Retrospective Cohort Study on the Impact of Travel Distance on Late-Stage Oral Cancer Treatment and Outcomes: An NCDB Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Cohort Characteristics and Distance Traveled
3.2. Cancer Subsite and Treatment Type
3.3. Pathology
3.4. Distance Traveled for the Treatment and Treatment Facility
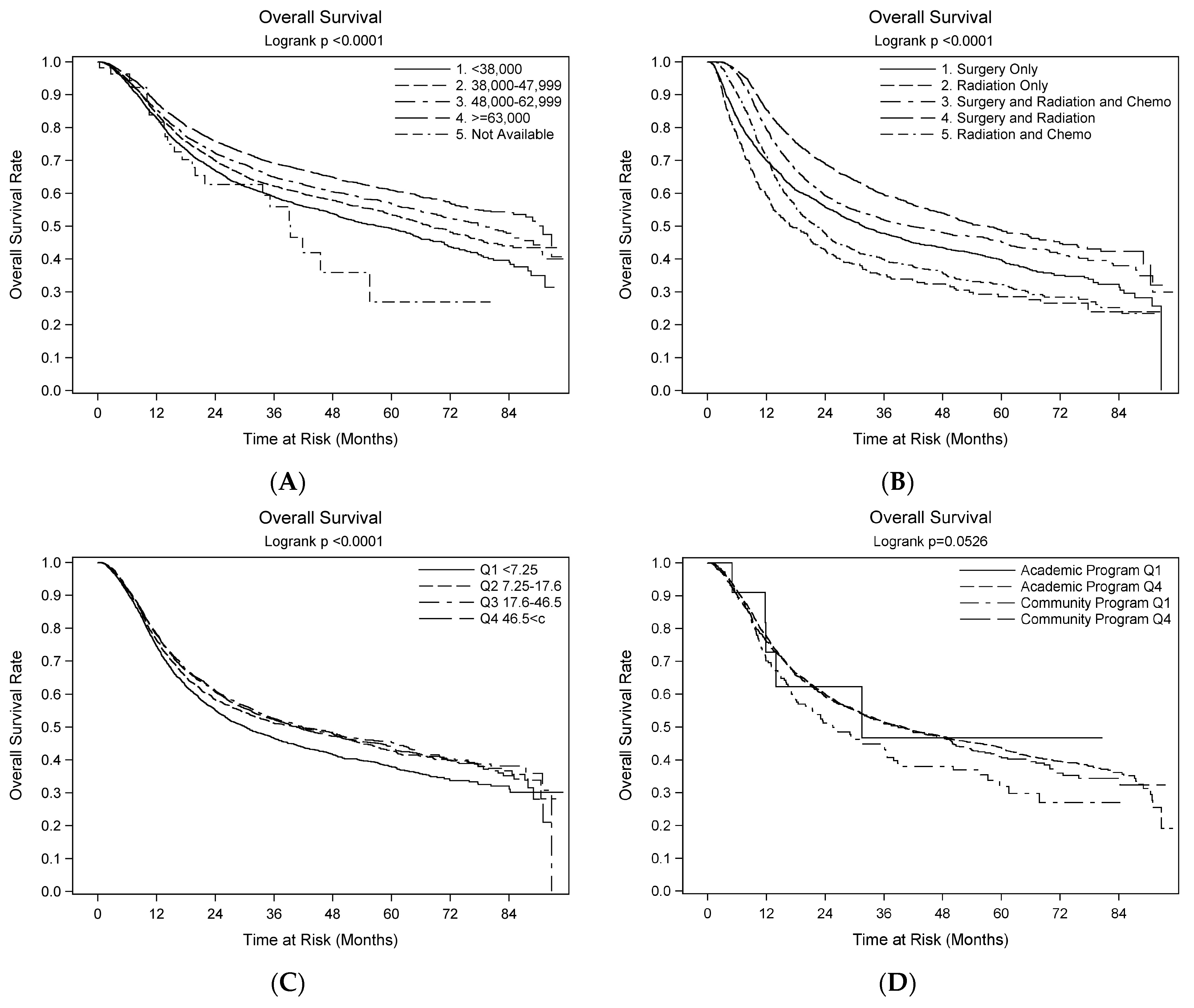
3.5. Survival Outcomes—Univariate
3.6. Survival Outcomes—Multivariate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Osazuwa-Peters, N.; Adjei Boakye, E.; Hussaini, A.S.; Sujijantarat, N.; Ganesh, R.N.; Snider, M.; Thompson, D.; Varvares, M.A. Characteristics and predictors of oral cancer knowledge in a predominantly African American community. PLoS ONE 2017, 12, e0177787. [Google Scholar] [CrossRef] [PubMed]
- Curtis, D.C.; Eckhart, S.C.; Morrow, A.C.; Sikes, L.C.; Mridha, T. Demographic and Behavioral Risk Factors for Oral Cancer among Florida Residents. J. Int. Soc. Prev. Community Dent. 2020, 10, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.T.; Treister, N.S.; Sollecito, T.P.; Schmidt, B.L.; Patton, L.L.; Yang, Y.; Lin, A.; Elting, L.S.; Hodges, J.S.; Lalla, R.V. Epidemiologic factors in patients with advanced head and neck cancer treated with radiation therapy. Head Neck 2021, 43, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Murphy, B.A. Lower levels of education and household income mediate lower dental care utilization among survivors of early life cancers. Prev. Med. Rep. 2019, 14, 100868. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Agrawal, R.R.; Jones, E.A.; Devaiah, A.K. Social Determinants of Health and Oral Cavity Cancer Treatment and Survival: A Competing Risk Analysis. Laryngoscope 2020, 130, 2160–2165. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Peres, M.A.; Watt, R.G. The Relationship between Income and Oral Health: A Critical Review. J. Dent. Res. 2019, 98, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Ambroggi, M.; Biasini, C.; Del Giovane, C.; Fornari, F.; Cavanna, L. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncologist 2015, 20, 1378–1385. [Google Scholar] [CrossRef]
