Drug Combination Nanoparticles Containing Gemcitabine and Paclitaxel Enable Orthotopic 4T1 Breast Tumor Regression
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
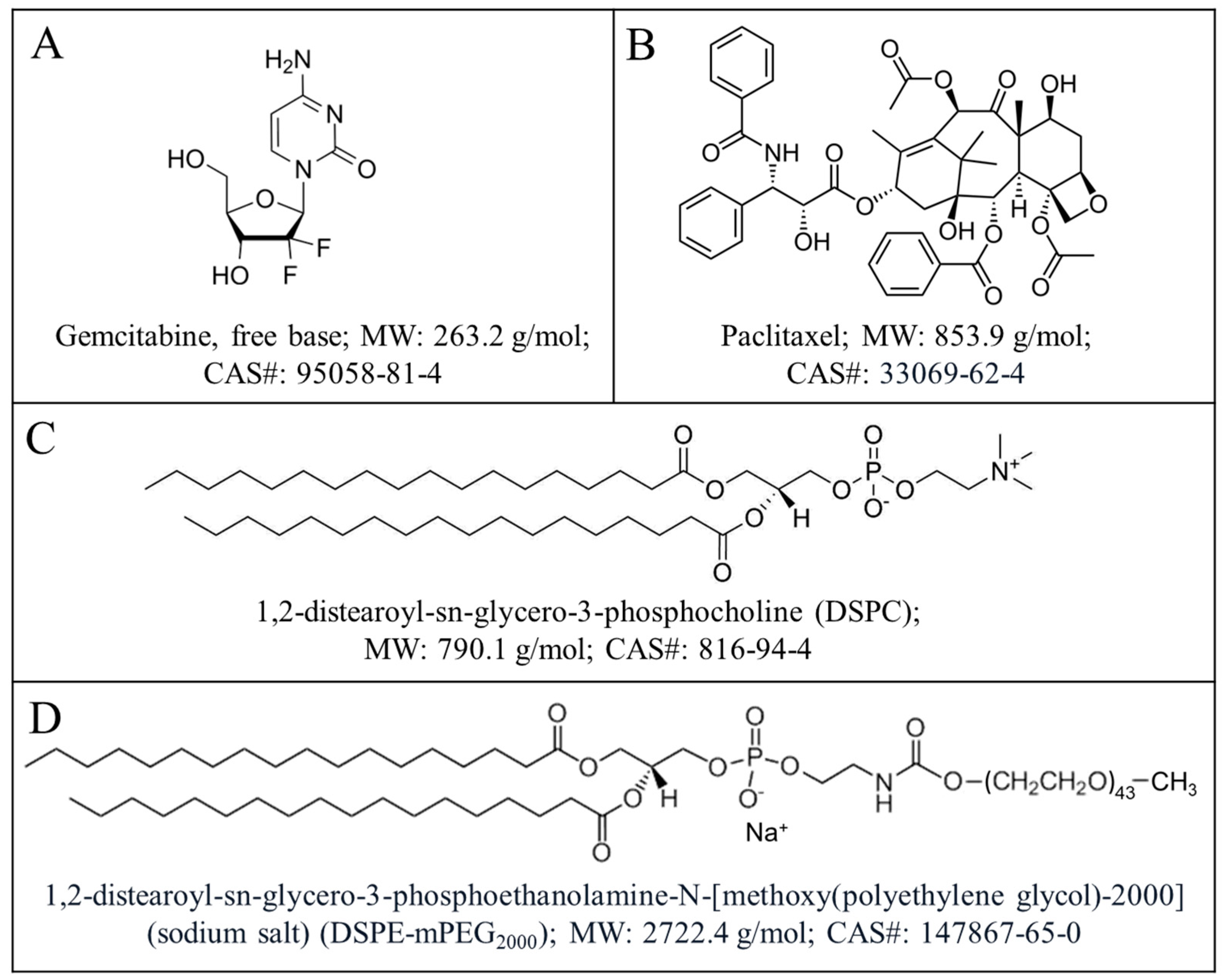
2.1. Materials
2.2. Preparation and Characterization of Gemcitabine and Paclitaxel (GT) in Drug Combination Nanoparticles
2.3. Preparation of Freely and Soluble Gemcitabine and Paclitaxel (GT) Combination
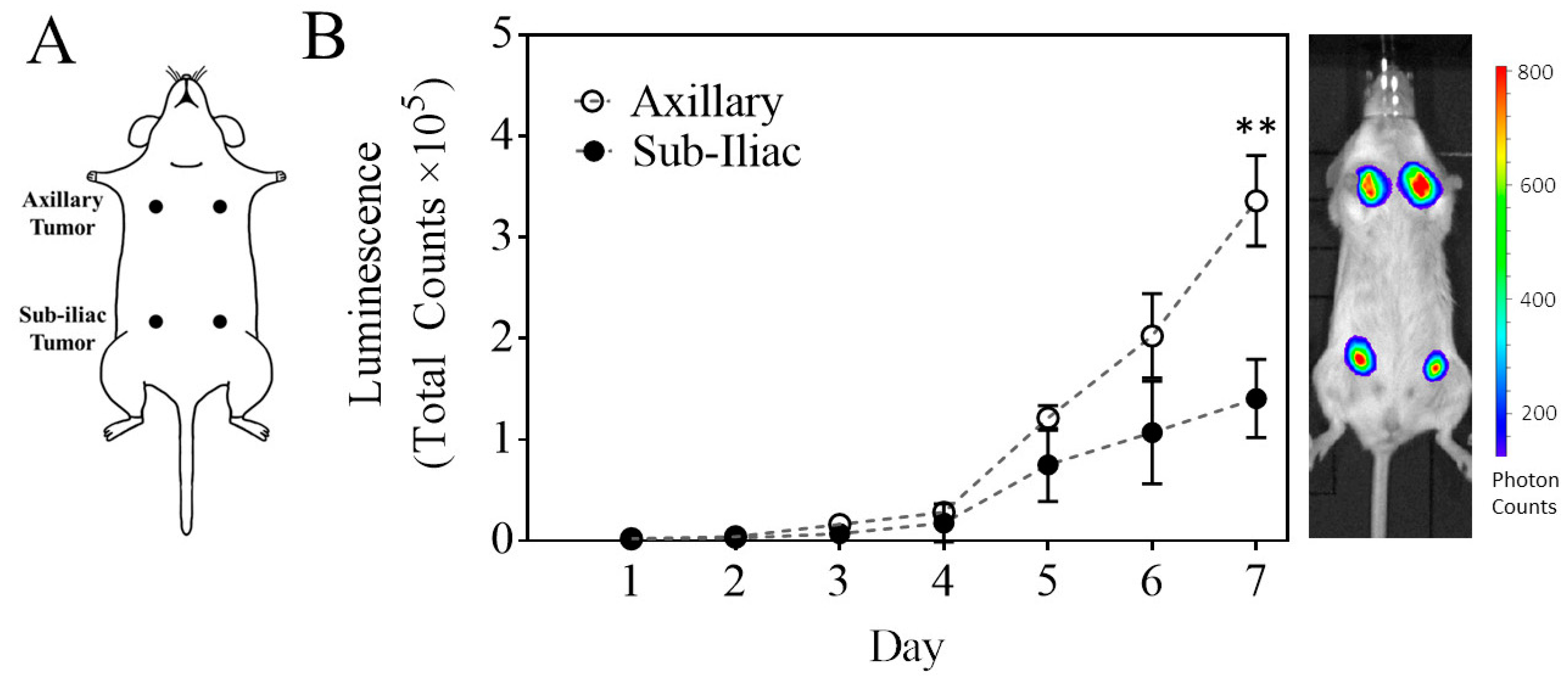
2.4. Development and Characterization of 4T1 Breast Tumor in Mouse Mammary Fat Pads
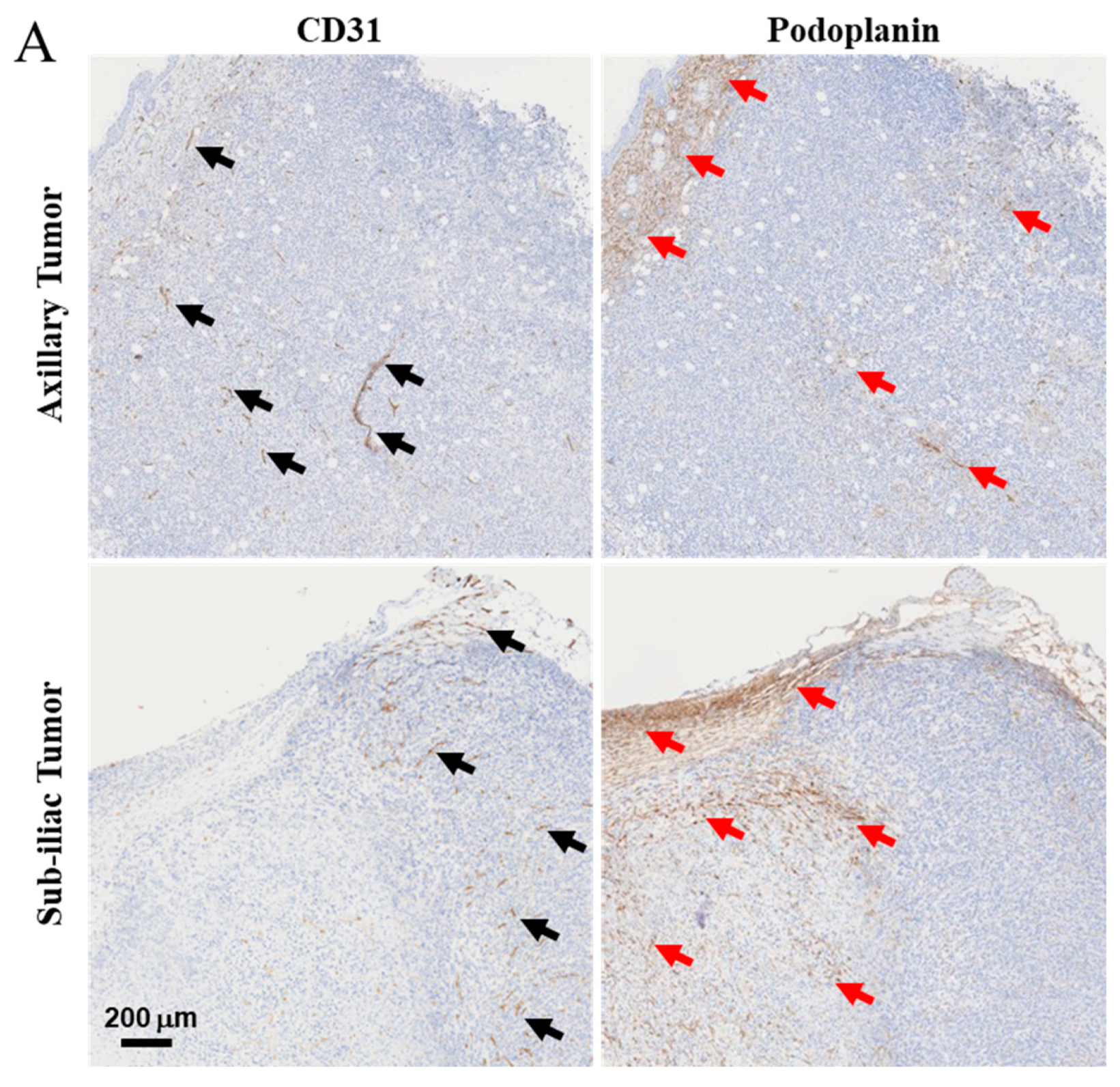
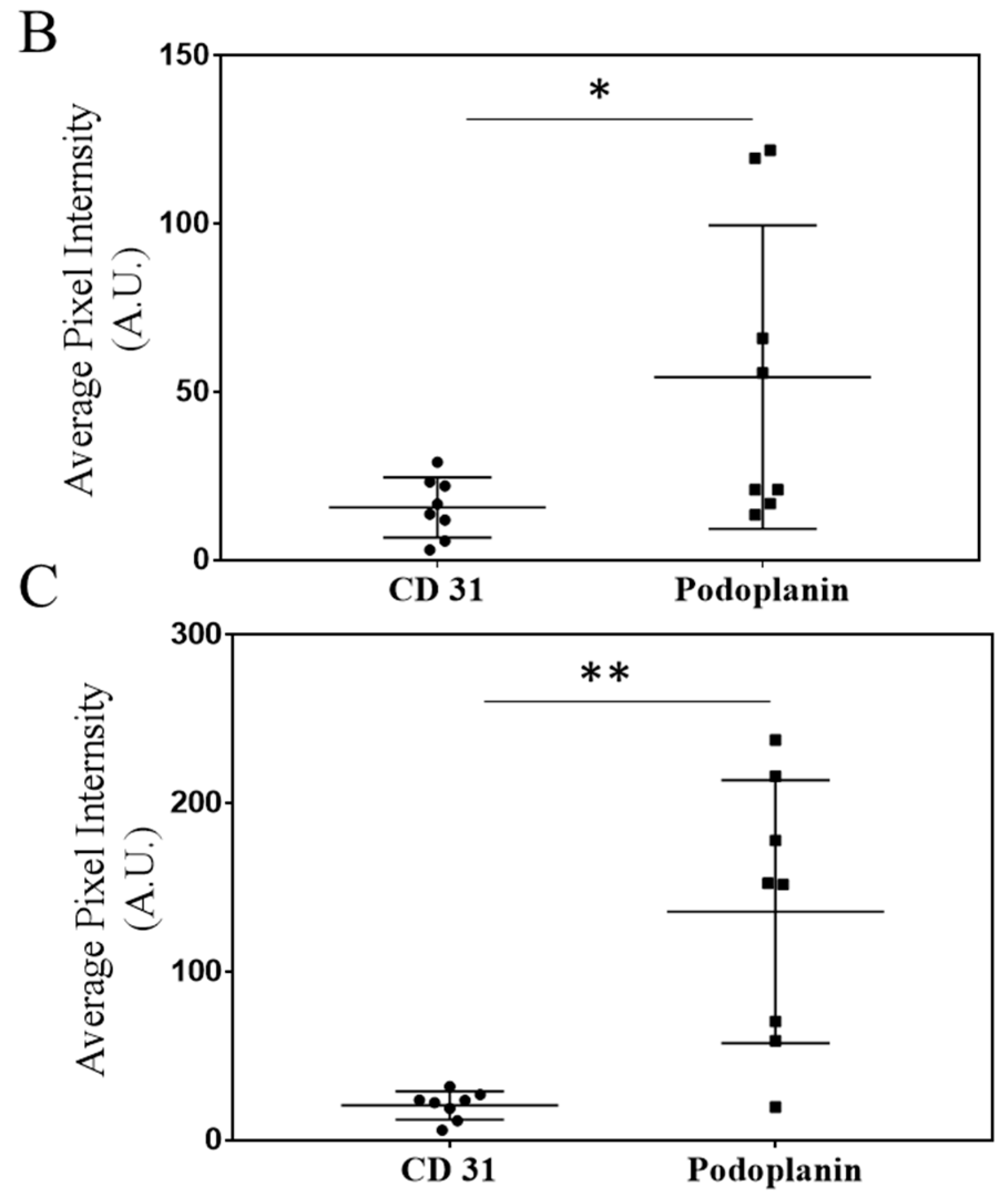
