Hematologic Toxicity and Bone Marrow-Sparing Strategies in Chemoradiation for Locally Advanced Cervical Cancer: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Search Terms
2.3. Eligibility Criteria
2.4. Data Extraction and Analysis
2.5. Quality Assessment
3. Results
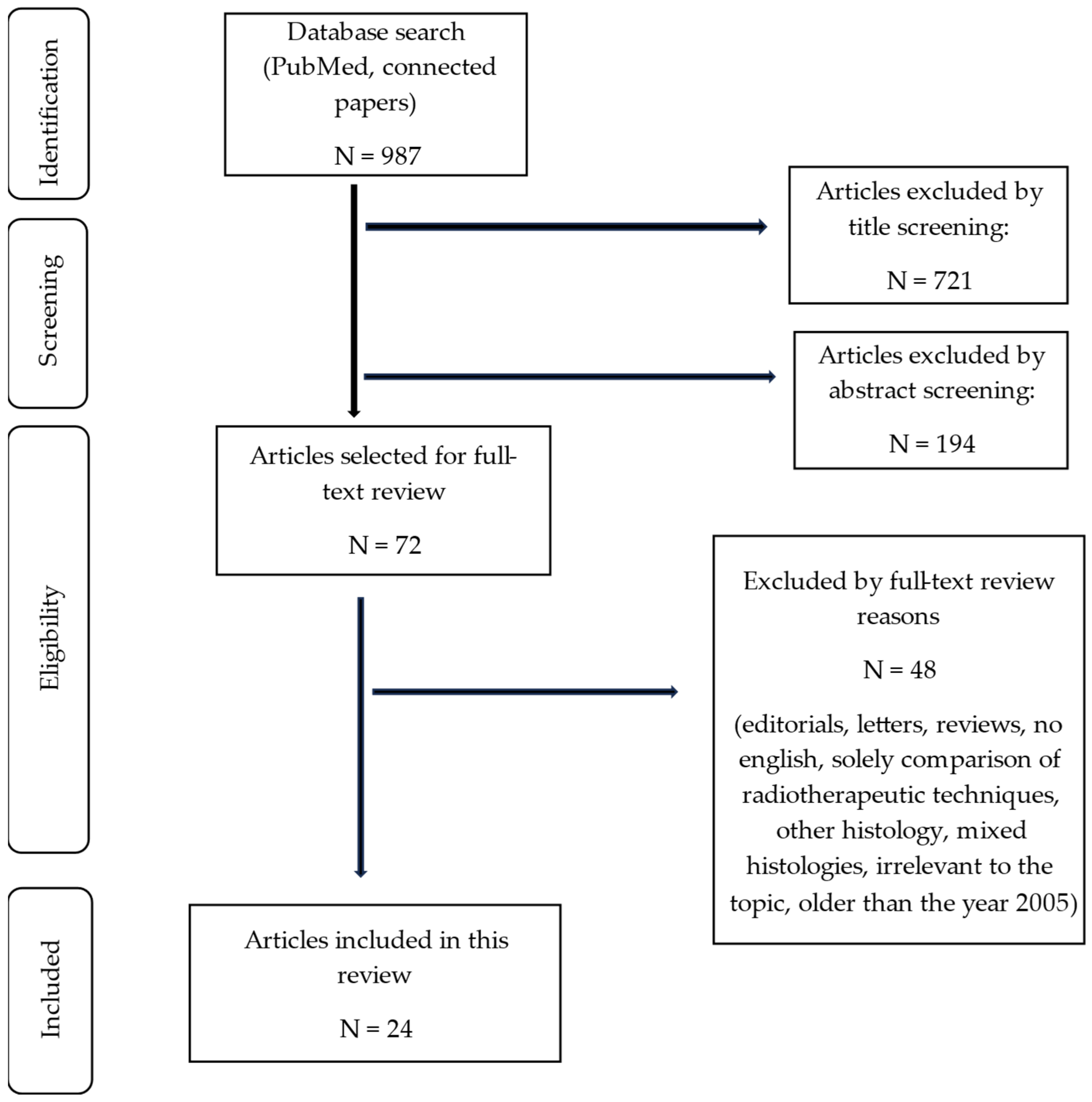
3.1. Search Results
3.2. Population Characteristics
3.3. Therapy Regimens
3.4. Hematologic Toxicity
3.5. Pelvic Bone
3.5.1. Delineation Methods
3.5.2. Correlation between Dose Received by the Bone Marrow (Pelvic Bone) and HT Grades
Whole Bone
Substructures
Correlation between Active Bone Marrow and HT
Low-Density Bone Marrow Spaces
Recommended Cut-off Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D: | three-dimensional conformal radiotherapy |
| [18F]FDG-PET: | 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography |
| ABM: | active bone marrow |
| ANC: | absolute neutrophil count |
| AP/PA: | anterior–posterior/posterior–anterior |
| BM: | bone marrow |
| BMS: | bone marrow sparing |
| BSA: | body surface area |
| BT: | brachytherapy |
| CRT: | chemoradiotherapy |
| CT: | computed tomography |
| CTCAE: | Common Terminology Criteria for Adverse Events |
| CTx: | chemotherapy |
| Cy: | cycles |
| FB: | femoral bone |
| FU: | follow-up |
| GI: | gastrointestinal |
| GU: | genitourinary |
| Gy: | Gray |
| Hb: | hemoglobin |
| HPV: | human papillomavirus |
| HT: | hematologic toxicity |
| IBM: | inactive bone marrow |
| IC: | iliac crest |
| IL: | os ilium |
| IMRT: | intensity-modulated radiation therapy |
| IS: | os ischium |
| LKP: | leukopenia |
| LPB: | lower pelvic bone |
| LPBM: | lower pelvic bone marrow |
| LS: | lumbar spine |
| LSS: | lumbosacral spine |
| LSSBM: | lumbosacral spine marrow |
| LYM: | lymphocytes |
| LYP: | lymphopenia |
| NLR: | neutrophil-to-lymphocyte ratio |
| NTP: | neutropenia |
| OAR: | organ at risk |
| PABM: | pelvic active bone marrow |
| PB: | pelvic bone |
| PBM: | pelvic bone marrow |
| PBMS: | pelvic bone marrow sparing |
| PLT: | platelets (thrombocytes) |
| PTV: | planning target volume |
| RCT: | randomized controlled trial |
| RT: | radiation therapy |
| SB: | sacral bone |
| SUV: | standardized uptake value |
| Tc-99m SPET: | technetium-99m sulfur colloid single-photon emission tomography |
| TOMO: | tomotherapy |
| VMAT: | volumetric modulated arc therapy |
| WB: | whole body |
| WBC: | white blood cells |
| WPB: | whole pelvic bone |
| Vx: | volume receiving x Gy |
| WPBM: | whole pelvic bone marrow |
References
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Lortet-Tieulent, J.; Plummer, M.; Franceschi, S.; Bray, F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur. J. Cancer 2013, 49, 3262–3273. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparen, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Rositch, A.; Qeadan, F.; Gravitt, P.E.; Blaakaer, J. Age-specific prevalence of HPV16/18 genotypes in cervical cancer: A systematic review and meta-analysis. Int. J. Cancer 2016, 138, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Gennigens, C.; De Cuypere, M.; Hermesse, J.; Kridelka, F.; Jerusalem, G. Optimal treatment in locally advanced cervical cancer. Expert Rev. Anticancer Ther. 2021, 21, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Lee, L.J.; Eswara, J.R.; Horowitz, N.S.; Konstantinopoulos, P.A.; Mirabeau-Beale, K.L.; Rose, B.S.; Von Keudell, A.G.; Wo, J.Y. Complications of pelvic radiation in patients treated for gynecologic malignancies. Cancer 2014, 120, 3870–3883. [Google Scholar] [CrossRef] [PubMed]