- Lamont, E.B.; Hayreh, D.; Pickett, K.E.; Dignam, J.J.; List, M.A.; Stenson, K.M.; Haraf, D.J.; Brockstein, B.E.; Sellergren, S.A.; Vokes, E.E. Is patient travel distance associated with survival on phase II clinical trials in oncology? J. Natl. Cancer Inst. 2003, 95, 1370–1375. [Google Scholar] [CrossRef]
- Xia, L.; Taylor, B.L.; Mamtani, R.; Christodouleas, J.P.; Guzzo, T.J. Associations Between Travel Distance, Hospital Volume, and Outcomes Following Radical Cystectomy in Patients With Muscle-invasive Bladder Cancer. Urology 2018, 114, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Graboyes, E.M.; Ellis, M.A.; Li, H.; Kaczmar, J.M.; Sharma, A.K.; Lentsch, E.J.; Day, T.A.; Hughes Halbert, C. Racial and Ethnic Disparities in Travel for Head and Neck Cancer Treatment and the Impact of Travel Distance on Survival. Cancer 2018, 124, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, G.; Comini, L.V.; Piazza, C. Surgical margins in oral squamous cell cancer: Intraoperative evaluation and prognostic impact. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Fathy, R.; Shah, R.R.; Rajasekaran, K.; Cannady, S.B.; Newman, J.G.; Ibrahim, S.A.; Brant, J.A. Association of Type of Treatment Facility With Overall Survival After a Diagnosis of Head and Neck Cancer. JAMA Netw. Open 2020, 3, e1919697. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Ho, A.S.; Luu, M.; Yoshida, E.J.; Kim, S.; Mita, A.C.; Scher, K.S.; Shiao, S.L.; Tighiouart, M.; Zumsteg, Z.S. Treatment at high-volume facilities and academic centers is independently associated with improved survival in patients with locally advanced head and neck cancer. Cancer 2017, 123, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- The American College of Surgeons. About the National Cancer Database. Available online: www.facs.org/quality-programs/cancer-programs/national-cancer-database/about/ (accessed on 26 June 2024).