2.5. Immunohistochemistry (IHC) Staining of CD31 and Podoplanin to Detect Developing Tumor Blood and Lymphatic Vasculature
2.6. Comparison of Tumor Inhibition Effects of Equivalent Doses of Gemcitabine and Paclitaxel in DcNP or Free Form
2.7. Dose Dependence of Gemcitabine and Paclitaxel in DcNP on Tumor Growth Inhibition
2.8. Time Course of Gemcitabine and Paclitaxel (GT) in Tumors and Plasma in 4T1 Tumor Bearing Mouse Model and Biodistribution Study
2.9. Extraction of Drugs from Plasma and Tissues
2.10. Quantification of Gemcitabine and Paclitaxel in Plasma and Tumors by HPLC-MS/MS
2.11. Effect of GT-in-DcNP on Human MDA-231-HM Tumor Implanted in Fat-Pad of Athymic Mice
2.12. Statistical Analysis
3. Results
3.1. Characterization of a Primary Multi-Site Mammary Fat Pad 4T1 Breast Cancer Model
3.2. Characterization of Lymph and Blood Vasculature Development in Primary 4T1 Tumors
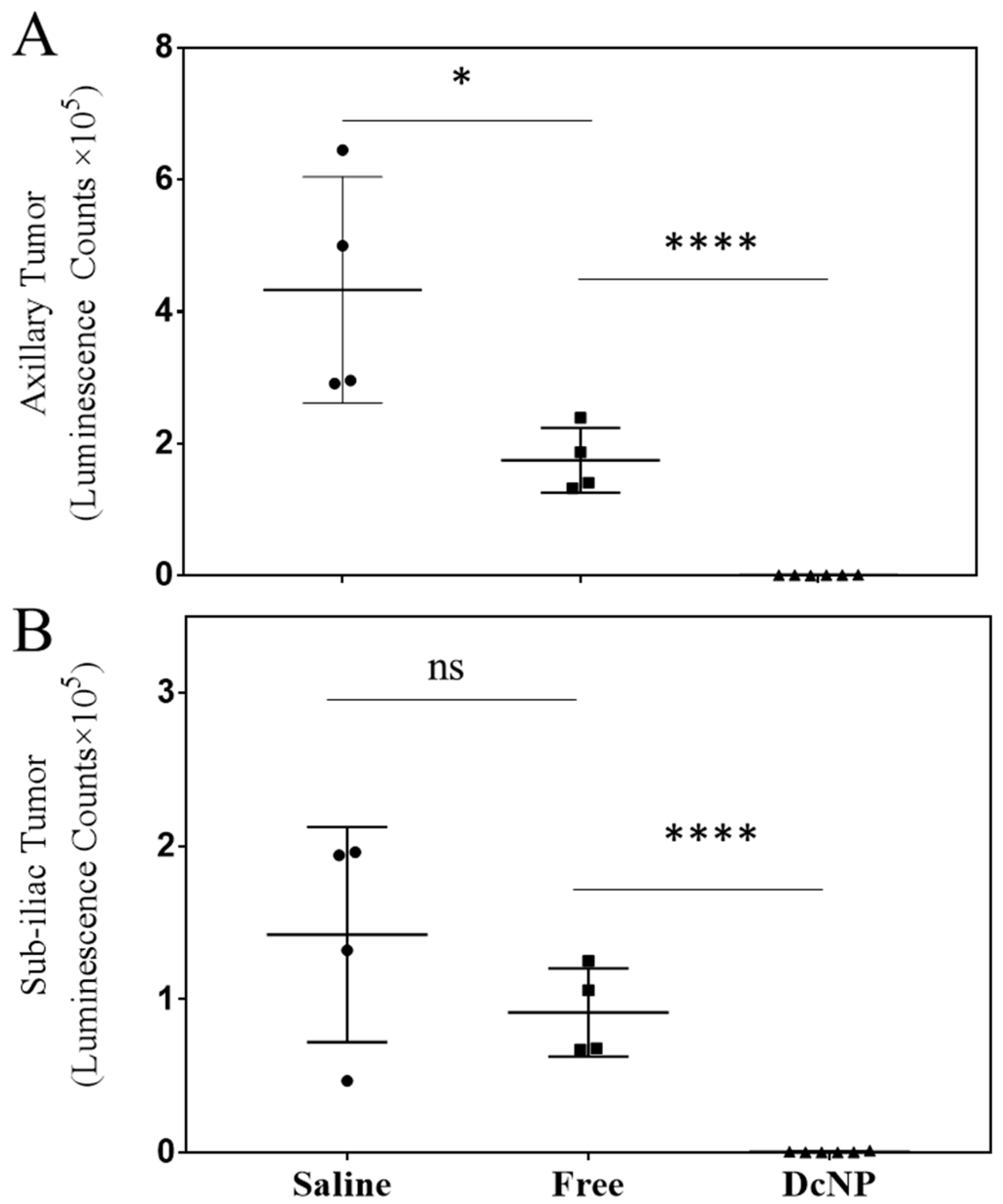
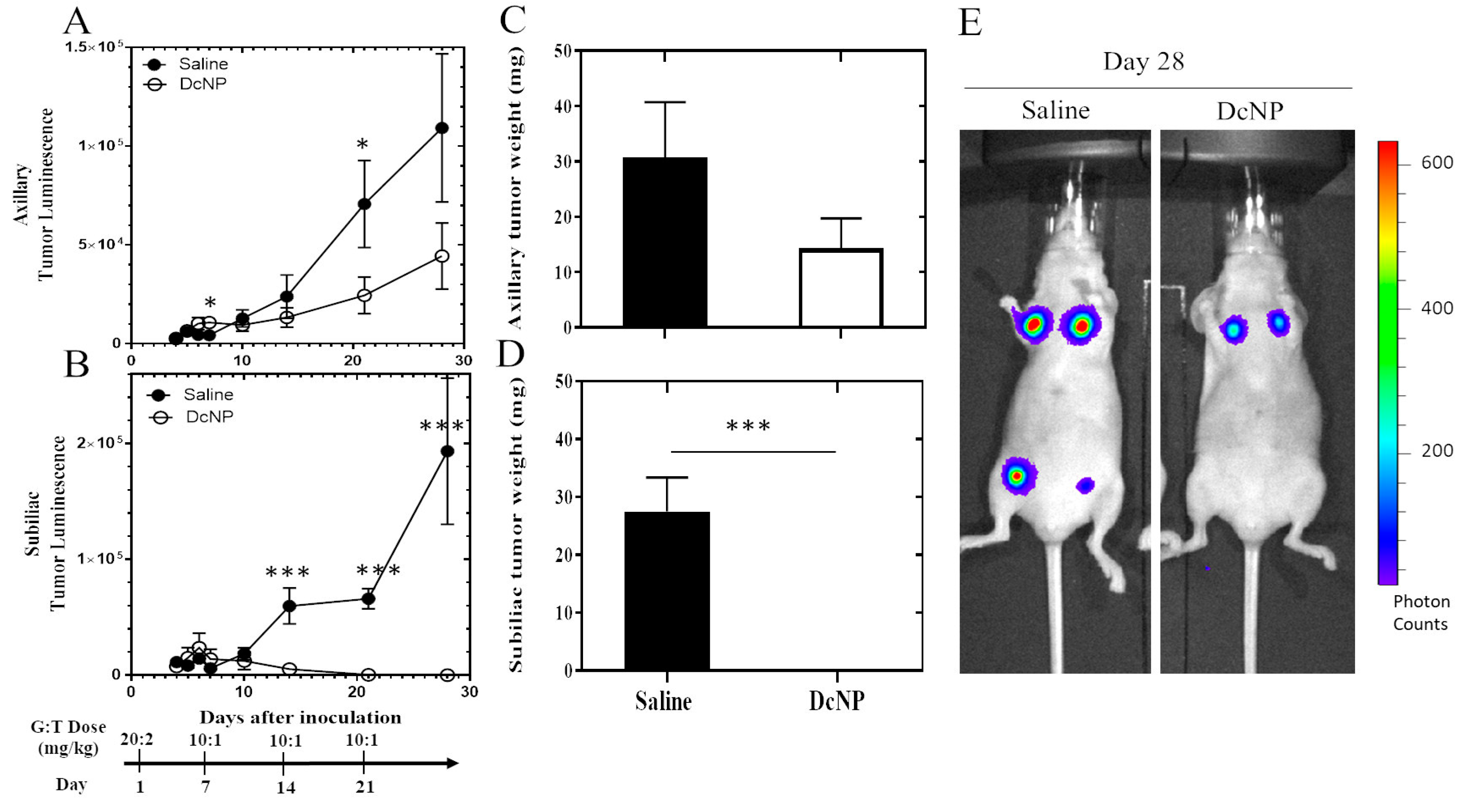
3.3. Effects of DcNP on Gemcitabine and Paclitaxel Combination to Inhibit 4T1 Mammary Tumor
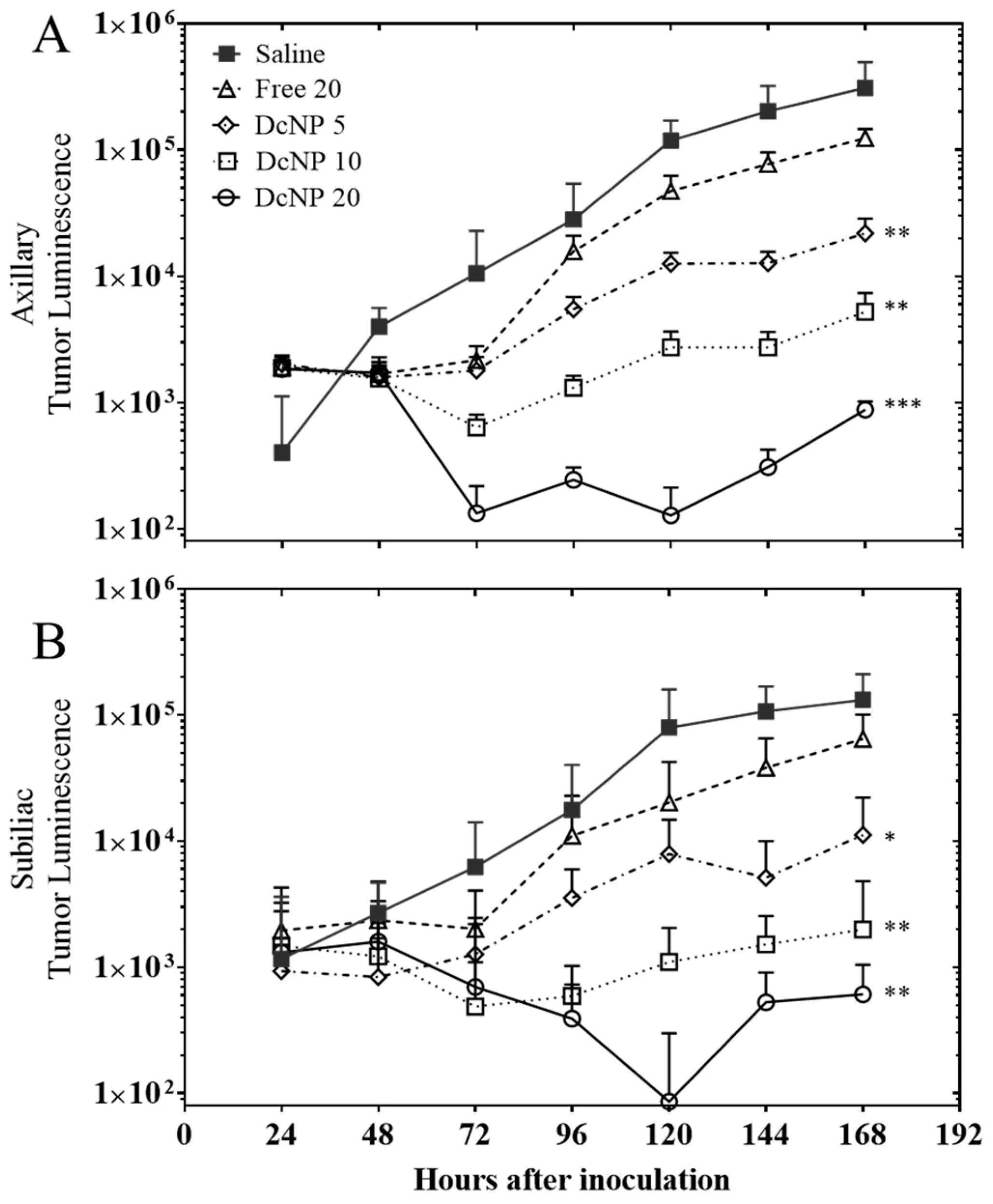
3.4. Dose–Response Study of Gemcitabine and Paclitaxel in DcNP Form to Suppress 4T1 Breast Tumor
3.5. Mechanisms Relating to DcNP Mediated Enhancement in GT Exposure Leading to 4T1 Mammary Tumor Regression and Inhibition
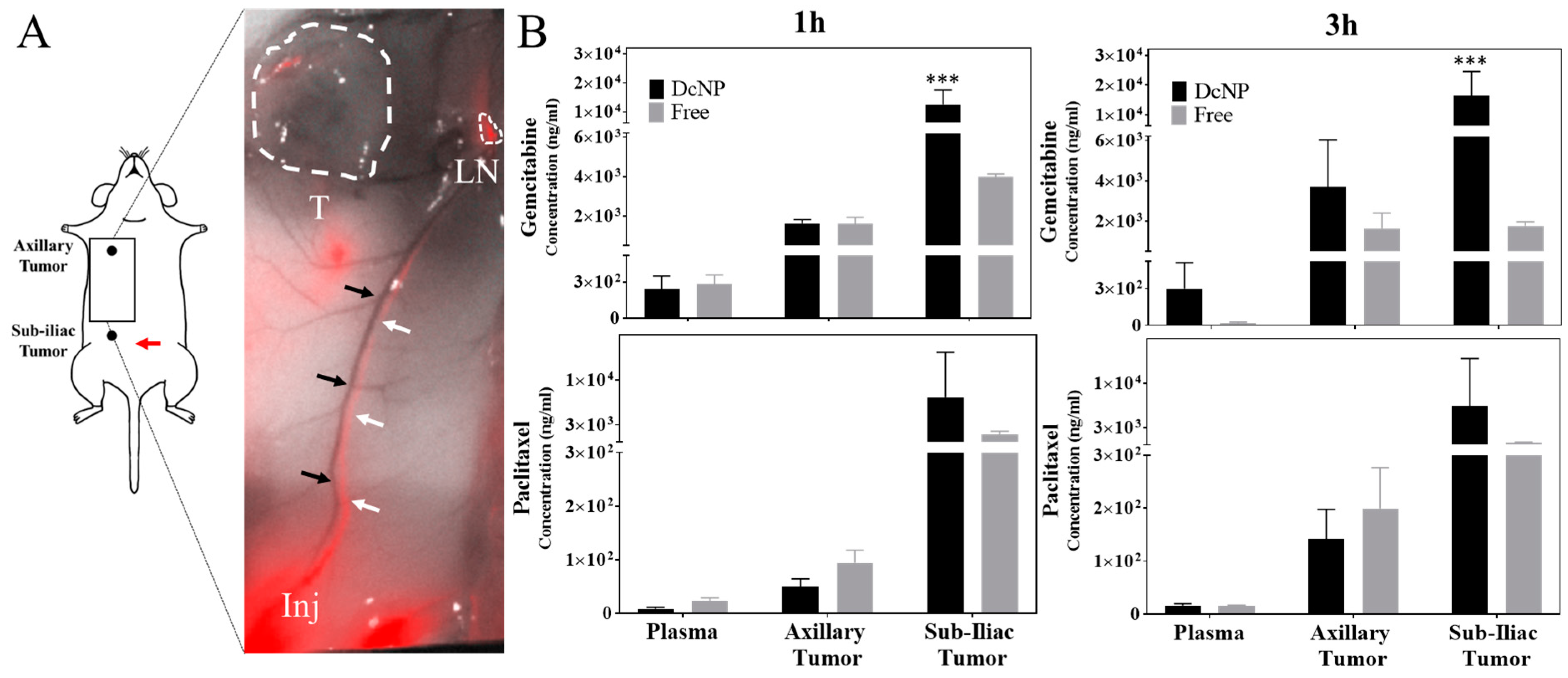