- Small, W.; Winter, K.; Levenback, C.; Iyer, R.; Gaffney, D.; Asbell, S.; Erickson, B.; Jhingran, A.; Greven, K. Extended-Field Irradiation and Intracavitary Brachytherapy Combined with Cisplatin Chemotherapy for Cervical Cancer with Positive Para-Aortic or High Common Iliac Lymph Nodes: Results of ARM 1 of RTOG 0116. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.J.; Yoshinaga, H.; Mizuno, M. Active bone marrow distribution in the adult. Br. J. Radiol. 1966, 39, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Muirhead, R.; McGowan, D.R.; Chu, K.Y.; Jacobs, C.; Hawkins, M.A. Differential Response of Pelvic Bone Marrow Fluorodeoxyglucose Uptake in Patients Receiving Chemoradiotherapy. Clin. Oncol. 2023, 35, e622–e627. [Google Scholar] [CrossRef] [PubMed]
- Hui, B.; Zhang, Y.; Shi, F.; Wang, J.; Wang, T.; Wang, J.; Yuan, W.; Li, Y.; Liu, Z. Association between Bone Marrow Dosimetric Parameters and Acute Hematologic Toxicity in Cervical Cancer Patients Undergoing Concurrent Chemoradiotherapy: Comparison of Three-Dimensional Conformal Radiotherapy and Intensity-Modulated Radiation Therapy. Int. J. Gynecol. Cancer 2014, 24, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, M.H.; Chen, J.; Liu, W.; Zhu, B.Q.; Lu, E.M. Correlation between bone marrow dose volumes and acute hematological toxicity in postoperative gynecological cancer patients. Pak. J. Med. Sci. 2016, 32, 1547. [Google Scholar] [CrossRef] [PubMed]
- Nurgalieva, Z.; Liu, C.C.; Du, X.L. Chemotherapy use and risk of bone marrow suppression in a large population-based cohort of older women with breast and ovarian cancer. Med. Oncol. 2011, 28, 716–725. [Google Scholar] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Bai, Y.L.; Feng, Y.; Wang, L.; Yun, W.K.; Li, X.; Song, J.Y.; Yang, S.S.; Zhang, Y.Y. Which Bone Marrow Sparing Strategy and Radiotherapy Technology Is Most Beneficial in Bone Marrow-Sparing Intensity Modulated Radiation Therapy for Patients with Cervical Cancer? Front. Oncol. 2020, 10, 554241. [Google Scholar] [PubMed]
- Mell, L.K.; Tiryaki, H.; Ahn, K.H.; Mundt, A.J.; Roeske, J.C.; Aydogan, B. Dosimetric Comparison of Bone Marrow-Sparing Intensity-Modulated Radiotherapy versus Conventional Techniques for Treatment of Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1504–1510. [Google Scholar] [CrossRef]
- Mauricio, D.; Zeybek, B.; Tymon-Rosario, J.; Harold, J.; Santin, A.D. Immunotherapy in Cervical Cancer. Curr. Oncol. Rep. 2021, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Vora, C.; Gupta, S. Targeted therapy in cervical cancer. ESMO Open 2019, 3, e000462. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.; Clark, J.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H.; Goddard, O.; et al. OCEBM Levels of Evidence Working Group*. “The Oxford Levels of Evidence 2”. Oxford Centre for Evidence-Based Medicine. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 24 February 2024).
