The Impact of Biliary Injury on the Recurrence of Biliary Cancer and Benign Disease after Liver Transplantation: Risk Factors and Mechanisms
Abstract
Simple Summary
Abstract
1. Introduction
2. Biliary Inflammation, Cholangiocarcinoma, and Liver Transplantation
2.1. A Mechanistic Overview of Ischemic Cholangiopathy
2.2. Inflammation Triggered by Ischemia-Reperfusion Injury and Cancer Recurrence
2.3. Biliary Inflammation and Biliary Cancer Recurrence
3. Inflammation and Benign Disease Recurrence
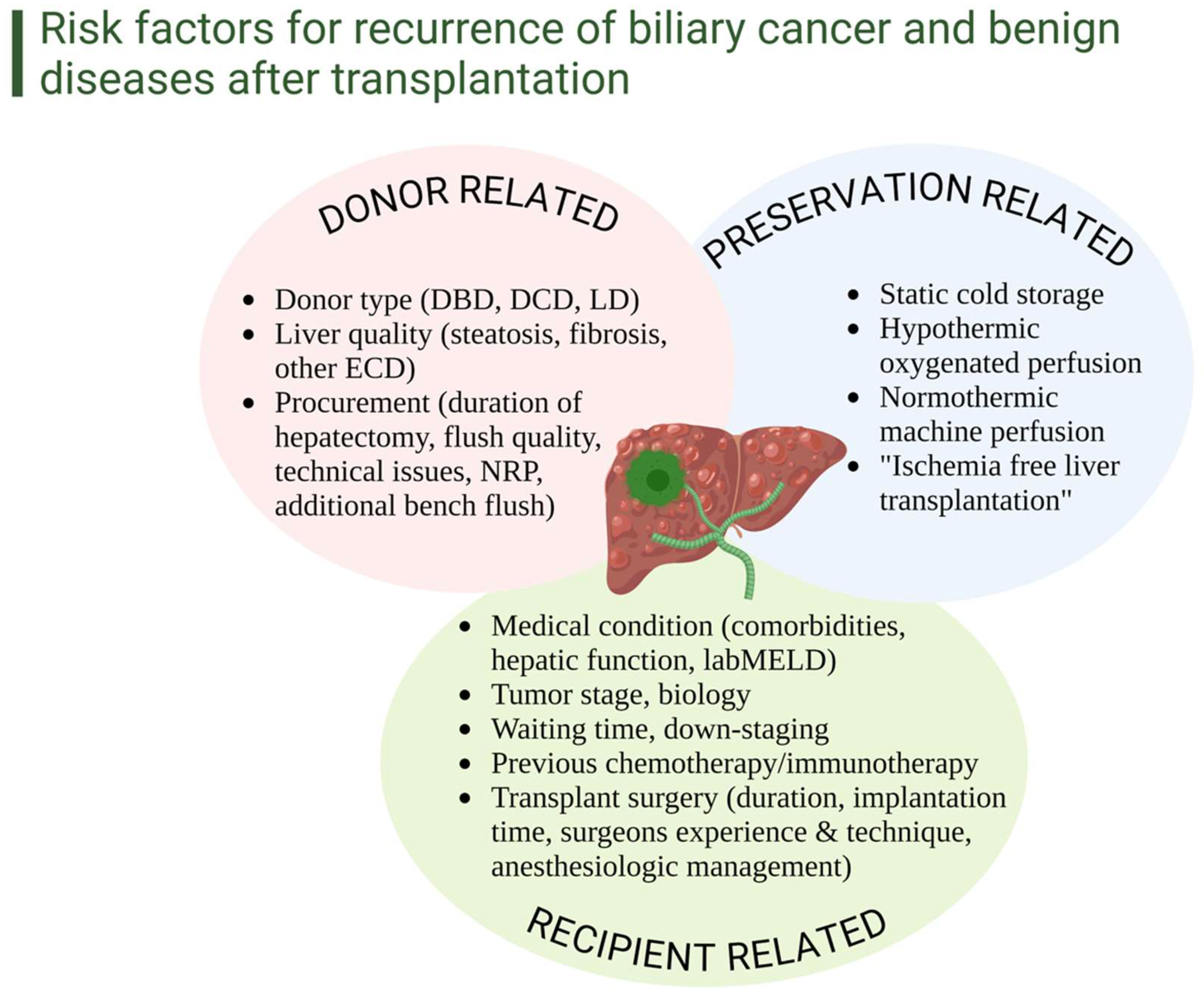
4. Clinical Strategies, Novel Interventions, and Future Perspectives
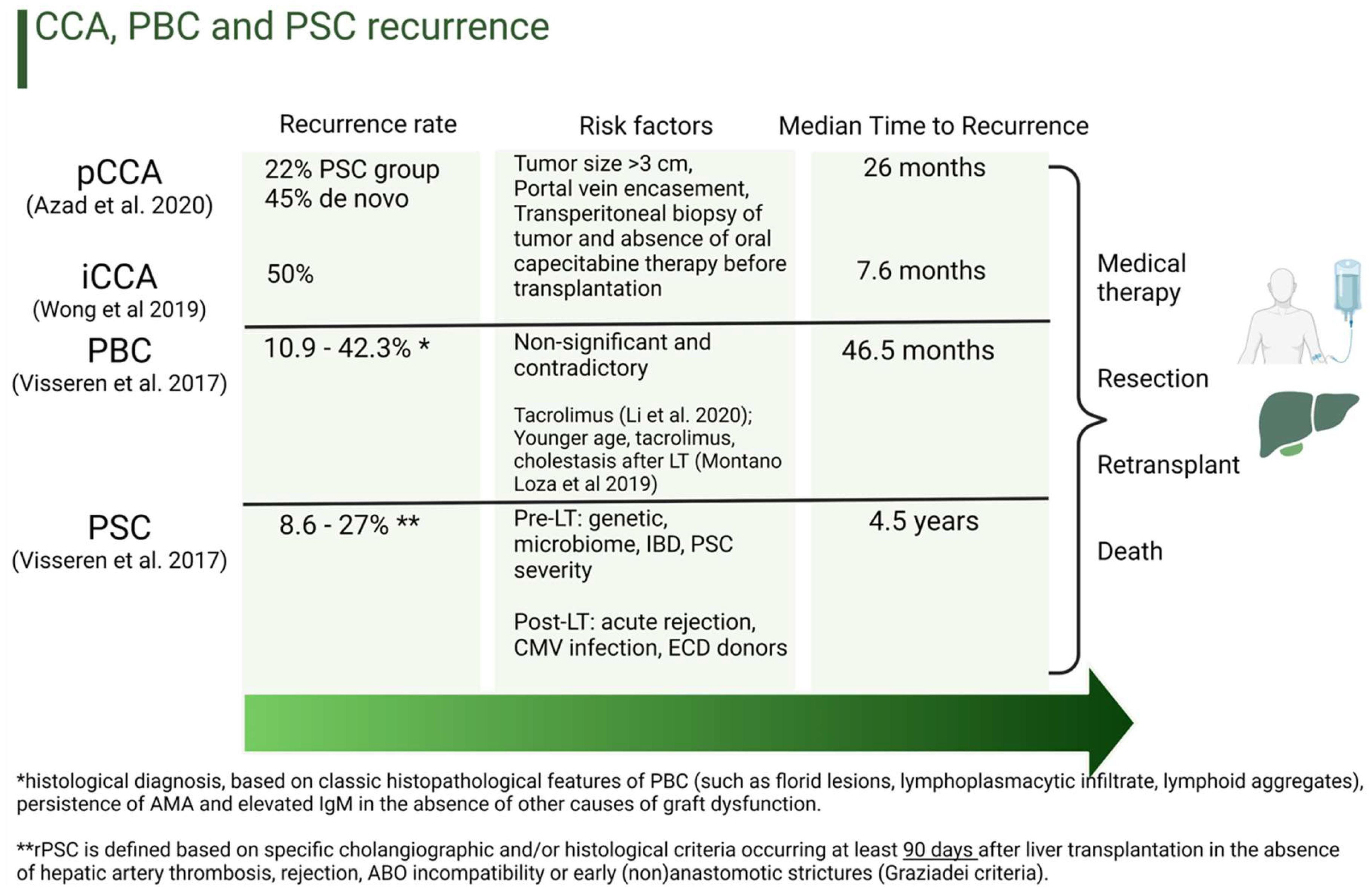
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IRI | Ischemia-Reperfusion Injury |
| CCA | Cholangiocarcinoma |
| PSC | Primary Sclerosing Cholangitis |
| ROS | Reactive Oxygen Species |
| iCCA | Intrahepatic Cholangiocarcinoma |
| pCCA | Peri-hilar Cholangiocarcinoma |
| LT | Liver Transplantation |
| CIT | Cold Ischemia Time |
| dWIT | Donor warm ischemia time |
| rWIT | Recipient Warm Ischemia Time (implantation time) |
| MAP | Mean Arterial Pressure |
| AS | Anastomotic stricture |
| NAS | Non-Anastomotic Stricture |
| ICU | Intensive Care Unit |
| ATP | Adenosine Tri-phosphate |
| TCA | Tricarboxylic Acid |
| DCD | Donation after Cardiac Death |
| DBD | Donation after Brain Death |
| IC | Ischemic Cholangiopathy |
| FMN | Flavin Mononucleotide |
| cTC | Circulating Tumor Cells |
| TME | Tumor Microenvironment |
| SEC | Sinusoidal Epithelial Cells |
| GRWR | Graft-to-recipient weight ratio |
| LDLT | Living donor Liver Transplant |
| CSC | Cancer Stem Cells |
| MASH | Metabolic Associated Steato-hepatitis |
| ACR | Acute Cellular Rejection |
| HOPE | Hypothermic Oxygenated Perfusion |
| PBC | Primary Biliary Cirrhosis |
| AIH | Autoimmune Hepatitis |
| HCC | Hepatocellular Carcinoma |
| CRLM | Colorectal Liver Metastasis |
| NET | Neuroendocrine Tumor |
References
- Goffaux, A.; Delorme, A.; Dahlqvist, G.; Lanthier, N. Improving the prognosis before and after liver transplantation: Is muscle a game changer? World J. Gastroenterol. 2022, 28, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R., Jr.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144–1165. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Hernaez, R.; Liu, Y.; Taylor, T.J.; Rana, A.; Kramer, J.R.; Naik, A.D.; Smith, D.; Taddei, T.; Asch, S.M. Factors Associated with Access to and Receipt of Liver Transplantation in Veterans with End-stage Liver Disease. JAMA Intern. Med. 2021, 181, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.A.; Guidinger, M.K.; Finlayson, S.; Schaubel, D.E.; Goodman, D.C.; Chobanian, M.; Merion, R.M. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA 2008, 299, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Moylan, C.A.; Brady, C.W.; Johnson, J.L.; Smith, A.D.; Tuttle-Newhall, J.E.; Muir, A.J. Disparities in liver transplantation before and after introduction of the MELD score. JAMA 2008, 300, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, N. Selection for Liver Transplantation: Indications and Evaluation. Curr. Hepatol. Rep. 2020, 19, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results. Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer; NCIS: Bethesda, MD, USA, 2023. [Google Scholar]
- Watson, J.; Hydon, K.; Lodge, P. Primary and secondary liver tumours. InnovAiT 2016, 9, 477–482. [Google Scholar] [CrossRef]
- Van der Bilt, J.D.; Kranenburg, O.; Nijkamp, M.W.; Smakman, N.; Veenendaal, L.M.; Te Velde, E.A.; Voest, E.E.; van Diest, P.J.; Borel Rinkes, I.H. Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology 2005, 42, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Ng, K.T.; Lo, C.M.; Ho, J.W.; Sun, B.S.; Sun, C.K.; Lee, T.K.; Poon, R.T.; Fan, S.T. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases--activation of cell invasion and migration pathways. Liver Transpl. 2007, 13, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Van der Bilt, J.D.; Kranenburg, O.; Verheem, A.; van Hillegersberg, R.; Borel Rinkes, I.H. Selective portal clamping to minimize hepatic ischaemia-reperfusion damage and avoid accelerated outgrowth of experimental colorectal liver metastases. Br. J. Surg. 2006, 93, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Panconesi, R.; Flores Carvalho, M.; Mueller, M.; Dutkowski, P.; Muiesan, P.; Schlegel, A. Mitochondrial Reprogramming—What Is the Benefit of Hypothermic Oxygenated Perfusion in Liver Transplantation? Transplantology 2021, 2, 149–161. [Google Scholar] [CrossRef]