3.5.1. Effects of Drug Combination Nanoparticles on Gemcitabine and Paclitaxel Transit and Accumulation in Lymphatic Vessels and Tumors
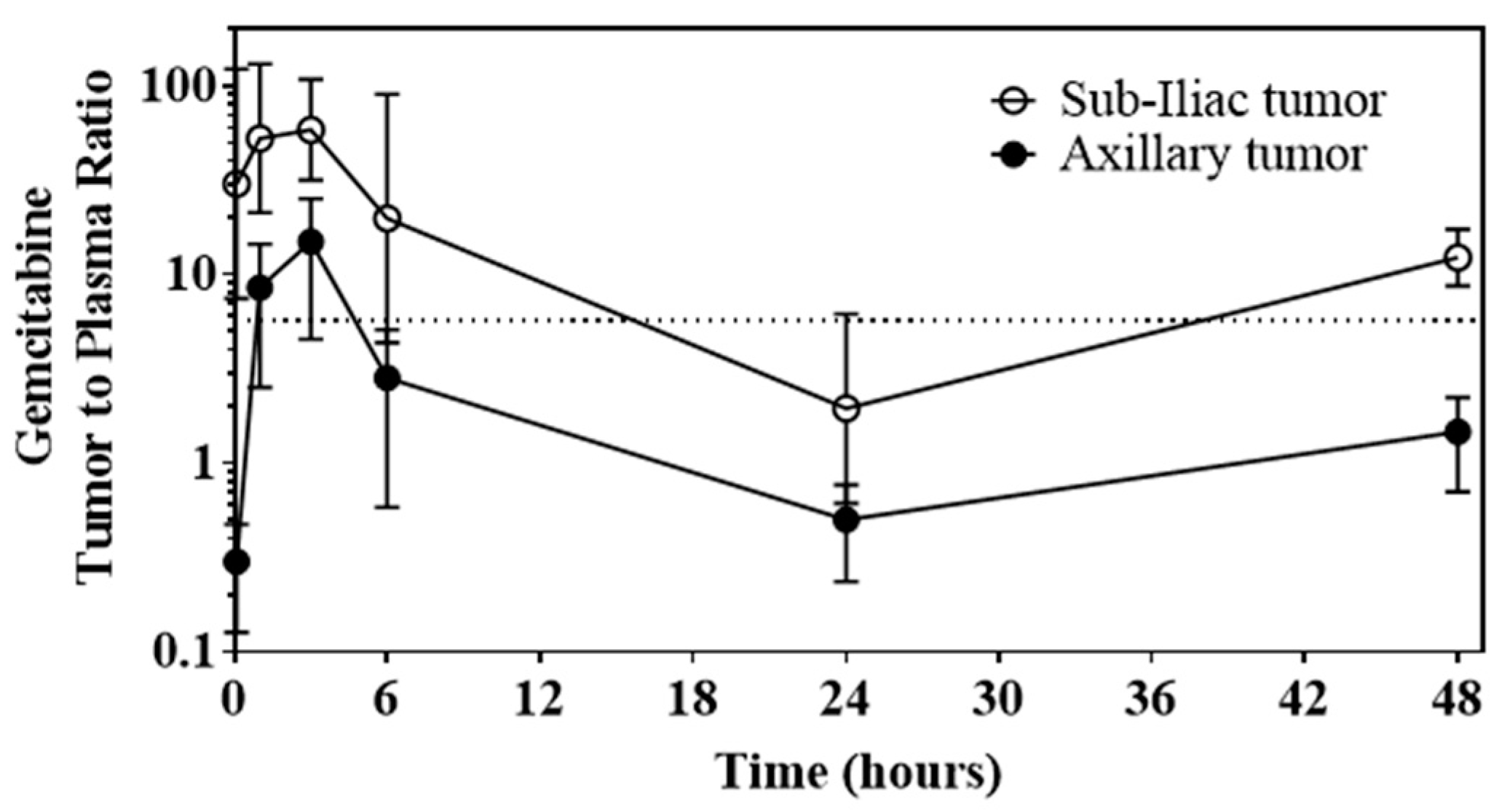
3.5.2. Ability of DcNP to Enhance Gemcitabine and Paclitaxel Retention in Tumors and Extend Persistence of Plasma Drug Concentrations
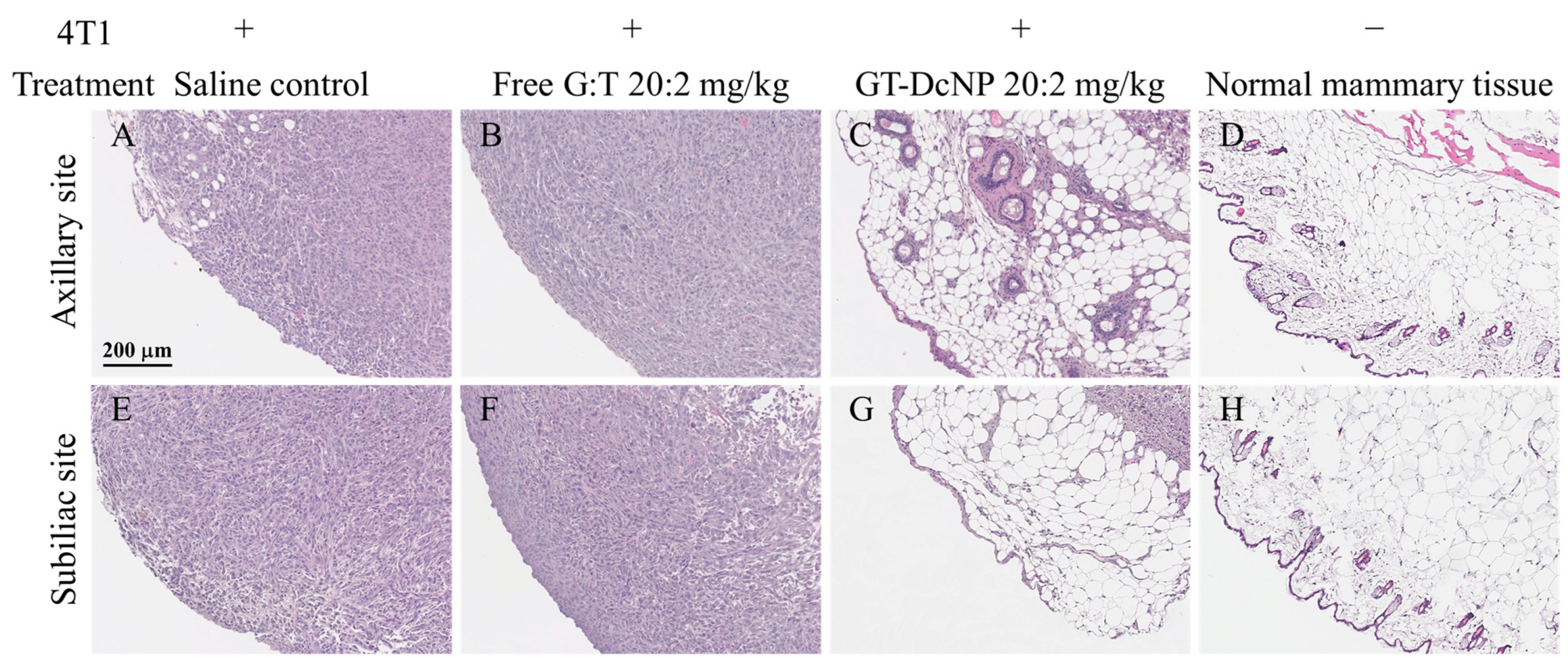
3.6. Safety and Pathology of DcNP Effects on GT Drug Combination Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| G | Gemcitabine |
| T | Paclitaxel |
| DcNP | Drug combination nanoparticles |
| IV | Intravenous/intravenously |
| SC | Subcutaneous/subcutaneously |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| DSPE-mPEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] |