- Flanagin, A.; Frey, T.; Christiansen, S.L.; AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021, 326, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Moro, J.D.S.; Maroneze, M.C.; Ardenghi, T.M.; Barin, L.M.; Danesi, C.C. Oral and oropharyngeal cancer: Epidemiology and survival analysis. Einstein 2018, 16, eAO4248. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D. Determining Adequate Margins in Head and Neck Cancers: Practice and Continued Challenges. Curr. Oncol. Rep. 2016, 18, 54. [Google Scholar] [CrossRef]
- Otsuru, M.; Hasegawa, T.; Yamakawa, N.; Okura, M.; Yamada, S.I.; Hirai, E.; Inomata, T.; Saito, H.; Miura, K.I.; Furukawa, K.; et al. A Multicenter Study on the Effect of Margin Distance on Survival and Local Control in Stage 1–2 Squamous Cell Carcinoma of the Tongue. Ann. Surg. Oncol. 2023, 30, 1158–1166. [Google Scholar] [CrossRef]
- Gogna, S.; Kashyap, S.; Gupta, N. Neck Cancer Resection and Dissection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ho, A.S.; Kim, S.; Tighiouart, M.; Gudino, C.; Mita, A.; Scher, K.S.; Laury, A.; Prasad, R.; Shiao, S.L.; Ali, N.; et al. Association of Quantitative Metastatic Lymph Node Burden With Survival in Hypopharyngeal and Laryngeal Cancer. JAMA Oncol. 2018, 4, 985–989. [Google Scholar] [CrossRef] [PubMed]
- de Ridder, M.; Marres, C.C.; Smeele, L.E.; van den Brekel, M.W.; Hauptmann, M.; Balm, A.J.; van Velthuysen, M.L. A critical evaluation of lymph node ratio in head and neck cancer. Virchows Arch. 2016, 469, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Roh, J.L.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Number of positive lymph nodes better predicts survival for oral cavity cancer. J. Surg. Oncol. 2019, 119, 675–682. [Google Scholar] [CrossRef]
- Adrien, J.; Bertolus, C.; Gambotti, L.; Mallet, A.; Baujat, B. Why are head and neck squamous cell carcinoma diagnosed so late? Influence of health care disparities and socio-economic factors. Oral Oncol. 2014, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Stock, S.; Aboalela, A.; Lerman, M.A.; Woo, S.B.; Sonis, S.T.; Treister, N.S. Oral Medicine referrals at a hospital-based practice in the United States. Oral. Surg. Oral. Med. Oral. Pathol. Oral Radiol. 2015, 119, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, D.R.; Masood, M.M.; Lenze, N.R.; McDaniel, P.; Mazul, A.; Sheth, S.; Zanation, A.M.; Hackman, T.G.; Weissler, M.; Zevallos, J.P.; et al. Travel time to provider is associated with advanced stage at diagnosis among low income head and neck squamous cell carcinoma patients in North Carolina. Oral Oncol. 2019, 89, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.; Jarrett, N.; Jeffs, D. The impact of travel on cancer patients’ experiences of treatment: A literature review. Eur. J. Cancer Care 2000, 9, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Luryi, A.L.; Chen, M.M.; Mehra, S.; Roman, S.A.; Sosa, J.A.; Judson, B.L. Treatment Factors Associated With Survival in Early-Stage Oral Cavity Cancer: Analysis of 6830 Cases From the National Cancer Data Base. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 593–598. [Google Scholar] [CrossRef]