- Mell, L.K.; Kochanski, J.D.; Roeske, J.C.; Haslam, J.J.; Mehta, N.; Yamada, S.D.; Hurteau, J.A.; Collins, Y.C.; Lengyel, E.; Mundt, A.J. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1356–1365. [Google Scholar] [CrossRef]
- Rose, B.S.; Aydogan, B.; Liang, Y.; Yeginer, M.; Hasselle, M.D.; Dandekar, V.; Bafana, R.; Yashar, C.M.; Mundt, A.J.; Roeske, J.C.; et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 800–807. [Google Scholar] [CrossRef]
- Albuquerque, K.; Giangreco, D.; Morrison, C.; Siddiqui, M.; Sinacore, J.; Potkul, R.; Roeske, J. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow-sparing pelvic IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Klopp, A.H.; Moughan, J.; Portelance, L.; Miller, B.E.; Salehpour, M.R.; Hildebrandt, E.; Nuanjing, J.; D’Souza, D.; Souhami, L.; Small, W.; et al. Hematologic toxicity in RTOG 0418: A phase 2 study of postoperative IMRT for gynecologic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zakeri, K.; Vaida, F.; Carmona, R.; Dadachanji, K.K.; Bair, R.; Aydogan, B.; Hasan, Y.; Yashar, C.M.; Mell, L.K. Longitudinal study of acute haematologic toxicity in cervical cancer patients treated with chemoradiotherapy. J. Med. Imaging Radiat. Oncol. 2015, 59, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, Z.Y.; Li, G.L.; Li, Q.; Yang, Q.; Fan, J.Q.; Zhao, Y.C.; Song, Y.Q.; Wu, G. Correlations between Radiation Dose in Bone Marrow and Hematological Toxicity in Patients with Cervical Cancer: A Comparison of 3DCRT, IMRT, and RapidARC. Int. J. Gynecol. Cancer 2016, 26, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Bosque, M.Á.S.D.; Cervantes-Bonilla, M.Á.; Palacios-Saucedo, G.d.C. Clinical and dosimetric factors associated with the development of hematologic toxicity in locally advanced cervical cancer treated with chemotherapy and 3D conformal radiotherapy. Rep. Pract. Oncol. Radiother. 2018, 23, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Ajayakumar, T. Association between Acute Hematological Toxicities and Bone Marrow Dosimetric Parameters in Cervical Cancer Patients Undergoing Concurrent Chemoradiation—A Comparison between Three Dimensional Conformal Radiotherapy and Intensity Modulated Radiotherapy. Int. J. Contemp. Med. Res. 2018, 5, 2393–2915. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Li, Y.; Xu, L.M.; Chai, Y.L.; Qu, C.; Cao, Y.J.; Wang, J.; Hou, H.L.; Zhang, J. The relationship between the radiation dose of pelvic-bone marrow and lymphocytic toxicity in concurrent chemoradiotherapy for cervical cancer. Radiat. Oncol. 2023, 18, 12. [Google Scholar] [CrossRef]
- Chen, M.; Wang, D.; Bao, Z.; Yi, Z.; Mei, Z.; Sun, S.; Xiang, Q.; Yang, C.; Yang, H.; Qiu, H.; et al. The impact of bone marrow irradiation dose on acute haematologic toxicity in cervical cancer patients treated with concurrent chemoradiotherapy. Radiat. Oncol. 2023, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, Z.; Li, P.; Wu, J.; Zhu, B.; Zhang, X.; Wu, C.; Lin, R.; Zhou, Y.; Chen, W. Clinical study of acute toxicity of pelvic bone marrow-sparing intensity-modulated radiotherapy for cervical cancer. Ginekol. Pol. 2023, 94, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Mahantshetty, U.; Krishnatry, R.; Chaudhari, S.; Kanaujia, A.; Engineer, R.; Chopra, S.; Shrivastava, S. Comparison of 2 contouring methods of bone marrow on CT and correlation with hematological toxicities in non-bone marrow-sparing pelvic intensity-modulated radiotherapy with concurrent cisplatin for cervical cancer. Int. J. Gynecol. Cancer 2012, 22, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Chopra, S.; Naga, P.; Pant, S.; Dandpani, E.; Bharadwaj, N.; Mahantshetty, U.; Engineer, R.; Swamidas, J.; Ghosh, J.; et al. Acute hematological toxicity during post-operative bowel sparing image-guided intensity modulated radiation with concurrent cisplatin. Br. J. Radiol. 2018, 91, 20180005. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Schernberg, A.; Busato, F.; Laurans, M.; Fumagalli, I.; Dumas, I.; Deutsch, E.; Haie-Meder, C.; Chargari, C. Correlation between pelvic bone marrow radiation dose and acute hematological toxicity in cervical cancer patients treated with concurrent chemoradiation. Cancer Manag. Res. 2019, 11, 6285–6297. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, F.; Ji, T.; Zhao, J.; Li, G. Pelvic bone marrow sparing intensity modulated radiotherapy reduces the incidence of the hematologic toxicity of patients with cervical cancer receiving concurrent chemoradiotherapy: A single-center prospective randomized controlled trial. Radiat. Oncol. 