- Parente, A.; Cho, H.D.; Kim, K.H.; Schlegel, A. Association between Hepatocellular Carcinoma Recurrence and Graft Size in Living Donor Liver Transplantation: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6224. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Wehrle, C.J.; Ferreira-Gonzalez, S.; Jiao, C.; Hong, H.; Dadgar, N.; Arpi-Palacios, J.; Phong, Y.P.; Kim, J.; Sun, K.; et al. Tumor Organoids for Primary Liver Cancers: A Systematic Review of Current Applications in Diagnostics, Disease Modeling, and Drug Screening. JHEP Rep. 2024; 101164, in press. [Google Scholar] [CrossRef]
- Furukawa, H.; Todo, S.; Imventarza, O.; Casavilla, A.; Wu, Y.M.; Scotti-Foglieni, C.; Broznick, B.; Bryant, J.; Day, R.; Starzl, T.E. Effect of cold ischemia time on the early outcome of human hepatic allografts preserved with UW solution. Transplantation 1991, 51, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.C.; Yersiz, H.; Kositamongkol, P.; Xia, V.W.; Kaldas, F.M.; Petrowsky, H.; Farmer, D.G.; Lipshutz, G.; Markovic, D.; Hiatt, J.R.; et al. Liver transplantation using organ donation after cardiac death: A clinical predictive index for graft failure-free survival. Arch. Surg. 2011, 146, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Panconesi, R.; Carvalho, M.F.; Muiesan, P.; Dutkowski, P.; Schlegel, A. Liver perfusion strategies: What is best and do ischemia times still matter? Curr. Opin. Organ Transplant. 2022, 27, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Kalisvaart, M.; de Haan, J.E.; Polak, W.G.; IJzermans, J.N.M.; Gommers, D.; Metselaar, H.J.; de Jonge, J. Onset of Donor Warm Ischemia Time in Donation after Circulatory Death Liver Transplantation: Hypotension or Hypoxia? Liver Transpl. 2018, 24, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; He, S.; Liu, Y.; Chen, H.; He, S.; Yin, M.; Zou, D.; Chen, S.; Luo, T.; et al. Resolving the graft ischemia-reperfusion injury during liver transplantation at the single cell resolution. Cell Death Dis. 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.L.; Soroka, C.J. Bile formation and secretion: An update. J. Hepatol. 2021, 75, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.Y.; Olson, L.C.; Kisthard, J.A.; Perkins, J.D.; Bakthavatsalam, R.; Halldorson, J.B.; Reyes, J.D.; Larson, A.M.; Levy, A.E. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transplant. 2008, 14, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Porte, R.; Dutkowski, P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J. Hepatol. 2022, 76, 1330–1347. [Google Scholar] [CrossRef] [PubMed]
- Jay, C.L.; Lyuksemburg, V.; Kang, R.; Preczewski, L.; Stroupe, K.; Holl, J.L.; Abecassis, M.M.; Skaro, A.I. The increased costs of donation after cardiac death liver transplantation: Caveat emptor. Ann. Surg. 2010, 251, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Buis, C.I.; Verdonk, R.C.; Van der Jagt, E.J.; van der Hilst, C.S.; Slooff, M.J.; Haagsma, E.B.; Porte, R.J. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007, 13, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Mathur, A.K.; Aqel, B.; Yang, L.; Taner, T.; Heimbach, J.K.; Rosen, C.B.; Paz-Fumagalli, R.; Taner, C.B. Classification of Distinct Patterns of Ischemic Cholangiopathy Following DCD Liver Transplantation: Distinct Clinical Courses and Long-term Outcomes from a Multicenter Cohort. Transplantation 2022, 106, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Manay, P.; Seth, A.; Jackson, K.; Lentine, K.L.; Schnitzler, M.A.; Xiao, H.; Segev, D.L.; Axelrod, D.A. Biliary Complications After Liver Transplantation in the United States: Changing Trends and Economic Implications. Transplantation 2023, 107, e127–e138. [Google Scholar] [CrossRef] [PubMed]
- Kalisvaart, M.; de Haan, J.E.; Polak, W.G.; Metselaar, H.J.; Wijnhoven, B.P.L.; IJzermans, J.N.M.; de Jonge, J. Comparison of Postoperative Outcomes between Donation after Circulatory Death and Donation after Brain Death Liver Transplantation Using the Comprehensive Complication Index. Ann. Surg. 2017, 266, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Taner, C.B.; Bulatao, I.G.; Perry, D.K.; Sibulesky, L.; Willingham, D.L.; Kramer, D.J.; Nguyen, J.H. Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors. Transpl. Int. 2012, 25, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Goussous, N.; Alvarez-Casas, J.; Dawany, N.; Xie, W.; Malik, S.; Gray, S.H.; Barth, R.N.; LaMattina, J.C. Ischemic Cholangiopathy Postdonation after Circulatory Death Liver Transplantation: Donor Hepatectomy Time Matters. Transplant. Direct 2022, 8, e1277. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Hunt, F.; Messer, S.; Currie, I.; Large, S.; Sutherland, A.; Crick, K.; Wigmore, S.J.; Fear, C.; Cornateanu, S.; et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transplant. 2019, 19, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.-A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2018, 68, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.I.; Rosen, C.B.; Taner, T.; Heimbach, J.K.; Gores, G.J. Selected Patients with Unresectable Perihilar Cholangiocarcinoma (pCCA) Derive Long-Term Benefit from Liver Transplantation. Cancers 2020, 12, 3157. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Kim, J.; George, B.; Eriksen, C.; Pearson, T.; Robbins, J.; Zimmerman, M.A.; Hong, J.C. Downstaging Locally Advanced Cholangiocarcinoma Pre-Liver Transplantation: A Prospective Pilot Study. J. Surg. Res. 2019, 242, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Visseren, T.; Darwish Murad, S. Recurrence of primary sclerosing cholangitis, primary biliary cholangitis and auto-immune hepatitis after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Babboni, S.; Vacca, P.G.; Simonini, L.; Pezzati, D.; Martinelli, C.; Frongillo, F.; Bianco, G.; Marciano, E.; Basta, G.; Ghinolfi, D.; et al. Cholangiocyte Organoids: The New Frontier in Regenerative Medicine for the Study and Treatment of Cholangiopathies. J. Clin. Med. 2024, 13, 1804. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. eBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Noack, K.; Bronk, S.F.; Kato, A.; Gores, G.J. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation 1993, 56, 495–500. [Google Scholar] [CrossRef] [PubMed]
- De Jong, I.E.M.; Overi, D.; Carpino, G.; Gouw, A.S.H.; van den Heuvel, M.C.; van Kempen, L.C.; Mancone, C.; Onori, P.; Cardinale, V.; Casadei, L.; et al. Persistent biliary hypoxia and lack of regeneration are key mechanisms in the pathogenesis of posttransplant nonanastomotic strictures. Hepatology 2022, 75, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Methner, C.; Nadtochiy, S.M.; Logan, A.; Pell, V.R.; Ding, S.; James, A.M.; Cochemé, H.M.; Reinhold, J.; Lilley, K.S.; et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013, 19, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Eden, J.; Breuer, E.; Birrer, D.; Müller, M.; Pfister, M.; Mayr, H.; Sun, K.; Widmer, J.; Huwyler, F.; Ungethüm, U.; et al. Screening for mitochondrial function before use-routine liver assessment during hypothermic oxygenated perfusion impacts liver utilization. eBioMedicine 2023, 98, 104857. [Google Scholar] [CrossRef] [PubMed]
- Kahl, A.; Stepanova, A.; Konrad, C.; Anderson, C.; Manfredi, G.; Zhou, P.; Iadecola, C.; Galkin, A. Critical Role of Flavin and Glutathione in Complex I-Mediated Bioenergetic Failure in Brain Ischemia/Reperfusion Injury. Stroke 2018, 49, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, W.; Wang, Z.; Chen, T. Production of riboflavin and related cofactors by biotechnological processes. Microb. Cell Factories 2020, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.B.; Russell, M. Hydrolysis of flavin mononucleotide by acid phosphatases from animal tissues. Comp. Biochem. Physiol. 1962, 5, 113–121. [Google Scholar] [CrossRef]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function during Hypothermic Oxygenated Machine Perfusion before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Pan, J.; Shen, Q.; Li, M.; Peng, Y. Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J. Neuroinflamm. 2018, 15, 242. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Girardin, S.E. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011, 21, 558–560. [Google Scholar] [CrossRef]
- Parente, A.; Flores Carvalho, M.; Eden, J.; Dutkowski, P.; Schlegel, A. Mitochondria and Cancer Recurrence after Liver Transplantation-What Is the Benefit of Machine Perfusion? Int. J. Mol. Sci. 2022, 23, 9747. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sharma, L.K.; Bai, Y. Implications of mitochondrial DNA mutations and mitochondrial dysfunction in tumorigenesis. Cell Res. 2009, 19, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Scheid, A.D.; Beadnell, T.C.; Welch, D.R. Roles of mitochondria in the hallmarks of metastasis. Br. J. Cancer 2021, 124, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Payen, V.L.; Pérez-Escuredo, J.; De Saedeleer, C.J.; Danhier, P.; Copetti, T.; Dhup, S.; Tardy, M.; Vazeille, T.; Bouzin, C.; et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014, 8, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.; Dondossola, D.; Dutkowski, P.; Schlegel, A. Current evidence on the beneficial HOPE-effect based on systematic reviews and meta-analyses in liver transplantation. J. Hepatol. 