- Vetterlein, M.W.; Loppenberg, B.; Karabon, P.; Dalela, D.; Jindal, T.; Sood, A.; Chun, F.K.; Trinh, Q.D.; Menon, M.; Abdollah, F. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer 2017, 123, 3241–3252. [Google Scholar] [CrossRef]
- Ringstrom, M.J.; Christian, J.; Bush, M.L.; Levy, J.E.; Huang, B.; Gal, T.J. Travel distance: Impact on stage of presentation and treatment choices in head and neck cancer. Am. J. Otolaryngol. 2018, 39, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Korte, J.E. Racial disparities in being recommended to surgery for oral and oropharyngeal cancer in the United States. Community Dent. Oral Epidemiol. 2012, 40, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Stitzenberg, K.B.; Sigurdson, E.R.; Egleston, B.L.; Starkey, R.B.; Meropol, N.J. Centralization of cancer surgery: Implications for patient access to optimal care. J. Clin. Oncol. 2009, 27, 4671–4678. [Google Scholar] [CrossRef] [PubMed]
- Osarogiagbon, R.U.; Yu, X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann. Thorac. Surg. 2013, 96, 1178–1189. [Google Scholar] [CrossRef]
- Sullivan, C.B.; Al-Qurayshi, Z.; Anderson, C.M.; Seaman, A.T.; Pagedar, N.A. Factors Associated With the Choice of Radiation Therapy Treatment Facility in Head and Neck Cancer. Laryngoscope 2021, 131, 1019–1025. [Google Scholar] [CrossRef]
- Tassone, P.; Topf, M.C.; Dooley, L.; Galloway, T.; Biedermann, G.; Trendle, M. Going Off Guidelines: An NCDB Analysis of Missed Adjuvant Therapy Among Surgically Treated Oral Cavity Cancer. Otolaryngol. Head Neck Surg. 2023, 168, 1420–1432. [Google Scholar] [CrossRef]
| D1 <7.25 miles | D2 7.25–17.6 | D3 17.6–46.5 | D4 >46.5 miles | Total | p Value | ||
|---|---|---|---|---|---|---|---|
| Overall | N (%) | 2764 (24.9%) | 2714 (24.2%) | 2715 (24.5%) | 2910 (26.2%) | 11,121 (100%) | |
| Age | Mean ± SD | 62.5 ± 13.4 | 62.5 ± 13.2 | 62.0 ± 13.0 | 62.0 ± 13.1 | 62.2 ± 13.2 | 0.490 |
| Sex | Male | 1736 (62.8%) | 1689 (62.2%) | 1716 (62.2%) | 1869 (64.2%) | 7019 (63.1%) | 0.462 |
| Female | 1028 (37.2%) | 1025 (37.8%) | 999 (36.8%) | 1041 (35.8%) | 4102 (36.9%) | ||
| Race | White | 2183 (79.0%) | 2297 (84.6%) | 2414 (88.8%) | 2652 (91.1%) | 9559 (86.0%) | <0.001 |
| Black | 370 (13.4%) | 206 (7.7%) | 147 (5.4%) | 175 (6.0%) | 904 (8.1%) | ||
| Spanish-speaking | No | 2473 (89.5%) | 2499 (92.1%) | 2566 (94.5%) | 2777 (95.4%) | 10,330 (92.9%) | <0.001 |
| Yes | 223 (8.1%) | 155 (5.7%) | 101 (3.7%) | 81 (2.8%) | 563 (5.1%) | ||
| Median Household Income | <$38,000 | 685 (24.8%) | 258 (9.5%) | 372 (13.7%) | 758 (26.0%) | 2075 (18.7%) | <0.001 |
| $38,000–$47,999 | 687 (24.9%) | 454 (16.7%) | 712 (26.2%) | 1060 (36.4%) | 2913 (26.2%) | ||
| $48,000–$62,999 | 653 (23.6%) | 855 (31.5%) | 745 (27.4%) | 715 (24.6%) | 2968 (26.2%) | ||
| ≥$63,000 | 736 (26.6%) | 1141 (42.1%) | 885 (32.6%) | 371 (12.7%) | 3135 (28.2%) | ||