| ICG | Indocyanine green |
| GFP | Green fluorescence protein |
| IVIS | In vivo imaging system |
| IHC | Immunohistochemistry |
| AE% | Association efficiency |
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pereira, E.R.; Kedrin, D.; Seano, G.; Gautier, O.; Meijer, E.F.J.; Jones, D.; Chin, S.-M.; Kitahara, S.; Bouta, E.M.; Chang, J.; et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 2018, 359, 1403–1407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, M.; Assen, F.P.; Leithner, A.; Abe, J.; Schachner, H.; Asfour, G.; Bago-Horvath, Z.; Stein, J.V.; Uhrin, P.; Sixt, M.; et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018, 359, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Cote, B.; Rao, D.; Alany, R.G.; Kwon, G.S.; Alani, A.W. Lymphatic changes in cancer and drug delivery to the lymphatics in solid tumors. Adv. Drug Deliv. Rev. 2019, 144, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tufail, M.; Cui, J.; Wu, C. Breast cancer: Molecular mechanisms of underlying resistance and therapeutic approaches. Am. J. Cancer Res. 2022, 12, 2920–2949. [Google Scholar] [PubMed]
- National Comprehensive Cancer Network (NCCN). Practice Guidelines in Oncology: Breast Cancer; Version 3; National Comprehensive Cancer Network (NCCN): Plymouth Meeting, PA, USA, 2021; Available online: http://www.nccn.org (accessed on 2 June 2022).