2020, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Singareddy, R.; Kaur Bajwa, H.; Reddy, M.M.; Krishnam Raju, A. Dosimetric predictors of acute bone marrow toxicity in carcinoma cervix-experience from a tertiary cancer centre in India. Oncol. Radiother. 2021, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.S.; Liang, Y.; Lau, S.K.; Jensen, L.G.; Yashar, C.M.; Hoh, C.K.; Mell, L.K. Correlation between radiation dose to 18F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Elicin, O.; Callaway, S.; Prior, J.O.; Bourhis, J.; Ozsahin, M.; Herrera, F.G. [18F]FDG-PET standard uptake value as a metabolic predictor of bone marrow response to radiation: Impact on acute and late hematological toxicity in cervical cancer patients treated with chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Khullar, K.; Sudhoff, M.; Elson, J.; Herzog, T.; Jackson, A.; Billingsley, C.; Lamba, M.; Kharofa, J. A comparison of dosimetric parameters in PET-based active bone marrow volume and total bone volume in prediction of hematologic toxicity in cervical cancer patients treated with chemoradiation. J. Radiat. Oncol. 2016, 6, 161–165. [Google Scholar] [CrossRef]
- Yan, K.; Ramirez, E.; Xie, X.J.; Gu, X.; Xi, Y.; Albuquerque, K. Predicting severe hematologic toxicity from extended-field chemoradiation of para-aortic nodal metastases from cervical cancer. Pract. Radiat. Oncol. 2018, 8, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; Freese, C.; Meier, T.; Go, D.; Khullar, K.; Sudhoff, M.; Lamba, M.; Kharofa, J. The absolute volume of PET-defined, active bone marrow spared predicts for high grade hematologic toxicity in cervical cancer patients undergoing chemoradiation. Clin. Transl. Oncol. 2018, 20, 713–718. [Google Scholar] [PubMed]
- Wang, S.B.; Liu, J.P.; Lei, K.J.; Jia, Y.M.; Xu, Y.; Rong, J.F.; Wang, C.X. The volume of 99mTc sulfur colloid SPET-defined active bone marrow can predict grade 3 or higher acute hematologic toxicity in locally advanced cervical cancer patients who receive chemoradiotherapy. Cancer Med. 2019, 8, 7219–7226. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.W.; Sirák, I.; Xu, R.; Portelance, L.; Wei, L.; Tarnawski, R.; Mahantshetty, U.; Heide, E.S.; Yashar, C.M.; McHale, M.T.; et al. Positron Emission Tomography-Guided Bone Marrow-Sparing Radiation Therapy for Locoregionally Advanced Cervix Cancer: Final Results from the INTERTECC Phase II/III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Eter, P.; Ose, G.R.; Undy, R.N.B.; Dwin, E.; Atkins, B.W.; Ate, J.T.; Higpen, T.; Unther, G.; Eppe, D.; Aiman, I.A.M.; et al. Concurrent Cisplatin-Based Radiotherapy and Chemotherapy for Locally Advanced Cervical Cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP n.d. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 1 March 2024).
- Cox, J.D.; Stetz, J.A.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Bese, N.S.; Hendry, J.; Jeremic, B. Effects of Prolongation of Overall Treatment Time Due to Unplanned Interruptions during Radiotherapy of Different Tumor Sites and Practical Methods for Compensation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Girinsky, T.; Rey, A.; Roche, B.; Haie, C.; Gerbaulet, A.; Randrianarivello, H.; Chassagne, D. Overall treatment time in advanced cervical carcinomas: A critical parameter in treatment outcome. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- van Meir, H.; Nout, R.A.; Welters, M.J.P.; Loof, N.M.; de Kam, M.L.; van Ham, J.J.; Samuels, S.; Kenter, G.G.; Cohen, A.F.; Melief, C.J.M.; et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 2017, 6, e1267095. [Google Scholar] [CrossRef] [PubMed]
- Sacks, E.L.; Goris, M.L.; Glatstein, E.; Gilbert, E.; Kaplan, H.S. Bone marrow regeneration following large field radiation: Influence of volume, age, dose and time. Cancer 1978, 42, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Testart-Paillet, D.; Girard, P.; You, B.; Freyer, G.; Pobel, C.; Tranchand, B. Contribution of modelling chemotherapy-induced hematological toxicity for clinical practice. Crit. Rev. Oncol. Hematol. 2007, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mangioni, C.; Bolis, G.; Pecorelli, S.; Bragman, K.; Epis, A.; Favalli, G.; Gambino, A.; Landoni, F.; Presti, M.; Torri, W.; et al. Randomized trial in advanced ovarian cancer comparing cisplatin and carboplatin. J. Natl. Cancer Inst. 1989, 81, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Lokich, J. What is the “best” platinum: Cisplatin, carboplatin, or oxaliplatin? Cancer Investig. 2001, 19, 756–760. [Google Scholar]