2023, 80, e116–e119. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Hwang, S.; Lee, K.W.; Kim, J.M.; You, Y.K.; Choi, D.; Ryu, J.H.; Kim, B.W.; Kim, D.S.; Cho, J.Y.; et al. Small graft size and hepatocellular carcinoma outcomes in living donor liver transplantation: A retrospective multicentric cohort study. Int. J. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Kim, S.H.; Shim, J.R.; Park, S.J. Small-for-size grafts increase recurrence of hepatocellular carcinoma in liver transplantation beyond milan criteria. Liver Transpl. 2018, 24, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K. Liver regeneration. J. Cell Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Vernon, H.; Wehrle, C.; Kasi, A. Anatomy, Abdomen and Pelvis: Liver; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Maspero, M.; Yilmaz, S.; Cazzaniga, B.; Raj, R.; Ali, K.; Mazzaferro, V.; Schlegel, A. The role of ischemia-reperfusion injury and liver regeneration in hepatic tumor recurrence. JHEP Rep. 2023, 5, 100846. [Google Scholar] [CrossRef] [PubMed]
- Cressman, D.E.; Diamond, R.H.; Taub, R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology 1995, 21, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Kono, S.; Nagaike, M.; Matsumoto, K.; Nakamura, T. Marked induction of hepatocyte growth factor mRNA in intact kidney and spleen in response to injury of distant organs. Biochem. Biophys. Res. Commun. 1992, 186, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Goralski, M.; Prokofiev, D.; Dammann, P.; Grünewald, P.; Trippler, M.; Biglarnia, A.; Kamler, M.; Niehues, E.M.; Frilling, A.; et al. VEGF is important for early liver regeneration after partial hepatectomy. J. Surg. Res. 2007, 138, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Konsavage, W.M., Jr.; Yochum, G.S. Intersection of Hippo/YAP and Wnt/β-catenin signaling pathways. Acta Biochim. Biophys. Sin. 2013, 45, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wolfe, A.; Septer, S.; Edwards, G.; Zhong, X.; Abdulkarim, A.B.; Ranganathan, S.; Apte, U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. 2012, 32, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, H.; Sasaki, K.; Fujiki, M.; Uso, T.D.; Aucejo, F.; Kwon, C.H.D.; Eghtesad, B.; Miller, C.; Quintini, C.; Hashimoto, K. Too Much, Too Little, or Just Right? The Importance of Allograft Portal Flow in Deceased Donor Liver Transplantation. Transplantation 2020, 104, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.; Flores Carvalho, M.; Schlegel, A. Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 10091. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rammohan, A.; Gunasekaran, V.; Hong, S.; Chih-Yi Chen, I.; Kim, J.; Hervera Marquez, K.A.; Hsu, S.-C.; Kirimker, E.O.; Akamatsu, N.; et al. Biliary complications after adult-to-adult living-donor liver transplantation: An international multicenter study of 3633 cases. Am. J. Transplant. 2024, 24, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, Y.; Ren, H.; Wang, J.; Shang, L.; Liu, Y.; Zhu, W.; Shi, X. Ischemia reperfusion injury promotes recurrence of hepatocellular carcinoma in fatty liver via ALOX12-12HETE-GPR31 signaling axis. J. Exp. Clin. Cancer Res. 2019, 38, 489. [Google Scholar] [CrossRef] [PubMed]
- Grąt, M.; Krawczyk, M.; Wronka, K.M.; Stypułkowski, J.; Lewandowski, Z.; Wasilewicz, M.; Krawczyk, P.; Grąt, K.; Patkowski, W.; Zieniewicz, K. Ischemia-reperfusion injury and the risk of hepatocellular carcinoma recurrence after deceased donor liver transplantation. Sci. Rep. 2018, 8, 8935. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lu, X.; Liu, Z.; Chen, L.; Xu, Y.; Wang, Y.; Wei, G.; Chen, Y. FBXW7 suppresses epithelial-mesenchymal transition, stemness and metastatic potential of cholangiocarcinoma cells. Oncotarget 2015, 6, 6310–6325. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X. An overview of extrahepatic cholangiocarcinoma: From here to where? Front. Oncol. 2023, 13, 1171098. [Google Scholar] [CrossRef] [PubMed]