| Not Available | 3 (0.1%) | 5 (0.2%) | 1 (0.0%) | 6 (0.2%) | 30 (0.3%) | ||
| Insurance | No Insurance | 181 (6.5%) | 162 (6.0%) | 163 (6.0%) | 167 (5.7%) | 673 (6.1%) | 0.002 |
| Private Insurance | 937 (33.9%) | 1074 (39.6%) | 1406 (38.5%) | 1054 (36.2%) | 4118 (37.0%) | ||
| Government Insurance | 1605% (58.1%) | 1435 (52.9%) | 1467 (54.0%) | 1635 (56.2%) | 6153 (55.3%) | ||
| Urban | Rural | 33 (1.2%) | 29 (1.1%) | 119 (4.5%) | 611 (21.6%) | 793 (7.3%) | <0.001 |
| Urban | 2680 (98.8%) | 2637 (98.9%) | 2539 (95.5%) | 2217 (78.4%) | 10,080 (92.7%) | ||
| Cancer Stage | Stage III | 731 (26.4%) | 691 (25.5%) | 688 (25.3%) | 658 (22.6%) | 2772 (24.9%) | 0.006 |
| Stage IV | 2033 (73.6%) | 2023 (74.5%) | 2027 (74.7%) | 2252 (77.4%) | 8349 (75.1%) | ||
| Facility | Community Cancer Program | 989 (44.4%) | 785 (35.4%) | 633 (27.2%) | 293 (11.3%) | 2702 (28.8%) | <0.001 |
| Academic/Research Program | 1237 (55.6%) | 1434 (64.6%) | 1692 (72.8%) | 2302 (88.7%) | 6680 (71.2%) | ||
| Distance | Mean ± SD | 3.8 ± 1.9 | 11.0 ± 3.0 | 29.3 ± 9.3 | 134.1 ± 200.3 | 46.1 ± 115.6 |
| D1 <7.25 miles | D2 7.25–17.6 | D3 17.6–46.5 | D4 >46.5 miles | Total | p Value | ||
|---|---|---|---|---|---|---|---|
| Site Recorded | Oral tongue | 1219 (44.1%) | 1249 (46.0%) | 1131 (41.7%) | 1086 (37.3%) | 4696 (42.2%) | <0.001 |
| Gingiva and alveolus | 383 (13.9%) | 412 (15.2%) | 470 (17.3%) | 602 (37.3%) | 1869 (16.8%) | ||
| Floor of mouth | 525 (19.0%) | 445 (16.4%) | 459 (16.9%) | 555 (19.1%) | 1988 (17.9%) | ||
| Hard palate | 122 (4.4%) | 92 (3.4%) | 100 (3.7%) | 103 (3.5%) | 417 (3.7%) | ||
| Buccal mucosa | 213 (7.7%) | 217 (8.0%) | 224 (8.3%) | 217 (7.5%) | 871 (7.8%) | ||
| Retromolar trigone | 169 (6.1%) | 182 (6.7%) | 205 (7.6%) | 208 (7.1%) | 765 (6.9%) | ||
| Other and unspecified mouth | 133 (4.8%) | 117 (4.3%) | 126 (4.6%) | 139 (4.8%) | 515 (4.6%) | ||
| Chemo | No | 1422 (51.4%) | 1465 (54.0%) | 1584 (58.3%) | 1878 (64.5%) | 6356 (57.2%) | <0.001 |
| Yes | 1342 (48.6%) | 1249 (46.0%) | 1131 (41.7%) | 1032 (35.5%) | 4765 (42.8%) | ||
| Radiation | No | 498 (18.0%) | 568 (20.9%) | 400 (14.7%) | 231 (7.9%) | 1866 (16.8%) | <0.001 |
| Yes | 2266 (82.0%) | 2146 (79.1%) | 2315 (85.3%) | 2679 (92.1%) | 9255 (83.2%) | ||
| Surgery | No | 684 (24.7%) | 548 (79.8%) | 400 (14.7%) | 231 (7.9%) | 1866 (16.8%) | <0.001 |
| Yes | 2080 (75.3%) | 2166 (79.8%) | 2315 (85.3%) | 2679 (92.1%) | 9255 (83.2%) | ||
| Treatment | Surgery Only | 498 (18.0%) | 568 (20.9%) | 702 (25.9%) | 1017 (34.9%) | 2787 (25.1%) | <0.001 |
| Radiation Only | 206 (7.5%) | 168 (6.2%) | 105 (3.9%) | 72 (2.5%) | 552 (5.0%) | ||
| Surgery Radiation & Chemo | 864 (31.3%) | 869 (32.0%) | 836 (30.8%) | 873 (30.0%) | 3451 (31.0%) | ||
| Surgery and Radiation | 718 (26.0%) | 729 (26.9%) | 777 (28.6%) | 789 (27.1%) | 3017 (27.1%) | ||
| Radiation and Chemo | 478 (17.3%) | 380 (14.0%) | 295 (10.9%) | 158 (5.5%) | 1314 (11.8%) | ||