- Terrones-Campos, C.; Ledergerber, B.; Vogelius, I.R.; Helleberg, M.; Specht, L.; Lundgren, J. Hematological toxicity in patients with solid malignant tumors treated with radiation—Temporal analysis, dose response and impact on survival. Radiother. Oncol. 2021, 158, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, S.G. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv. Radiat. Oncol. 2018, 3, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Heylmann, D.; Rödel, F.; Kindler, T.; Kaina, B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim. Biophys. Acta 2014, 1846, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Cucinotta, F.A. Characterization of the radiation-damaged precursor cells in bone marrow based on modeling of the peripheral blood granulocytes response. Health Phys. 2011, 101, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Green, D.E.; Rubin, C.T. Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone 2014, 63, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cristy, M. Active bone marrow distribution as a function of age in humans. Phys. Med. Biol. 1981, 26, 389. [Google Scholar] [CrossRef] [PubMed]
- Hayman, J.A.; Callahan, J.W.; Herschtal, A.; Everitt, S.; Binns, D.S.; Hicks, R.J.; Mac Manus, M. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 847–852. [Google Scholar] [CrossRef]
- Mauch, P.; Constine, L.; Greenberger, J.; Knospe, W.; Sullivan, J.; Liesveld, J.L.; Deeg, H. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1319–1339. [Google Scholar] [CrossRef] [PubMed]
- Blebea, J.S.; Houseni, M.; Torigian, D.A.; Fan, C.; Mavi, A.; Zhuge, Y.; Iwanaga, T.; Mishra, S.; Udupa, J.; Zhuang, J.; et al. Structural and Functional Imaging of Normal Bone Marrow and Evaluation of Its Age-Related Changes. Semin. Nucl. Med. 2007, 37, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, E.; Shafrir, E. Yellow bone marrow as adipose tissue. Proc. Soc. Exp. Biol. Med. 1967, 124, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Klopp, A.H.; Yeung, A.R.; Deshmukh, S.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gifford, K.; Gaffney, D.K.; Small, W.; Thompson, S.; et al. Patient-Reported Toxicity during Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. J. Clin. Oncol. 2018, 36, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Gupta, S.; Kannan, S.; Dora, T.; Engineer, R.; Mangaj, A.; Maheshwari, A.; Surapp, S.T.; Ghosh, J.; Paul, S.N.; et al. Late Toxicity after Adjuvant Conventional Radiation versus Image-Guided Intensity-Modulated Radiotherapy for Cervical Cancer (PARCER): A Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 3682–3692. [Google Scholar] [CrossRef] [PubMed]
- Brixey, C.J.; Roeske, J.C.; Lujan, A.E.; Yamada, S.D.; Rotmensch, J.; Mundt, A.J. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Avinash, H.U.; Arul Ponni, T.R.; Janaki, M.G.; Kirthi Koushik, A.S.; Mohan Kumar, S. A prospective dosimetric and clinical comparison of acute hematological toxicities in three-dimensional conformal radiation therapy and intensity modulated radiation therapy with concurrent chemotherapy in carcinoma cervix. J. Cancer Res. Ther. 2015, 11, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Sood, S.; Sidhu, M.; Singh, K.; Sood, D.; Mupasana, V.; Palanisamy, M. Analysis of Rapid arc-based Radiation Therapy on Dosimetric Parameters in Cervical Cancer Patients with and without Bone Marrow Sparing. Asian Pac. J. Cancer Prev. 2022, 23, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Okamoto, H.; Kasamatsu, T.; Kobayashi, K.; Harada, K.; Kitaguchi, M.; Sekii, S.; Takahashi, K.; Yoshio, K.; Inaba, K.; et al. A dosimetric analysis of intensity-modulated radiation therapy with bone marrow sparing for cervical cancer. Anticancer Res. 2014, 34, 5091–5098. [Google Scholar] [PubMed]
- Jodda, A.; Urbański, B.; Piotrowski, T.; Malicki, J. Relations between doses cumulated in bone marrow and dose delivery techniques during radiation therapy of cervical and endometrial cancer. Phys. Med. 2017, 36, 54–59. [Google Scholar] [CrossRef]
- Wang, S.B.; Liu, J.; Lei, K.; Jia, Y.; Wang, C.; Zhang, X.; Li, T. Single-photon emission computed tomography-defined active bone marrow-sparing volumetric-modulated arc therapy reduces the incidence of acute hematologic toxicity in locally advanced cervical cancer patients who receive chemoradiotherapy: A single-center prospective randomized controlled trial. Cancer 2023, 129, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Pembrolizumab Plus Chemoradiotherapy for FIGO 2014 Stage III–IVA Cervical Cancer—The ASCO Post n.d. Available online: https://ascopost.com/news/january-2024/fda-approves-pembrolizumab-plus-chemoradiotherapy-for-figo-2014-stage-iii-iva-cervical-cancer/ (accessed on 29 February 2024).