- Seehawer, M.; Heinzmann, F.; D’Artista, L.; Harbig, J.; Roux, P.F.; Hoenicke, L.; Dang, H.; Klotz, S.; Robinson, L.; Doré, G.; et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 2018, 562, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sarcognato, S.; Jong, I.E.M.; Fabris, L.; Cadamuro, M.; Guido, M. Necroptosis in Cholangiocarcinoma. Cells 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.H. Tumor Microenvironment: Necroptosis Switches the Subtype of Liver Cancer While Necrosis Promotes Tumor Recurrence and Progression. Exp. Clin. Transplant. 2023, 21, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Thongchot, S.; Yongvanit, P.; Loilome, W.; Seubwai, W.; Phunicom, K.; Tassaneeyakul, W.; Pairojkul, C.; Promkotra, W.; Techasen, A.; Namwat, N. High expression of HIF-1α, BNIP3 and PI3KC3: Hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac. J. Cancer Prev. 2014, 15, 5873–5878. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sinha, S.; Shrivastava, N. Therapeutic Targeting Hypoxia-Inducible Factor (HIF-1) in Cancer: Cutting Gordian Knot of Cancer Cell Metabolism. Front. Genet. 2022, 13, 849040. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.Y.; Ho, A.F.W.; Bulluck, H.; Gao, F.; Chong, J.; Koh, Y.X.; Tan, E.K.; Abdul Latiff, J.B.; Chua, S.H.; Goh, B.K.P.; et al. Effect of remote ischemic preConditioning on liver injury in patients undergoing liver resection: The ERIC-LIVER trial. HPB 2020, 22, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Colgan, S.P.; Eltzschig, H.K. Hypoxia-inducible factors as molecular targets for liver diseases. J. Mol. Med. 2016, 94, 613–627. [Google Scholar] [CrossRef]
- Taneja, S.; Roy, A. Nonalcoholic steatohepatitis recurrence after liver transplant. Transl. Gastroenterol. Hepatol. 2020, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Ali, A.H.; Lindor, K.D. Primary Sclerosing Cholangitis, Part 1: Epidemiology, Etiopathogenesis, Clinical Features, and Treatment. Gastroenterol. Hepatol. 2018, 14, 293–304. [Google Scholar]
- Trivella, J.; John, B.V.; Levy, C. Primary biliary cholangitis: Epidemiology, prognosis, and treatment. Hepatol. Commun. 2023, 7, e0179. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Chen, S.; Li, M.; Zhang, D.; Kong, Y.; Jia, J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Vinaixa, C.; Rubín, A.; Aguilera, V.; Berenguer, M. Recurrence of hepatitis C after liver transplantation. Ann. Gastroenterol. 2013, 26, 304–313. [Google Scholar] [PubMed]
- Carbone, M.; Lenci, I.; Baiocchi, L. Prevention of hepatitis C recurrence after liver transplantation: An update. World J. Gastrointest. Pharmacol. Ther. 2012, 3, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Retortillo, M.; Forns, X. Prevention and treatment of hepatitis C virus recurrence after liver transplantation. J. Hepatol. 2004, 41, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Olaru, I.D.; Beliz Meier, M.; Mirzayev, F.; Prodanovic, N.; Kitchen, P.J.; Schumacher, S.G.; Denkinger, C.M. Global prevalence of hepatitis B or hepatitis C infection among patients with tuberculosis disease: Systematic review and meta-analysis. eClinicalMedicine 2023, 58, 101938. [Google Scholar] [CrossRef] [PubMed]
- Visseren, T.; Erler, N.S.; Heimbach, J.K.; Eaton, J.E.; Selzner, N.; Gulamhusein, A.; van der Heide, F.; Porte, R.J.; van Hoek, B.; Alwayn, I.P.J.; et al. Inflammatory conditions play a role in recurrence of PSC after liver transplantation: An international multicentre study. JHEP Rep. 2022, 4, 100599. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Della Penna, A.; Mazzarelli, C.; De Martin, E.; Villard, C.; Bergquist, A.; Line, P.D.; Neuberger, J.M.; Al-Shakhshir, S.; Trivedi, P.J.; et al. Liver Transplantation for Primary Sclerosing Cholangitis (PSC) with or without Inflammatory Bowel Disease (IBD)—A European Society of Organ Transplantation (ESOT) Consensus Statement. Transpl. Int. 2023, 36, 11729. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Reece, J.; Laing, R.W.; Slaney, E.; Cooney, R.; Gunson, B.K.; Kamarajah, S.K.; Pinkney, T.; Thompson, F.; Muiesan, P.; et al. The impact of ileal pouch-anal anastomosis on graft survival following liver transplantation for primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2018, 48, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Maspero, M.; Holubar, S.D.; Raj, R.; Yilmaz, S.; Prien, C.; Lavryk, O.; Pita, A.; Hashimoto, K.; Steele, S.R.; Hull, T.L. Ileal Pouch-anal Anastomosis in Primary Sclerosing Cholangitis-inflammatory Bowel Disease (PSC-IBD): Long-term Pouch and Liver Transplant Outcomes. Ann. Surg. 2023, 278, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Gringeri, E.; Cazzagon, N.; Floreani, A.; Cillo, U.; Burra, P.; Gambato, M. Risk Factors for Recurrence of Primary Sclerosing Cholangitis after Liver Transplantation: Single-Center Data. J. Pers. Med. 2024, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Steenstraten, I.C.; Sebib Korkmaz, K.; Trivedi, P.J.; Inderson, A.; van Hoek, B.; Rodriguez Girondo, M.D.M.; Maljaars, P.W.J. Systematic review with meta-analysis: Risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment. Pharmacol. Ther. 2019, 49, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Alabraba, E.; Nightingale, P.; Gunson, B.; Hubscher, S.; Olliff, S.; Mirza, D.; Neuberger, J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009, 15, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Gordon, F.D.; Goldberg, D.S.; Goodrich, N.P.; Lok, A.S.; Verna, E.C.; Selzner, N.; Stravitz, R.T.; Merion, R.M. Recurrent primary sclerosing cholangitis in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study: Comparison of risk factors between living and deceased donor recipients. Liver Transpl. 