| Reason for no surgery | Surgery of the primary site was performed | 2222 (80.4%) | 2320 (85.5%) | 2426 (89.4%) | 2757 (94.7%) | 9741 (87.6%) | <0.001 |
| Surgery was not a part of the planned first course treatment | 457 (16.5%) | 333 (12.3%) | 251 (9.2%) | 118 (4.1%) | 1161 (10.4%) | ||
| Surgery was contraindicated due to patient risk factors | 34 (1.2%) | 29 (1.1%) | 16 (0.6%) | 12 (0.4%) | 91 (0.8%) | ||
| TNM Path N (%) | 0 | 507 (20.0%) | 533 (20.9%) | 660 (25.5%) | 787 (27.9%) | 2489 (23.7%) | <0.001 |
| 1 | 581 (23.0%) | 600 (23.5%) | 564 (21.8%) | 656 (23.2%) | 2405 (22.5%) | ||
| 2 | 1006 (39.8%) | 1080 (42.3%) | 1093 (42.2%) | 1196 (42.4%) | 4382 (41.7%) | ||
| 3 | 21 (0.8%) | 18 (0.7%) | 18 (0.7%) | 20 (0.7%) | 78 (0.7%) | ||
| X | 415 (16.4%) | 322 (12.5%) | 253 (9.8%) | 159 (5.6%) | 1150 (10.9%) | ||
| Regional Nodes Examined | Mean ± SD | 25.6 ± 23.2 | 27.9 ± 22.3 | 30.1 ± 22.5 | 33.7 ± 22.2 | 29.0 ± 22.8 | <0.001 |
| RNE (cat.) | No nodes examined | 666 (24.1%) | 487 (17.9%) | 392 (14.4%) | 254 (8.7%) | 1801 (16.2%) | <0.001 |
| Nodes examined | 2084 (75.4%) | 2213 (81.5%) | 2313 (85.2%) | 2648 (91.0%) | 9273 (83.4%) | ||
| Unknown | 791 (28.6%) | 605 (22.3%) | 465 (17.1%) | 393 (13.5%) | 2256 (20.3%) | ||
| RNE (cat.) Regional Nodes Positive | Mean ± SD | 2.5 ± 3.5 | 2.4 ± 3.5 | 2.0 ± 3.8 | 2.3 ± 3.9 | 2.4 ± 3.7 | <0.001 |
| RNP (cat.) | No nodes positive | 469 (17.0%) | 494 (18.2%) | 624 (23.0%) | 763 (26.2%) | 2352 (21.1%) | <0.001 |
| Nodes positive | 1621 (58.6%) | 1724 (63.5%) | 1691 (62.3%) | 1886 (64.8%) | 6936 (62.4%) | ||
| Unknown | 674 (24.4%) | 496 (18.3%) | 400 (14.7%) | 261 (9.0%) | 1833 (16.5%) | ||
| RX_SUMM Surgical Margins | all margins grossly & microscopically negative | 1820 (65.8%) | 1951 (71.9%) | 2045 (75.3%) | 2340 (80.4%) | 8169 (73.5%) | <0.001 |
| margins positive | 161 (5.8%) | 155 (5.7%) | 160 (5.9%) | 197 (6.8%) | 675 (6.1%) |
| Model | Dependent Variable | Reference Level | Results Estimate (95% CL) p-Value |
|---|---|---|---|
| OS | Sex (Female) | (Ref: Male) | 0.99 (0.93, 1.06) p = 0.837 |
| Race (Black) | (Ref: White) | 1.05 (0.93, 1.19) p = 0.410 | |
| Treatment (Radiation only) | (Ref: Surgery Only) | 0.61 (0.48, 0.78) p ≤ 0.001 | |
| Treatment (Radiation and Chemo) | (Ref: Surgery Only) | 0.66 (0.53, 0.83) p ≤ 0.001 | |
| Treatment (Surgery and Radiation) | (Ref: Surgery Only) | 0.64 (0.59, 0.60) p ≤ 0.001 | |
| Treatment (Surgery and Radiation and Chemo) | (Ref: Surgery Only) | 0.80 (0.74, 0.86) p ≤ 0.001 | |
| Urban (Rural) | (Ref: Urban) | 1.07 (0.95, 1.20) p = 0.285 | |
| Facility (Academic/Research) | (Ref: Community Cancer Program) | 0.98 (0.91, 1.05) p = 0.560 | |
| Insurance (Government Insurance) | (Ref: No Insurance) | 1.39 (1.20, 1.60) p ≤ 0.001 | |
| Insurance (Private Insurance) | (Ref: No Insurance) | 0.86 (0.74, 1.35) p = 0.059 | |
| Surgical Margins (positive) | (Ref: negative) | 1.55 (1.42, 1.69) p ≤ 0.001 | |
| Distance (D2 7.25–17.6) | (Ref: D1 < 7.25) | 0.87 (0.79, 0.95) p = 0.004 | |