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients with cervical cancer who received (chemo)radiation therapy | Letters, reviews, abstracts and editorials |
| Studies reporting on the correlation between hematologic toxicity and the radiation dose received by the pelvic bone marrow | Studies published in languages other than English or German |
| Studies with a minimum of 10 patients | Mixed histologies that cannot be analyzed separately |
| Publications from 2006 onwards | Studies before the year 2006 |
| Both pre- and postoperative settings considered |
| Author and Year | Number of Patients | Study Design | RT Intention | Dose Prescription | RT Technique | Chemotherapeutic Regimen | CTx Cycles (Patients in %) |
|---|---|---|---|---|---|---|---|
| Mell, 2006 [21] | 37 | Retrospective case series | Definitive (91.9%)/postoperative (8.1%) | 39.6–50.4 Gy | IMRT + BT | Cisplatin 40 mg/m2 weekly | 6 cy: 2.7%, 5 cy: ≈46%, 4 cy: ≈35% |
| Rose, 2011 [22] | 81 | Retrospective case series and validation cohort | definitive | 39.6–50.4 Gy | IMRT + BT | Cisplatin 40 mg/m2 weekly | 4 cy: 20%, 5 cy: 34%, 6 cy: 25%, 55% at least 1 cy |
| Albuquerque, 2011 [23] | 40 | Retrospective case series | Definitive | 45 Gy | 3D | Cisplatin 40 mg/m2 weekly | Not mentioned |
| Klopp, 2013 [24] | 40 | Prospective cohort study | Postoperative | 50.4 Gy | IMRT | Cisplatin 40 mg/m2 weekly | 5 cy ≥ 83%, 4 cy ≥ 90%, 4 cy < 7.5% |
| Zhu, 2015 [25] | 102 | Retrospective cohort study | Postoperative/definitive | 39.6–50.4 Gy | IMRT (97.1%)/3D (2.9%) +BT | Cisplatin 40 mg/m2 weekly | 6 cy: 20%, 5 cy: 38%, 4 cy: 22%, 3 cy: 11%, 2 cy: 5%, 1 cy: 4% |
| Chang, 2016 [26] | 100 | Retrospective case series/cohort study | Definitive | 50.4–56 Gy | 3D (32%)/IMRT (37%)/ IMRT (RapidArc; 31%) + BT | Cisplatin 25 mg/m2 | 3–6 cy |
| Li, 2016 [12] | 100 | Retrospective case series | Postoperative | 40–50 Gy | 2D (AP/PA: 77%)/IMRT (23%) | 52% received CT (paclitaxel/nedaplatin). Concomitantly: 15%; induction CT: 47%. | 6 cy ≥ 4%, 5 cy: 1%, 4 cy: 5%, 3 cy: 4%, 2 cy: 17%, 1 cy: 21% |
| Bosque, 2018 [27] | 59 | Retrospective cohort study | Not mentioned | 45–50.4 Gy | 3D + BT | Cisplatin (93.2%)/carboplatin (6.7%) weekly | 5 cy ≥ 22%, 5 cy < 77.9% |
| Ajayakumar, 2018 [28] | 47 | Prospective observational study | Definitive | 45–50.4 Gy | 3D (53.2%)/IMRT (46.8%) + BT | Cisplatin 40 mg/m2 weekly | Not mentioned |
| Zhang, 2023 [29] | 117 | Retrospective case series | Definitive | 50.4 Gy | IMRT (24.79%)/VMAT (75.21%) + BT | Induction CT: (paclitaxel/cisplatin; 78.63%). Concomitant CT: cisplatin 25 mg/m2 weekly | 5 cy: 43.6%, 3–4 cy: 56.4% |
| Chen, 2023 [30] | 69 | Retrospective case series | Definitive (36.2%)/postoperative (63.8%) | 45–50.4 Gy | IMRT +BT | Cisplatin 40 mg/m2 weekly | 3–4 cy: 62.3%, 5–6 cy: 37.7% |
| Sun, 2023 [31] | 40 | Prospective observational study | Postoperative | 50 Gy | IMRT (50%) and PBMS-IMRT (50%) | Cisplatin 35–40 mg/m2 weekly | Not mentioned |
| Mahantshetty, 2012 [32] | 47 | Retrospective case series | Definitive | 50 Gy | IMRT + BT | Cisplatin 40 mg/m2 weekly | 4 cy ≥ 95%, 2 cy ≤ 5% |
| Lewis, 2018 [33] | 75 | Retrospective case series | Postoperative | 50 Gy | IMRT (TOMO) | Cisplatin 40 mg/m2 (98.7%)/carboplatin (1.3%) weekly | 6 cy: 1.3%, 5 cy: 69.3%, 4 cy: 24%, 3 cy: 1.3%, 2 cy: 2.7%, 1 cy: 1.3% |
| Kumar, 2019 [34] | 114 | Retrospective case series | Definitive | 45 Gy (+Boost up to 60 Gy) | 3D (75.4%)/IMRT (24.6%) + BT | Cisplatin 40 mg/m2 (89.5%) or carboplatin AUC2 (10.5%) weekly in case of renal impairment | 5 cy: 76.3%, 4 cy: 19.3%, 4 cy < 4.4% |
| Huang, 2020 [35] | 164 | Prospective RCT | Definitive | 50.4 Gy | IMRT + BT | Cisplatin 40 mg/m2 weekly | PBMS group: 6 cy: 48.8%, 5 cy: 40.2%, 4 cy: 8.5%, 2 cy: 1.2%, 1 cy: 1.2% Control group: 6 cy: 45.1%, 5 cy: 37.8%, 4 cy: 17.1% |
| Singareddy, 2021 [36] | 34 | Prospective observational study | Definitive | 50 Gy | IMRT (VMAT) + BT | Cisplatin 40 mg/m2 weekly | 5 cy: 85.2%, 4 cy: 11.8%, 3 cy: 2.9% |
| Rose, 2012 [37] | 26 | Retrospective case series | Definitive 81%; postoperative 19% | 45–50.4 Gy (+ Boost up to 60–66 Gy) | IMRT + BT | Cisplatin 40 mg/m2 weekly | 6 cy: 27%, 5 cy: 35%, 4 cy: 15%, 3 cy: 15%, 1 cy: 8% |
| Elicin, 2014 [38] | 17 | Retrospective case series | Definitive | 45–50.4 Gy (+ Boost up to 60 Gy) | IMRT (TOMO) + BT | Cisplatin 40 mg/m2 weekly | 5 cy: 100% |
| Khullar, 2017 [39] | 21 | Retrospective case series | Definitive | 45–50.4 Gy | 3D (76%)/IMRT (14%)/both (9%) | Cisplatin | 2–7 cy; ≥5 cy: 61.9% |
