic Review
Abstract
Simple Summary
Abstract
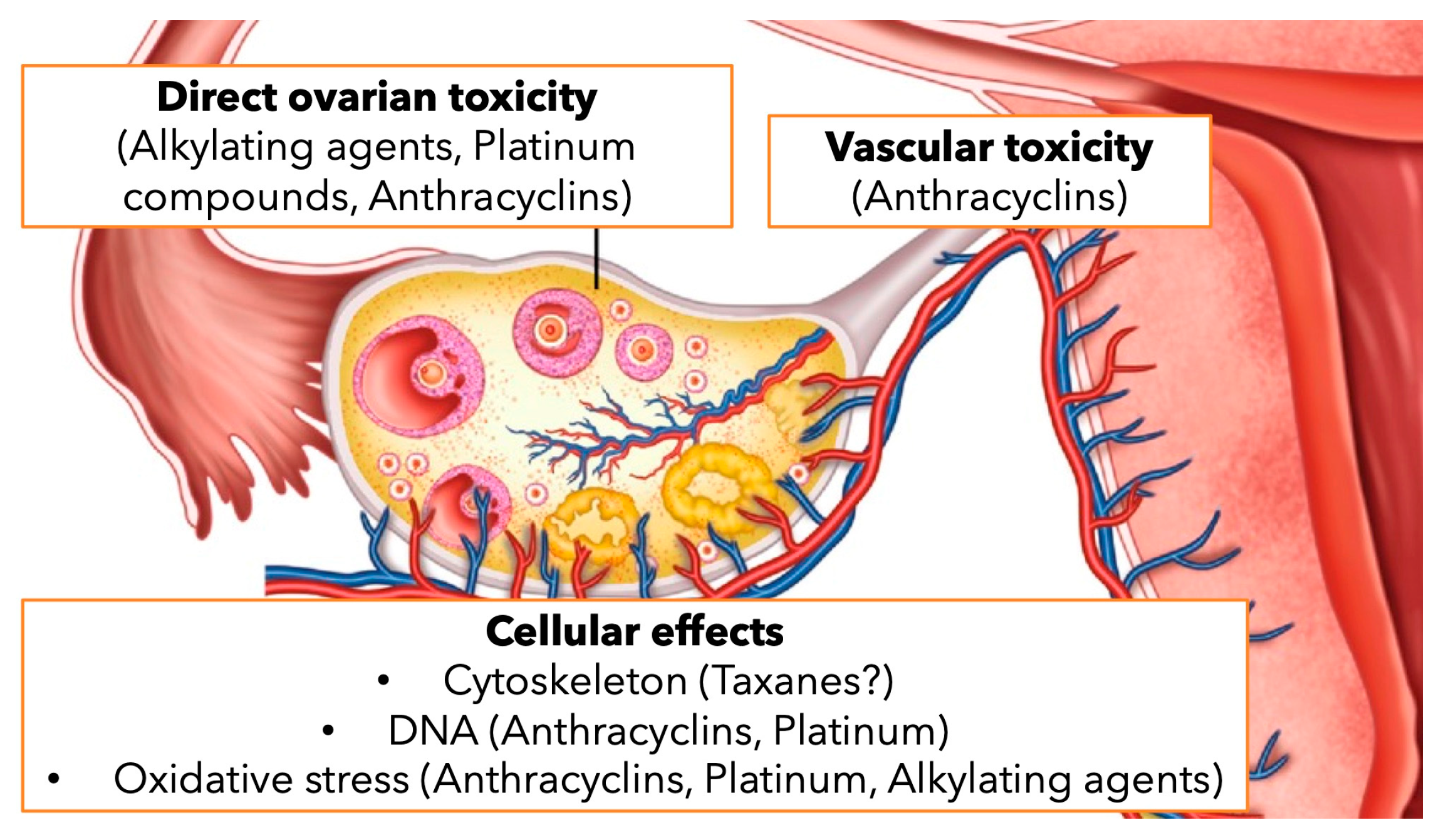
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
- Type of study (observational or interventional design, retrospective or prospective data, randomized or non-randomized allocation of patients);
- Country
- Number of patients
- Genes associated with CIOF assessed in the study
- Chemotherapy drugs studied
- Type of neoplasm
2.5. Assessment of Risk of Bias within Studies
3. Results
3.1. Study Selection
3.2. Studies and Patients’ Characteristics
3.3. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Canada, A.L.; Schover, L.R. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psychooncology 2012, 21, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garcia, C.; Domingo, J.; Garcia-Velasco, J.A.; Herraiz, S.; Mirabet, V.; Iniesta, I.; Cobo, A.; Remohí, J.; Pellicer, A. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: A prospective cohort study. Fertil. Steril. 2018, 109, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Aslan, E.; Ozkaya, E.; Sonmezer, M.; Oktay, K. Ovarian cryopreservation. Minerva Medica 2012, 103, 37–46. [Google Scholar] [PubMed]
- Gracia, C.R.; Sammel, M.D.; Freeman, E.; Prewitt, M.; Carlson, C.; Ray, A.; Vance, A.; Ginsberg, J.P. Impact of cancer therapies on ovarian reserve. Fertil. Steril. 2012, 97, 134–140.e1. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Barton, D.L.; Loprinzi, C.L. Chemotherapy-induced ovarian failure: Manifestations and management. Drug Saf. 2005, 28, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, J.; Marmé, F.; Seiler, S.; Thode, C.; Untch, M.; Schmatloch, S.; Schneeweiss, A.; Bassy, M.; Fasching, P.A.; Strik, D.; et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: Prospective analysis of four randomised neoadjuvant/adjuvant breast cancer trials. Eur. J. Cancer 2021, 152, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Tiong, V.; Rozita, A.M.; Taib, N.A.; Yip, C.H.; Ng, C.H. Incidence of chemotherapy-induced ovarian failure in premenopausal women undergoing chemotherapy for breast cancer. World J. Surg. 2014, 38, 2288–2296. [Google Scholar] [CrossRef]
- Cui, W.; Stern, C.; Hickey, M.; Goldblatt, F.; Anazodo, A.; Stevenson, W.S.; Phillips, K.A. Preventing ovarian failure associated with chemotherapy. Med. J. Aust. 2018, 209, 412–416. [Google Scholar] [CrossRef]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673. [Google Scholar] [CrossRef]
- Ben-Aharon, I.; Shalgi, R. What lies behind chemotherapy-induced ovarian toxicity? Reproduction 2012, 144, 153–163. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, M.L.; Huisbrink, J.; Hauptmann, M.; Kuenen, M.A.; Ouwens, G.M.; Van ’t Veer, M.B.; Aleman, B.M.P.; Van Leeuwen, F.E. Treatment-related risk factors for premature menopause following Hodgkin lymphoma. Blood 2008, 111, 101–108. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Oktay, K. Options on fertility preservation in female cancer patients. Cancer Treat. Rev. 2012, 38, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Bozza, C.; Puglisi, F.; Lambertini, M.; Osa, E.O.; Manno, M.; Del Mastro, L. Anti-Mullerian hormone: Determination of ovarian reserve in early breast cancer patients. Endocr. Relat. Cancer 2014, 21, R51–R65. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Hurd, W.; Jindal, S.; Kalra, S.; Mersereau, J.; et al. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Park, H.T.; Song, J.Y.; Kim, T. Genomic Consideration in Chemotherapy-Induced Ovarian Damage and Fertility Preservation. Genes 2021, 12, 1525. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (Minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Su, H.I.; Sammel, M.D.; Velders, L.; Horn, M.; Stankiewicz, C.; Matro, J.; Gracia, C.R.; Green, J.; DeMichele, A. Association of cyclophosphamide drug-metabolizing enzyme polymorphisms and chemotherapy-related ovarian failure in breast cancer survivors. Fertil. Steril. 2010, 94, 645–654. [Google Scholar] [CrossRef]
- Charo, L.M.; Homer, M.V.; Natarajan, L.; Haunschild, C.; Chung, K.; Mao, J.J.; DeMichele, A.M.; Su, H.I. Drug metabolising enzyme polymorphisms and chemotherapy-related ovarian failure in young breast cancer survivors. J. Obstet. Gynaecol. 2021, 41, 447–452. [Google Scholar] [CrossRef]
- Oktay, K.H.; Bedoschi, G.; Goldfarb, S.B.; Taylan, E.; Titus, S.; Palomaki, G.E.; Cigler, T.; Robson, M.; Dickler, M.N. Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double strand break repair deficiency. Fertil. Steril. 2020, 113, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Van Der Perk, M.E.M.; Broer, L.; Yasui, Y.; Robison, L.L.; Hudson, M.M.; Laven, J.S.E.; Van Der Pal, H.J.; Tissing, W.J.E.; Versluys, B.; Bresters, D.; et al. Effect of Genetic Variation in CYP450 on Gonadal Impairment in a European Cohort of Female Childhood Cancer Survivors, Based on a Candidate Gene Approach: Results from the PanCareLIFE Study. Cancers 2021, 13, 4598. [Google Scholar] [CrossRef]
- van der Kooi, A.L.L.F.; Clemens, E.; Broer, L.; Zolk, O.; Byrne, J.; Campbell, H.; van den Berg, M.; Berger, C.; Calaminus, G.; Dirksen, U.; et al. Genetic variation in gonadal impairment in female survivors of childhood cancer: A PanCareLIFE study protocol. BMC Cancer 2018, 18, 930. [Google Scholar] [CrossRef]
- Howell, C.R.; Bjornard, K.L.; Ness, K.K.; Alberts, N.; Armstrong, G.T.; Bhakta, N.; Brinkman, T.; Caron, E.; Chemaitilly, W.; Green, D.M.; et al. Cohort Profile: The St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. Int. J. Epidemiol. 2021, 50, 39–40I. [Google Scholar] [CrossRef]
- Porcu, E.; Cillo, G.M.; Cipriani, L.; Sacilotto, F.; Notarangelo, L.; Damiano, G.; Dirodi, M.; Roncarati, I. Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J. Assist. Reprod. Genet. 2020, 37, 709–715. [Google Scholar] [CrossRef]
- Lin, W.; Titus, S.; Moy, F.; Ginsburg, E.S.; Oktay, K. Ovarian Aging in Women with BRCA Germline Mutations. J. Clin. Endocrinol. Metab. 2017, 102, 3839. [Google Scholar] [CrossRef] [PubMed]
- Drechsel Katja, C.E.; van Tilborg Theodora, C.; Eijkemans Marinus, J.C.; Lentjes Eef, G.W.M.; Irene, H.; Mariette, G.; van Golde Ron, J.T.; Willem, V.; Lichtenbelt Klaske, D.; Broekmans Frank, J.M.; et al. The Impact of BRCA1- and BRCA2 Mutations on Ovarian Reserve Status. Reprod. Sci. 2023, 30, 270–282. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Hamish Wallace, W.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; De Muinck Keizer-Schrama, S.; Hogervorst, E.; Janse, F.; et al. ESHRE Guideline: Management of women with premature ovarian insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H.B. Toxicity of chemotherapy and radiation on female reproduction. Clin. Obstet. Gynecol. 