2016, 22, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elmagd, K.; Malinchoc, M.; Dickson, E.R.; Fung, J.J.; Murtaugh, P.A.; Langworthy, A.L.; Demetris, A.J.; Krom, R.A.; Van Thiel, D.H.; Starzl, T.E. Efficacy of hepatic transplantation in patients with primary sclerosing cholangitis. Surg. Gynecol. Obstet. 1993, 177, 335–344. [Google Scholar] [PubMed]
- De Carlis, R.; Schlegel, A.; Frassoni, S.; Olivieri, T.; Ravaioli, M.; Camagni, S.; Patrono, D.; Bassi, D.; Pagano, D.; Di Sandro, S.; et al. How to Preserve Liver Grafts from Circulatory Death with Long Warm Ischemia? A Retrospective Italian Cohort Study with Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation 2021, 105, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Muller, X.; Schlegel, A.; Würdinger, M.; Wendt, M.; Kron, P.; Eshmuminov, D.; Müllhaupt, B.; Clavien, P.A.; Dutkowski, P. Can hypothermic oxygenated perfusion (HOPE) rescue futile DCD liver grafts? HPB 2019, 21, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Zhang, L.; Jia, J.; Zhong, K.; Nie, Y. The conclusion of reducing acute rejection after liver transplantation by machine perfusion should be extrapolated with caution. Hepatobiliary Surg. Nutr. 2023, 12, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Ronca, V.; Wootton, G.; Milani, C.; Cain, O. The Immunological Basis of Liver Allograft Rejection. Front. Immunol. 2020, 11, 2155. [Google Scholar] [CrossRef]
- Silveira, M.G.; Talwalkar, J.A.; Lindor, K.D.; Wiesner, R.H. Recurrent Primary Biliary Cirrhosis After Liver Transplantation. Am. J. Transplant. 2010, 10, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Hansen, B.E.; Corpechot, C.; Roccarina, D.; Thorburn, D.; Trivedi, P.; Hirschfield, G.; McDowell, P.; Poupon, R.; Dumortier, J.; et al. Factors Associated with Recurrence of Primary Biliary Cholangitis after Liver Transplantation and Effects on Graft and Patient Survival. Gastroenterology 2019, 156, 96–107.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, S.S. Post-COVID-19 Cholangiopathy: Clinical and Radiologic Findings. Korean J. Radiol. 2023, 24, 1167–1171. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Ronca, V.; Ebadi, M.; Hansen, B.E.; Hirschfield, G.; Elwir, S.; Alsaed, M.; Milkiewicz, P.; Janik, M.K.; Marschall, H.-U.; et al. Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J. Hepatol. 2022, 77, 84–97. [Google Scholar] [CrossRef]
- Rosen, C.B.; Heimbach, J.K.; Gores, G.J. Surgery for cholangiocarcinoma: The role of liver transplantation. HPB 2008, 10, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Hoogwater, F.J.H.; Kuipers, H.; de Meijer, V.E.; Maulat, C.; Muscari, F.; Polak, W.G.; van Hoek, B.; Jézéquel, C.; Alwayn, I.P.J.; Ijzermans, J.N.M.; et al. Role of neoadjuvant chemoradiotherapy in liver transplantation for unresectable perihilar cholangiocarcinoma: Multicentre, retrospective cohort study. BJS Open 2023, 7, zrad025. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Javle, M.; Heyne, K.; Shroff, R.T.; Abdel-Wahab, R.; Gupta, N.; Mobley, C.M.; Saharia, A.; Victor, D.W.; Nguyen, D.T.; et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: A prospective case-series. Lancet Gastroenterol. Hepatol. 2018, 3, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xi, P.; Yao, Z.; Zhao, C.; Li, X.; Chen, Z.; Lin, X. The Clinical Association between the Inflammation-Nutritional Condition and Prognosis of Locally Advanced Intrahepatic Cholangiocarcinoma after R0 Resection: Evidence from Competing Risk and Propensity Matching Analysis. J. Inflamm. Res. 2024, 17, 2787–2799. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.; Tirotta, F.; Pini, A.; Eden, J.; Dondossola, D.; Manzia, T.M.; Dutkowski, P.; Schlegel, A. Machine perfusion techniques for liver transplantation—A meta-analysis of the first seven randomized-controlled trials. J. Hepatol. 2023, 79, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.; Isaac, J.R.