| Yan, 2018 [40] | 38 | Retrospective case series | Definitive | 45 Gy (+ Boost up to 55–60 Gy) | 3D/IMRT + BT | Cisplatin 40 mg/m2 weekly | Not mentioned |
| Zhou, 2018 [41] | 31 | Retrospective case series | Definitive/postoperative | 45–68 Gy | 3D (58%)/IMRT (42%) | Cisplatin 40 mg/m2 weekly | 0 cy: 29%, 1–3 cy: 19%, 4–5 cy: 32%, 6–7 cy: 19% |
| Wang, 2019 [42] | 39 | Prospective cohort study | Definitive | 45 Gy (+ Boost 10–20 Gy) | IMRT (VMAT) + BT | Cisplatin 30–40 mg/m2 weekly | 5 cy: 84.6%, 4 cy: 15.4% |
| Williamson, 2022 [43] | 101 | Prospective | Definitive | 45–50.4 Gy (+ Boost up to 59.4 Gy) | IMRT + BT | Cisplatin 40 mg/m2 weekly | ≥5 cy > 80% |
| Author and Year | HT Assessment Method | HT Assessment Frequency | Blood Components Analyzed | HT Incidence |
|---|---|---|---|---|
| Mell, 2006 [21] | RTOG | Weekly during CRT | WBC, ANC, Hb, PLT | G2+ LKP 43.2%, NTP 18.9% |
| Rose, 2011 [22] | RTOG | Weekly during CRT | WBC, ANC, Hb, PLT | G2+ LKP 74%, NTP 48% |
| Albuquerque, 2011 [23] | CTCAE 3.0 | During CRT | WBC, ANC, Hb, PLT | G2+ HT 67.5% |
| Klopp, 2013 [24] | CTCAE 3.0 | Not mentioned | Not mentioned | G2+ HT 58%, G3+ HT 25% |
| Zhu, 2015 [25] | Not mentioned | Weekly during CRT | WBC, ANC, Hb, PLT | Not mentioned |
| Chang, 2016 [26] | Not mentioned | Weekly during CRT | WBC, ANC, Hb, PLT | G2+ LKP: 3D/IMRT/RapidARC: 100%/78.4%/80.6% G3+ LKP: 78.1%/40.5%/48.4% G2+ NTP: 93.7%/64.9%/67.7% G3+ NTP: 65.6%/27.0%/29.0% |
| Li, 2016 [12] | RTOG | Weekly and 120 days after start of RT | WBC, ANC, Hb, PLT | G2+ HT 47% |
| Bosque, 2018 [27] | RTOG | Not mentioned | WBC, ANC, Hb, PLT | G2+ HT 50.8% |
| Ajayakumar, 2018 [28] | RTOG | Weekly during CRT | Not mentioned | G2+ 38.3% (3D: 72.2%/IMRT: 27.8%) |
| Zhang, 2023 [29] | CTCAE 4.0 | Baseline, weekly during CRT and at 1 mo FU | LYM | G3+ LYP 68.38% |
| Chen, 2023 [30] | RTOG | Weekly during CRT | LYM, ANC, Hb, PLT | G2+ NTP: 50.7%, Anemia: 21.7%, Thrombocytopenia: 24.6%. |
| Sun, 2023 [31] | Not mentioned | Until 3-month FU | Not mentioned | IMRT: G2+ 45% vs. PBMS-IMRT:G2+ 25%. (p = 0.038). |
| Mahantshetty, 2012 [32] | RTOG | Weekly during CRT | WBC, ANC, Hb, PLT | G2+ LKP 53%, NTP 29.8% |
| Lewis, 2018 [33] | CTCAE 3.0 | Weekly during CRT | WBC, ANC, Hb, PLT | G2+ 57.4%, G3+ HT 14.7% |
| Kumar, 2019 [34] | CTCAE 4.0 | Baseline, weekly during CRT and at least once within 2 weeks FU | WBC, LYM, ANC, Hb, PLT | G4+ LKP 2.6%, LYM 12.3% |
| Huang, 2020 [35] | RTOG | Weekly during CRT | WBC, ANC, Hb, PLT | G2+ HT 50% |
| Singareddy, 2021 [36] | RTOG | Baseline and weekly during CRT | WBC, Hb, PLT | G2+ HT 50% |
| Rose, 2012 [37] | RTOG | Weekly during CRT | WBC, ANC, Hb, PLT | G3+ LKP 38.5%, NTP 23.1% |
| Elicin, 2014 [38] | RTOG | 1 week before and weekly during CRT 3 months after CRT, and at last FU | WBC, ANC, Hb, PLT | G3+ LKP 35%, NTP 35% |
| Khullar, 2017 [39] | CTCAE 4.0 | Weekly to 6 weeks after end of CRT | Not mentioned | G3+ HT 71.4% |
| Yan, 2018 [40] | CTCAE 4.0 | Weekly during RCT | WBC, ANC, Hb, PLT | G3+ HT 50% |
| Zhou, 2018 [41] | CTCAE 4.0 | Weekly and one week after CRT | WBC, ANC, Hb, PLT | G3+ HT 77% |
| Wang, 2019 [42] | CTCAE 3.0 | Weekly to two weeks after CRT | WBC, ANC, Hb, PLT | G3+ LKP 46.2%, NTP 2.5% |
| Williamson, 2022 [43] | Not mentioned | Not mentioned | LYM, ANC, Hb, PLT | PET-BMS-IMRT vs. IMRT: significantly reduced G3+ neutropenia (13% vs. 35%) |
| Author and Year | Bone Marrow Delineation Method | Bone Marrow Substructure Definition | Bone Marrow Dose Constraint during Treatment Planning | Distinction between ABM and IBM | Extended-Field RT (Para-Aortic) | Dosimetric Predictors |
|---|---|---|---|---|---|---|
| Mell, 2006 [21] | CT-based PB contour | yes (LSS, IL, LPB) | no | no | no | PB-V10, V20 |
| Rose, 2011 [22] | CT-based PB contour | no | no | no | no | PB V10, V20 |
| Albuquerque, 2011 [23] | CT-based PB contour | yes (LSS, IL, LPB, PB+IL+LPB, WPB+LSS) | no | no | no | PB-V20 |
| Klopp, 2013 [24] | CT-based PB contour | no | no | no | no | PB-V40, Dmean |
| Zhu, 2015 [25] | CT-based PB contour | Yes (LSS, IC, LPB) | individual | no | no | PB-Dmean, V20, V30, V40, LSS-V10, V40, LPB-V20, V30 |
| Chang, 2016 [26] | CT-based PB contour | no | no | no | no | PB-V20, V40 |
| Li, 2016 [12] | CT-based PB contour | Yes (WPB, LSS)) | no | no | no | LSS-V5-40 and LPB-V5-40 |
| Bosque, 2018 [27] | CT-based PB contour | no | no | no | not mentioned | none |
| Ajayakumar, 2018 [28] | CT-based PB contour | Yes (WPB, LSS) | yes | no | no | PBM: V20, 30, 40. LSSBM: V40 |
| Zhang, 2023 [29] | CT-based PB contour | no | no | no | not mentioned | PB V5, V10, V20 and V30 |