- Goormaghtigh, E.; Chatelain, P.; Caspers, J.; Ruysschaert, J. Evidence of a complex between adriamycin derivatives and cardiolipin: Possible role in cardiotoxicity. Biochem. Pharmacol. 1980, 29, 3003–3010. [Google Scholar] [CrossRef]
- Hu, X.-C.; Zhang, J.; Xu, B.-H.; Cai, L.; Ragaz, J.; Wang, Z.-H.; Wang, B.-Y.; Teng, Y.-E.; Tong, Z.-S.; Pan, Y.-Y.; et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Albain, K.S.; Nag, S.M.; Calderillo-Ruiz, G.; Jordaan, J.P.; Llombart, A.C.; Pluzanska, A.; Rolski, J.; Melemed, A.S.; Reyes-Vidal, J.M.; Sekhon, J.S.; et al. Gemcitabine Plus Paclitaxel Versus Paclitaxel Monotherapy in Patients With Metastatic Breast Cancer and Prior Anthracycline Treatment. J. Clin. Oncol. 2008, 26, 3950–3957. [Google Scholar] [CrossRef] [PubMed]
- Allouache, D.; Gawande, S.R.; Tubiana-Hulin, M.; Tubiana-Mathieu, N.; Piperno-Neumann, S.; Mefti, F.; Bozec, L.; Genot, J.-Y. First-line therapy with gemcitabine and paclitaxel in locally, recurrent or metastatic breast cancer: A phase II study. BMC Cancer 2005, 5, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mu, Q.; Yu, J.; Griffin, J.I.; Wu, Y.; Zhu, L.; McConnachie, L.A.; Ho, R.J.Y. Novel drug combination nanoparticles exhibit enhanced plasma exposure and dose-responsive effects on eliminating breast cancer lung metastasis. PLoS ONE 2020, 15, e0228557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.; Mu, Q.; Perazzolo, S.I.; Griffin, J.; Zhu, L.; McConnachie, L.A.; Shen, D.D.; Ho, R.J. Novel Long-Acting Drug Combination Nanoparticles Composed of Gemcitabine and Paclitaxel Enhance Localization of Both Drugs in Metastatic Breast Cancer Nodules. Pharm. Res. 2020, 37, 197. [Google Scholar] [CrossRef]
- Zhu, L.; Mu, Q.; Yu, J.; Griffin, J.I.; Xu, X.; Ho, R.J.Y. ICAM-1 Targeted Drug Combination Nanoparticles Enhanced Gemcitabine-Paclitaxel Exposure and Breast Cancer Suppression in Mouse Models. Pharmaceutics 2022, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohammed, R.A.; Ellis, I.O.; Lee, A.H.; Martin, S.G. Vascular invasion in breast cancer; an overview of recent prognostic developments and molecular pathophysiological mechanisms. Histopathology 2009, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression Using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio-Protocol 2019, 9, e3465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Li, X.; Tse, B.W.-C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraft, J.C.; Treuting, P.M.; Ho, R.J.Y. Indocyanine green nanoparticles undergo selective lymphatic uptake, distribution and retention and enable detailed mapping of lymph vessels, nodes and abnormalities. J. Drug Target. 2018, 26, 494–504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kodama, T.; Hatakeyama, Y.; Kato, S.; Mori, S. Visualization of fluid drainage pathways in lymphatic vessels and lymph nodes using a mouse model to test a lymphatic drug delivery system. Biomed. Opt. Express 2015, 6, 124–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamaji, Y.; Akita, S.; Akita, H.; Miura, N.; Gomi, M.; Manabe, I.; Kubota, Y.; Mitsukawa, N. Development of a mouse model for the visual and quantitative assessment of lymphatic trafficking and function by in vivo imaging. Sci. Rep. 2018, 8, 5921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377. [Google Scholar]
- Shaw, M.T.; Spector, M.H.; Ladman, A. Effects of cancer, radiotherapy and cytotoxic drugs on intestinal structure and function. Cancer Treat. Rev. 1979, 6, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, R.; Morrissey, L.; Sidky, Y. Regional differences in incidence and growth of mouse tumors following intradermal or subcutaneous inoculation. Cancer Res. 1978, 38, 1739–1744. [Google Scholar] [PubMed]
- McConnachie, L.A.; Kinman, L.M.; Koehn, J.; Kraft, J.C.; Lane, S.; Lee, W.; Collier, A.C.; Ho, R.J. Long-Acting Profile of 4 Drugs in 1 Anti-HIV Nanosuspension in Nonhuman Primates for 5 Weeks After a Single Subcutaneous Injection. J. Pharm. Sci. 2018, 107, 1787–1790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraft, J.C.; McConnachie, L.A.; Koehn, J.; Kinman, L.; Collins, C.; Shen, D.D.; Collier, A.C.; Ho, R.J. Long-acting combination anti-HIV drug suspension enhances and sustains higher drug levels in lymph node cells than in blood cells and plasma. AIDS 2017, 31, 765–770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, C.-N.; Berghuis, B.; Tsarfaty, G.; Bruch, M.; Kort, E.J.; Ditlev, J.; Tsarfaty, I.; Hudson, E.; Jackson, D.G.; Petillo, D.; et al. Preparing the “soil”: The primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006, 66, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-C. Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. Int. J. Mol. Sci. 2016, 18, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stanley, A.; Ashrafi, G.H.; Seddon, A.M.; Modjtahedi, H. Synergistic effects of various Her inhibitors in combination with IGF-1R, C-MET and Src targeting agents in breast cancer cell lines. Sci. Rep. 2017, 7, 3964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.; Yu, D.; Lane, S.; McConnachie, L.; Ho, R.J. Controlled Solvent Removal from Antiviral Drugs and Excipients in Solution Enables the Formation of Novel Combination Multi-Drug-Motifs in Pharmaceutical Powders Composed of Lopinavir, Ritonavir and Tenofovir. J. Pharm. Sci. 2020, 109, 3480–3489. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo, S.; Shireman, L.M.; Koehn, J.; McConnachie, L.A.; Kraft, J.C.; Shen, D.D.; Ho, R.J. Three HIV Drugs, Atazanavir, Ritonavir, and Tenofovir, Coformulated in Drug-Combination Nanoparticles Exhibit Long-Acting and Lymphocyte-Targeting Properties in Nonhuman Primates. J. Pharm. Sci. 2018, 107, 3153–3162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koehn, J.; Iwamoto, J.F.; Kraft, J.C.; McConnachie, L.A.; Collier, A.C.; Ho, R.J. Extended cell and plasma drug levels after one dose of a three-in-one nanosuspension containing lopinavir, efavirenz, and tenofovir in nonhuman primates. AIDS 2018, 32, 2463–2467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griffin, J.; Wu, Y.; Mu, Q.; Li, X.; Ho, R.J.Y. Design and Characterization of a Novel Venetoclax-Zanubrutinib Nano-Combination for Enhancing Leukemic Cell Uptake and Long-Acting Plasma Exposure. Pharmaceutics 2023, 15, 1016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic Polyglycerol-Derived Nano-Architectures as Delivery Platforms of Gemcitabine for Pancreatic Cancer. Macromol. Biosci. 2019, 19, e1900073. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kawano, I.; Iwase, H. Nab-paclitaxel for the treatment of breast cancer: Efficacy, safety, and approval. OncoTargets Ther. 2011, 4, 123–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernabeu, E.; Helguera, G.; Legaspi, M.J.; Gonzalez, L.; Hocht, C.; Taira, C.; Chiappetta, D.A. Paclitaxel-loaded PCL–TPGS nanoparticles: In vitro and in vivo performance compared with Abraxane®. Colloids Surf. B Biointerfaces 2014, 113, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Guo, Y.; Duan, Y.; Li, Z.; Wang, C.; Niu, L.; Wang, N.; Ma, M.; Shi, Y.; Zhang, M. Co-delivery of paclitaxel and gemcitabine by methoxy poly(ethylene glycol)–poly(lactide-coglycolide)-polypeptide nanoparticles for effective breast cancer therapy. Anti-Cancer Drugs 2018, 29, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, P.; Zou, Q.; Li, X.; Fu, J.; Luo, Y.; Liang, X.; Jin, Y. Co-Delivery of Gemcitabine and Paclitaxel in cRGD-Modified Long Circulating Nanoparticles with Asymmetric Lipid Layers for Breast Cancer Treatment. Molecules 2018, 23, 2906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, M.; Sha, S.; Wang, X.; Wang, J.; Du, X.; Miao, H.; Zhou, H.; Bai, E.; Shi, J.; Zhu, Y. Co-delivery of paclitaxel and gemcitabine via a self-assembling nanoparticle for targeted treatment of breast cancer. RSC Adv. 2019, 9, 5512–5520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.; Kang, S.; Park, H.; Gil Sun, J.; Kim, E.C.; Shim, G. Nanoparticles for Lymph Node-Directed Delivery. Pharmaceutics 2023, 15, 565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Cell Number | Volume | # of Tumors | Live Tumor Luminescence (Photon Counts) b | Tumor Volume at Day 5 (mm3) a | ||
|---|---|---|---|---|---|---|
| (Million) | Inoculant (µL) | Day 1 | Day 3 | Tumor Growth (Fold-Change) | ||
| 0.2 | 20 | 4 | 760 ± 894 | 7769 ± 6975 | 10.2 | N/A d |
| 0.5 | 50 | 4 | 2617 ± 3294 | 14,759 ± 17,201 | 5.6 | N/A d |
| 1 | 100 c | 4 | 4350 ± 995 | 23,435 ± 11,180 | 5.4 | N/A d |
| 1 | 50 | 12 | 3244 ± 1942 | 16,021 ± 13,247 | 4.9 | 40 ± 17 |
| 1 | 50 | 16 | 1386 ± 1950 | 8389 ± 9889 | 6 | 53 ± 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xu, X.; Griffin, J.I.; Mu, Q.; Ho, R.J.Y. Drug Combination Nanoparticles Containing Gemcitabine and Paclitaxel Enable Orthotopic 4T1 Breast Tumor Regression. Cancers 2024, 16, 2792. https://doi.org/10.3390/cancers16162792
Yu J, Xu X, Griffin JI, Mu Q, Ho RJY. Drug Combination Nanoparticles Containing Gemcitabine and Paclitaxel Enable Orthotopic 4T1 Breast Tumor Regression. Cancers. 2024; 16(16):2792. https://doi.org/10.3390/cancers16162792
Chicago/Turabian StyleYu, Jesse, Xiaolin Xu, James Ian Griffin, Qingxin Mu, and Rodney J. Y. Ho. 2024. "Drug Combination Nanoparticles Containing Gemcitabine and Paclitaxel Enable Orthotopic 4T1 Breast Tumor Regression" Cancers 16, no. 16: 2792. https://doi.org/10.3390/cancers16162792
APA StyleYu, J., Xu, X., Griffin, J. I., Mu, Q., & Ho, R. J. Y. (2024). Drug Combination Nanoparticles Containing Gemcitabine and Paclitaxel Enable Orthotopic 4T1 Breast Tumor Regression. Cancers, 16(16), 2792. https://doi.org/10.3390/cancers16162792