2010, 53, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Lyttle Schumacher, B.; Grover, N.; Mesen, T.; Steiner, A.; Mersereau, J. Modeling of live-birth rates and cost-effectiveness of oocyte cryopreservation for cancer patients prior to high- and low-risk gonadotoxic chemotherapy. Hum. Reprod. 2017, 32, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, R.; Macciocca, M.; Vicenti, R.; Paradisi, R.; Rossi, S.; Sabattini, E.; Gazzola, A.; Seracchioli, R. First Italian birth after cryopreserved ovarian tissue transplantation in a patient affected by non-Hodgkin’s lymphoma. Int. J. Hematol. Oncol. 2018, 7, IJH08. [Google Scholar] [CrossRef] [PubMed]
- Hyman, J.H.; Tulandi, T. Fertility Preservation Options After Gonadotoxic Chemotherapy. Clin. Med. Insights. Reprod. Health 2013, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, R.; Vicenti, R.; Magnani, V.; Paradisi, R.; Lima, M.; De Meis, L.; Rossi, S.; Raimondo, D.; Casadio, P.; Venturoli, S.; et al. Ovarian tissue cryopreservation and transplantation: 20 years experience in Bologna University. Front. Endocrinol. 2022, 13, 1035109. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Fertility Preservation in Women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of cryopreserved ovarian tissue in a series of 285 women: A review of five leading European centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, D.; Giaquinto, I.; Maletta, M.; Vicenti, R.; Iodice, R.; Arena, A.; Del Forno, S.; Raffone, A.; Lenzi, J.; Casadio, P.; et al. Cost-effectiveness analysis of ovarian tissue cryopreservation and transplantation for preservation of fertility in post-pubertal oncological women submitted to high-risk gonadotoxic chemotherapy. Int. J. Gynaecol. Obstet. 2022, 159, 116. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.; Boivin, J.; Dancet, E.; De Klerk, C.; Emery, M.; Lewis-Jones, C.; Thorn, P.; Van Den Broeck, U.; Venetis, C.; Verhaak, C.M.; et al. ESHRE guideline: Routine psychosocial care in infertility and medically assisted reproduction-a guide for fertility staff. Hum. Reprod. 2015, 30, 2476–2485. [Google Scholar] [CrossRef]
- Zhao, G.; Yan, G.; Cheng, J.; Zhou, X.; Fang, T.; Sun, H.; Hou, Y.; Hu, Y. Hyaluronic acid prevents immunosuppressive drug-induced ovarian damage via up-regulating PGRMC1 expression. Sci. Rep. 2015, 5, 7647. [Google Scholar] [CrossRef]
- Huang, C.C.; Chou, C.H.; Yang, Y.S.; Ho, H.N.; Shun, C.T.; Wen, W.F.; Chen, S.U.; Chen, M.J. Metformin: A novel promising option for fertility preservation during cyclophosphamide-based chemotherapy. Mol. Hum. Reprod. 2021, 27, gaaa084. [Google Scholar] [CrossRef] [PubMed]
- Delkhosh, A.; Delashoub, M.; Tehrani, A.A.; Bahrami, A.M.; Niazi, V.; Shoorei, H.; Banimohammad, M.; Kalarestaghi, H.; Shokoohi, M.; Agabalazadeh, A.; et al. Upregulation of FSHR and PCNA by administration of coenzyme Q10 on cyclophosphamide-induced premature ovarian failure in a mouse model. J. Biochem. Mol. Toxicol. 2019, 33, e22398. [Google Scholar] [CrossRef] [PubMed]