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Panconesi, R.; Widmer, J.; Carvalho, M.F.; Eden, J.; Dondossola, D.; Dutkowski, P.; Schlegel, A. Mitochondria and ischemia reperfusion injury. Curr. Opin. Organ Transplant. 2022, 27, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Mueller, M.; Muller, X.; Eden, J.; Panconesi, R.; von Felten, S.; Steigmiller, K.; Sousa Da Silva, R.X.; de Rougemont, O.; Mabrut, J.-Y.; et al. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J. Hepatol. 2023, 78, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Vasuri, F.; Riefolo, M.; Ravaioli, M.; Cescon, M.; Pasquinelli, G.; Germinario, G.; D’Errico, A. Predictive value of portal fibrosis and inflammation in transplanted liver grafts treated with hypothermic oxygenated perfusion. Pathol. Res. Pract. 2023, 243, 154361. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Cussa, D.; Sciannameo, V.; Montanari, E.; Panconesi, R.; Berchialla, P.; Lepore, M.; Gambella, A.; Rizza, G.; Catalano, G.; et al. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am. J. Transplant. 2022, 22, 1382–1395. [Google Scholar] [CrossRef]
- Thorne, A.M.; Wolters, J.C.; Lascaris, B.; Bodewes, S.B.; Lantinga, V.A.; van Leeuwen, O.B.; de Jong, I.E.M.; Ustyantsev, K.; Berezikov, E.; Lisman, T.; et al. Bile proteome reveals biliary regeneration during normothermic preservation of human donor livers. Nat. Commun. 2023, 14, 7880. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Colli, F.; Colangelo, M.; De Stefano, N.; Apostu, A.L.; Mazza, E.; Catalano, S.; Rizza, G.; Mirabella, S.; Romagnoli, R. How Can Machine Perfusion Change the Paradigm of Liver Transplantation for Patients with Perihilar Cholangiocarcinoma? J. Clin. Med. 2023, 12, 2026. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, I.W.; Wiesner, R.H.; Batts, K.P.; Marotta, P.J.; LaRusso, N.F.; Porayko, M.K.; Hay, J.E.; Gores, G.J.; Charlton, M.R.; Ludwig, J.; et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology 1999, 29, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, J.; Ouyang, R.; Yang, Y.; Yu, C.; Lin, H. Risk factors for recurrent primary biliary cirrhosis after liver transplantation: A systematic review and meta-analysis. Dig. Liver Dis. 2021, 53, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; McMillan, R.; He, A.R.; Starr, J.S.; Dhani, H.; Aushev, V.N.; Koyen Malashevich, A.; Rattigan, N.H.; et al. Feasibility of disease recurrence monitoring in liver post-transplantation for patients with hepatocellular carcinoma via personalized and tumor-informed ctDNA test. J. Clin. Oncol. 2022, 40 (Suppl. 16), e16123. [Google Scholar] [CrossRef]
- Chen, H.; Lu, D.; Yang, X.; Hu, Z.; He, C.; Li, H.; Lin, Z.; Yang, M.; Xu, X. One Shoot, Two Birds: Alleviating Inflammation Caused by Ischemia/Reperfusion Injury to Reduce the Recurrence of Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 879552. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Guo, S.; Jia, Z.; Gao, Z.; You, K.; Gong, J.; Li, S. N-acetylcysteine in the Donor, Recipient, or Both Donor and Recipient in Liver Transplantation: A Systematic Review with Meta-analysis and Trial Sequential Analysis. Transplantation 2023, 107, 1976–1990. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.K.; Jones, D.E.J. UDCA prophylaxis for post-transplant PBC recurrence prevention: Time to change practice. J. Hepatol. 2020, 73, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Willing, A.R.; Kairouz, A.; Cunningham, E.B.; Wheeler, A.; O’Brien, N.; Perera, V.; Ward, J.W.; Hiebert, L.; Degenhardt, L.; et al. Direct-acting antiviral therapies for hepatitis C infection: Global registration, reimbursement, and restrictions. Lancet Gastroenterol. Hepatol. 2024, 9, 366–382. [Google Scholar] [CrossRef] [PubMed]
- Davey, S.; Costello, K.; Russo, M.; Davies, S.; Lalani, H.S.; Kesselheim, A.S.; Rome, B.N. Changes in Use of Hepatitis C Direct-Acting Antivirals After Access Restrictions Were Eased by State Medicaid Programs. JAMA Health Forum. 2024, 5, e240302. [Google Scholar] [CrossRef] [PubMed]
- Grigg, S.E.; Sarri, G.L.; Gow, P.J.; Yeomans, N.D. Systematic review with meta-analysis: Sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2019, 49, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Kugelmas, M.; Spiegelman, P.; Osgood, M.J.; Young, D.A.; Trotter, J.F.; Steinberg, T.; Wachs, M.E.; Bak, T.; Kam, I.; Everson, G.T. Different immunosuppressive regimens and recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl. 2003, 9, 727–732. [Google Scholar] [CrossRef]