| Distance (D3 17.6–46.5) | (Ref: D1 < 7.25) | 0.85 (0.78, 0.94) p ≤ 0.001 | |
| Distance (D4 46.5 < c) | (Ref: D1 < 7.25) | 0.90 (0.82, 0.98) p = 0.018 | |
| RNP (Nodes positive) | (Ref: Non nodes positive) | 1.87 (1.71, 2.04) p ≤ 0.001 | |
| RNP (Unknown) | (Ref: Non nodes positive) | 1.63 (1.39, 1.90) p ≤ 0.001 | |
| Age | 1.02 (1.02, 1.02) p ≤ 0.001 |
| Model | Dependent Variable | Reference Level | Results Estimate (95% CI) p-Value |
|---|---|---|---|
| OS | Sex (Female) | (Ref: Male) | 0.91 (0.85, 0.97) p = 0.007 |
| Race (Black) | (Ref: White) | 1.05 (0.93, 1.19) p = 0.394 | |
| Treatment (Radiation only) | (Ref: Surgery Only) | 0.57 (0.45, 0.73) p ≤ 0.001 | |
| Treatment (Radiation and Chemo) | (Ref: Surgery Only) | 0.63 (0.50, 0.79) p ≤ 0.001 | |
| Treatment (Surgery and Radiation) | (Ref: Surgery Only) | 0.67 (0.61, 0.63) p ≤ 0.001 | |
| Treatment (Surgery, Radiation & Chemo) | (Ref: Surgery Only) | 0.78 (0.71, 0.85) p ≤ 0.001 | |
| Urban (Rural) | (Ref: Urban) | 1.05 (0.93, 1.10) p = 0.416 | |
| Facility (Academic/Research) | (Ref: Community Cancer Program) | 1.03 (0.95, 1.11) p = 0.427 | |
| Insurance (Government Ins.) | (Ref: No Insurance) | 1.08 (0.93, 1.19) p = 0.328 | |
| Insurance (Private Insurance) | (Ref: No Insurance) | 0.82 (0.71, 0.96) p = 0.011 | |
| Surgical Margins (positive) | (Ref: negative) | 1.51 (1.39, 1.64) p ≤ 0.001 | |
| Distance (D2 7.25–17.6) | (Ref: D1 < 7.25) | 0.86 (0.78, 0.94) p = 0.002 | |
| Distance (D3 17.6–46.5) | (Ref: D1 < 7.25) | 0.86 (0.79, 0.95) p = 0.002 | |
| Distance (D4 46.5 < c) | (Ref: D1 < 7.25) | 0.87 (0.79, 0.96) p = 0.006 | |
| RNP (Nodes positive) | (Ref: Non nodes positive) | 2.00 (1.82, 2.20) p ≤ 0.001 | |
| RNP (Unknown) | (Ref: Non nodes positive) | 1.39 (1.18, 1.64) p ≤ 0.001 | |
| Age | 1.02 (1.02, 1.02) p ≤ 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, C.S.; Groman, A.; Sigurdson, S.L.; Magner, W.J.; Singh, A.K.; Gupta, V. Retrospective Cohort Study on the Impact of Travel Distance on Late-Stage Oral Cancer Treatment and Outcomes: An NCDB Analysis. Cancers 2024, 16, 2750. https://doi.org/10.3390/cancers16152750
Harris CS, Groman A, Sigurdson SL, Magner WJ, Singh AK, Gupta V. Retrospective Cohort Study on the Impact of Travel Distance on Late-Stage Oral Cancer Treatment and Outcomes: An NCDB Analysis. Cancers. 2024; 16(15):2750. https://doi.org/10.3390/cancers16152750
Chicago/Turabian StyleHarris, Courtney S., Adrienne Groman, S. Lynn Sigurdson, William J. Magner, Anurag K. Singh, and Vishal Gupta. 2024. "Retrospective Cohort Study on the Impact of Travel Distance on Late-Stage Oral Cancer Treatment and Outcomes: An NCDB Analysis" Cancers 16, no. 15: 2750. https://doi.org/10.3390/cancers16152750
APA StyleHarris, C. S., Groman, A., Sigurdson, S. L., Magner, W. J., Singh, A. K., & Gupta, V. (2024). Retrospective Cohort Study on the Impact of Travel Distance on Late-Stage Oral Cancer Treatment and Outcomes: An NCDB Analysis. Cancers, 16(15), 2750. https://doi.org/10.3390/cancers16152750