- Niringiyumukiza, J.D.; Cai, H.; Chen, L.; Li, Y.; Wang, L.; Zhang, M.; Xu, X.; Xiang, W. Protective properties of glycogen synthase kinase-3 inhibition against doxorubicin-induced oxidative damage to mouse ovarian reserve. Biomed. Pharmacother. 2019, 116, 108963. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.; Kim, B. Increasing blunting of inhibin responses to dynamic ovarian challenge is associated with reproductive aging in the rat. Reprod. Sci. 2007, 14, 10–19. [Google Scholar] [CrossRef] [PubMed]

| Study | Country | Patients n | Studied Genes | Chemotherapy | Type of Cancer |
|---|---|---|---|---|---|
| 2010 Su et al. [19] | USA | 127 | CYP2B6, CYP2C9, CYP3A4, CYP3A5, GSTM1, GSTP1, GSTA1 and GSTT1 (9 SNPs on a total of 8 DMEs) | Cyclophosphamide + Adriamycin ± (taxane OR 5-fluorouracil); others | Breast |
| 2021 Charo et al. [20] | USA | 115 | CYP3A4, CYP2C19, GSTP1 and GSTA1 | Adriamycin+ cyclophosphamide ± paclitaxel ORDocetaxel + cyclophosphamide | Breast |
| 2020 Oktay et al. [21] | USA | 108 | BRCA1 and BRCA 2 | Cyclophosphamide + doxorubicin + paclitaxel OR cyclophosphamide + methotrexate + 5-fluorouracile OR cyclophosphamide + epirubicina + docetaxel OR only Taxol; others | Breast |
| 2021 van der Perk et al. [22] | International (PanCareLIFE project + SJLIFE study) | 743 + 391 | CYP2C19, CYP3A4, CYP2B6 (9 SNPs on a total of 3 DMEs) | Alkylating agents [quantified using the CED-score] | Leukemia, HL, NHL, Brain, Neuroblastoma, Renal, Sarcomas, Germ Cell Tumor, Skin (incl. melanoma), Retinoblastoma, Liver, Thyroid, Colon, Other |
| Aim | Patient Selection | Prospective Data Collection | Appropriate Endpoints | Unbiased Assessment of the Study Endpoint | Appropriate Follow-Up Period | Loss to Follow-Up | |
|---|---|---|---|---|---|---|---|
| 2010 Su et al. [19] | + | ? | + | + | + | + | − |
| 2021 Charo et al. [20] | + | ? | + | + | + | + | ? |
| 2020 Oktay et al. [21] | + | ? | + | + | + | + | − |
| 2021 Van Der Perk et al. [22] | + | + | + | + | + | + | ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimondo, D.; Raffone, A.; Neola, D.; Genovese, F.; Travaglino, A.; Aguzzi, A.; De Gobbi, V.; Virgilio, A.; Di Santo, S.; Vicenti, R.; et al. Molecular Factors Predicting Ovarian Chemotoxicity in Fertile Women: A Systematic Review. Cancers 2024, 16, 2793. https://doi.org/10.3390/cancers16162793
Raimondo D, Raffone A, Neola D, Genovese F, Travaglino A, Aguzzi A, De Gobbi V, Virgilio A, Di Santo S, Vicenti R, et al. Molecular Factors Predicting Ovarian Chemotoxicity in Fertile Women: A Systematic Review. Cancers. 2024; 16(16):2793. https://doi.org/10.3390/cancers16162793
Chicago/Turabian StyleRaimondo, Diego, Antonio Raffone, Daniele Neola, Federica Genovese, Antonio Travaglino, Alberto Aguzzi, Valeria De Gobbi, Agnese Virgilio, Sara Di Santo, Rossella Vicenti, and et al. 2024. "Molecular Factors Predicting Ovarian Chemotoxicity in Fertile Women: A Systematic Review" Cancers 16, no. 16: 2793. https://doi.org/10.3390/cancers16162793
APA StyleRaimondo, D., Raffone, A., Neola, D., Genovese, F., Travaglino, A., Aguzzi, A., De Gobbi, V., Virgilio, A., Di Santo, S., Vicenti, R., Magnani, V., Guida, M., Pippucci, T., & Seracchioli, R. (2024). Molecular Factors Predicting Ovarian Chemotoxicity in Fertile Women: A Systematic Review. Cancers, 16(16), 2793. https://doi.org/10.3390/cancers16162793