- Fares, S.; Wehrle, C.J.; Hong, H.; Sun, K.; Jiao, C.; Zhang, M.; Gross, A.; Allkushi, E.; Uysal, M.; Kamath, S.; et al. Emerging and Clinically Accepted Biomarkers for Hepatocellular Carcinoma. Cancers 2024, 16, 1453. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Hong, H.; Kamath, S.; Schlegel, A.; Fujiki, M.; Hashimoto, K.; Kwon, D.C.H.; Miller, C.; Walsh, R.M.; Aucejo, F. Tumor Mutational Burden from Circulating Tumor DNA Predicts Recurrence of Hepatocellular Carcinoma after Resection: An Emerging Biomarker for Surveillance: An Emerging Biomarker for Surveillance. Ann. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Raj, R.; Aykun, N.; Orabi, D.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Fujiki, M.; Hashimoto, K.; Quintini, C.; et al. Liquid Biopsy by ctDNA in Liver Transplantation for Colorectal Cancer Liver Metastasis. J. Gastrointest. Surg. 2023, 27, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Raj, R.; Aykun, N.; Orabi, D.; Stackhouse, K.; Chang, J.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Walsh, R.M.; et al. Circulating Tumor DNA in Colorectal Cancer Liver Metastasis: Analysis of Patients Receiving Liver Resection and Transplant. JCO Clin. Cancer Inform. 2023, 7, e2300111. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Wehrle, C.J.; Zhang, M.; Fares, S.; Stitzel, H.; Garib, D.; Estfan, B.; Kamath, S.; Krishnamurthi, S.; Ma, W.W.; et al. Circulating Tumor DNA Profiling in Liver Transplant for Hepatocellular Carcinoma, Cholangiocarcinoma, and Colorectal Liver Metastases: A Programmatic Proof of Concept. Cancers 2024, 16, 927. [Google Scholar] [CrossRef]
- Visseren, T.; Fuhler, G.M.; Erler, N.S.; Nossent, Y.R.A.; Metselaar, H.J.; IJzermans, J.N.M.; Darwish Murad, S.; Peppelenbosch, M.P. Recurrence of primary sclerosing cholangitis after liver transplantation is associated with specific changes in the gut microbiome pretransplant—A pilot study. Transpl. Int. 2020, 33, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Assis, D.N.; Bowlus, C.L. Recent Advances in the Management of Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2023, 21, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, J.; Kandpal, M.; Guo, K.; Kleiboeker, S.; Sinha, R.; Abecassis, M. Donor-derived cell-free DNA levels predict graft injury in liver transplant recipients. Am. J. Transplant. 2022, 22, 532–540. [Google Scholar] [CrossRef] [PubMed]

| Authors and References | Disease | Country; Region; Center | Number of Patients | Donor Type | Recurrence Rate | Donor or Preservation-Related Risk Factors |
|---|---|---|---|---|---|---|
| Catanzaro et al. 2024 [96] | PSC | Padua, Italy | 33 | DBD | 27% | CIT Female donor |
| Steenstraten et al. 2019 [97] | PSC | Japan, USA, UK, Hungary, Germany, Canada, Nordic countries | 2159 | DBD, LD | 17.7% | Donor age |
| Alabraba et al. 2009 [98] | PSC | Birmingham, UK | 230 | DBD | 23% | ECD grafts |
| Gordon et al. 2016 [99] | PSC | North America (US and Canada) | 307 | DBD, DCD, LD | 11% | Donor age |
| Abu-Elmagd et al. 1997 [100] | PBC | - | 421 | DBD | 11% | CIT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehrle, C.J.; Panconesi, R.; Satish, S.; Maspero, M.; Jiao, C.; Sun, K.; Karakaya, O.; Allkushi, E.; Modaresi Esfeh, J.; Whitsett Linganna, M.; et al. The Impact of Biliary Injury on the Recurrence of Biliary Cancer and Benign Disease after Liver Transplantation: Risk Factors and Mechanisms. Cancers 2024, 16, 2789. https://doi.org/10.3390/cancers16162789
Wehrle CJ, Panconesi R, Satish S, Maspero M, Jiao C, Sun K, Karakaya O, Allkushi E, Modaresi Esfeh J, Whitsett Linganna M, et al. The Impact of Biliary Injury on the Recurrence of Biliary Cancer and Benign Disease after Liver Transplantation: Risk Factors and Mechanisms. Cancers. 2024; 16(16):2789. https://doi.org/10.3390/cancers16162789
Chicago/Turabian StyleWehrle, Chase J., Rebecca Panconesi, Sangeeta Satish, Marianna Maspero, Chunbao Jiao, Keyue Sun, Omer Karakaya, Erlind Allkushi, Jamak Modaresi Esfeh, Maureen Whitsett Linganna, and et al. 2024. "The Impact of Biliary Injury on the Recurrence of Biliary Cancer and Benign Disease after Liver Transplantation: Risk Factors and Mechanisms" Cancers 16, no. 16: 2789. https://doi.org/10.3390/cancers16162789
APA StyleWehrle, C. J., Panconesi, R., Satish, S., Maspero, M., Jiao, C., Sun, K., Karakaya, O., Allkushi, E., Modaresi Esfeh, J., Whitsett Linganna, M., Ma, W. W., Fujiki, M., Hashimoto, K., Miller, C., Kwon, D. C. H., Aucejo, F., & Schlegel, A. (2024). The Impact of Biliary Injury on the Recurrence of Biliary Cancer and Benign Disease after Liver Transplantation: Risk Factors and Mechanisms. Cancers, 16(16), 2789. https://doi.org/10.3390/cancers16162789