| Chen, 2023 [30] | CT-based PB contour | Yes (IL, LPB, LSS) | no | no | no | Hb: R(elative)-LPB-V10, R-LPB-V25, R-LPB-V50, R-LPB-mean, A(bsolute)-LPB-V15, A-LPB-V25 and A-LPB-V30. PLT: R-LPB-V40. ANC: R-IL-V15 and R-IL-V50 and A-LPB-V50. NLR: R-LPB-V15 and A-PBM-mean |
| Sun, 2023 [31] | CT-based PB contour | no | yes (V20 < 76% and V40 < 35% in the PBMS group) | no | no | none |
| Mahantshetty, 2012 [32] | CT-based PB contour + BM defined as the low-density regions within the corresponding bones | yes (SB, IL, IS, LPB, LSS, WPB) | no | no | not mentioned | PBM-V40 |
| Lewis, 2018 [33] | CT-based PB contour + BM defined as the low-density regions within the corresponding bones | yes (WPB+LS, LS, SB, IL, IS, FB, WPB, LPB) | no | no | not mentioned | Ilium-PB V20 |
| Kumar, 2019 [34] | CT-based PB contour + BM defined as the low-density regions within the corresponding bones | yes (LSS, IC, LPB) | no | no | yes (13.2%) | PB-V20, LPB-V5,20, Iliac crest-Dmean |
| Huang, 2020 [35] | CT-based PB contour + BM defined as the low-density regions within the corresponding bones | yes (WPB, LSS) | yes | no | no | PB: PB-V40, LSS-V10, Dmean; PBM: PBM-V40, LSSBM -V10, V20, V40, Dmean |
| Singareddy, 2021 [36] | CT-based PB contour + BM defined as the low-density regions within the corresponding bones | no | no | no | no | WPBM-V20,30,40 and Dmean |
| Rose, 2012 [37] | CT-based PB contour, [18F]FDG-PET based ABM (≥SUVmean WPB) | no | no | yes | yes (23%) | PABM Dmean |
| Elicin, 2014 [38] | CT-based PB contour, [18F]FDG-PET based ABM (≥SUVmean WPB) | no | no | yes | yes (23.5%) | none |
| Khullar, 2017 [39] | CT-based PB contour, [18F]FDG-PET based ABM (≥SUVmean WB) | no | no | yes | not mentioned | PABM volume, PABM-V40 |
| Yan, 2018 [40] | CT-based PB contour, [18F]FDG-PET based ABM (≥SUVmean WPB) | no | no | yes | yes (100%) | PB-V20, V30, V45, Dmean; PABM-V10,20,30,45, Dmean |
| Zhou, 2018 [41] | CT-based PB contour, [18F]FDG-PET ABM (≥SUVmean WB) | no | no | yes | not mentioned | PB-V10; PABM V10, 20,40 Gy |
| Wang, 2019 [42] | CT-based PB contour, Tc-99m SPET (≥SUVmean WB) | no | no | yes | no | PABM volume, PABM-V30, V40 |
| Williamson, 2022 [43] | CT-based PB contour, [18F]FDG-PET-based ABM (≥SUVmean WPBM) | no | yes (PBM and ABM mean doses were constrained to <27 Gy and <28.5 Gy; V10 <90% and V20 <75%) | yes | no | none |
| Parameter | Recommended Dose Constraints |
|---|---|
| Whole Pelvic Bone (Marrow) | |
| V10 | <90%, <95% |
| V20 | ≤65%, <71.7%, <76%, <78.6%, <79.4%, <86.6% |
| V30 | <47.1%, <49.7%, <57% |
| V40 | <22.8%, <28%, <29%, ≤37%, <40% |
| V45 | < 20.4% |
| Dmean | <28.8 Gy, <30.3 Gy, ≤34.2 Gy, <39.0 Gy, |
| Volume spared 10 Gy | ≥230 cc |
| Substructures (Bone Marrow) | |
| LPB-V5 | ≤95% |
| LSS-V10 | <87% |
| LPB-V20, IL-PB-V20 | ≤45%, ≤90% |
| LSS-V40 | <50.9% |
| Dmean IC | ≤31 Gy |
| Pelvic Active Bone Marrow | |
| V10 | <95.5% |
| V20 | <80.5% |
| V30 | <46.5%, <59.6% |
| V40 | <23.5% |
| V45 | <31.7% |
| Dmean | <26.8 Gy, <32.4 Gy |
| Baseline PABM volume | >387.5 cc, ≥1201 cc |
| Volume spared (V10, V20, V40) | ≥179 cc, ≥186 cc, ≥738 cc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konnerth, D.; Gaasch, A.; Zinn, A.; Rogowski, P.; Rottler, M.; Walter, F.; Knoth, J.; Sturdza, A.; Oelmann, J.; Grawe, F.; et al. Hematologic Toxicity and Bone Marrow-Sparing Strategies in Chemoradiation for Locally Advanced Cervical Cancer: A Systematic Review. Cancers 2024, 16, 1842. https://doi.org/10.3390/cancers16101842
Konnerth D, Gaasch A, Zinn A, Rogowski P, Rottler M, Walter F, Knoth J, Sturdza A, Oelmann J, Grawe F, et al. Hematologic Toxicity and Bone Marrow-Sparing Strategies in Chemoradiation for Locally Advanced Cervical Cancer: A Systematic Review. Cancers. 2024; 16(10):1842. https://doi.org/10.3390/cancers16101842
Chicago/Turabian StyleKonnerth, Dinah, Aurelie Gaasch, Annemarie Zinn, Paul Rogowski, Maya Rottler, Franziska Walter, Johannes Knoth, Alina Sturdza, Jan Oelmann, Freba Grawe, and et al. 2024. "Hematologic Toxicity and Bone Marrow-Sparing Strategies in Chemoradiation for Locally Advanced Cervical Cancer: A Systematic Review" Cancers 16, no. 10: 1842. https://doi.org/10.3390/cancers16101842
APA StyleKonnerth, D., Gaasch, A., Zinn, A., Rogowski, P., Rottler, M., Walter, F., Knoth, J., Sturdza, A., Oelmann, J., Grawe, F., Bodensohn, R., Belka, C., & Corradini, S. (2024). Hematologic Toxicity and Bone Marrow-Sparing Strategies in Chemoradiation for Locally Advanced Cervical Cancer: A Systematic Review. Cancers, 16(10), 1842. https://doi.org/10.3390/cancers16101842







