Axitinib after Treatment Failure with Sunitinib or Cytokines in Advanced Renal Cell Carcinoma—Systematic Literature Review of Clinical and Real-World Evidence
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
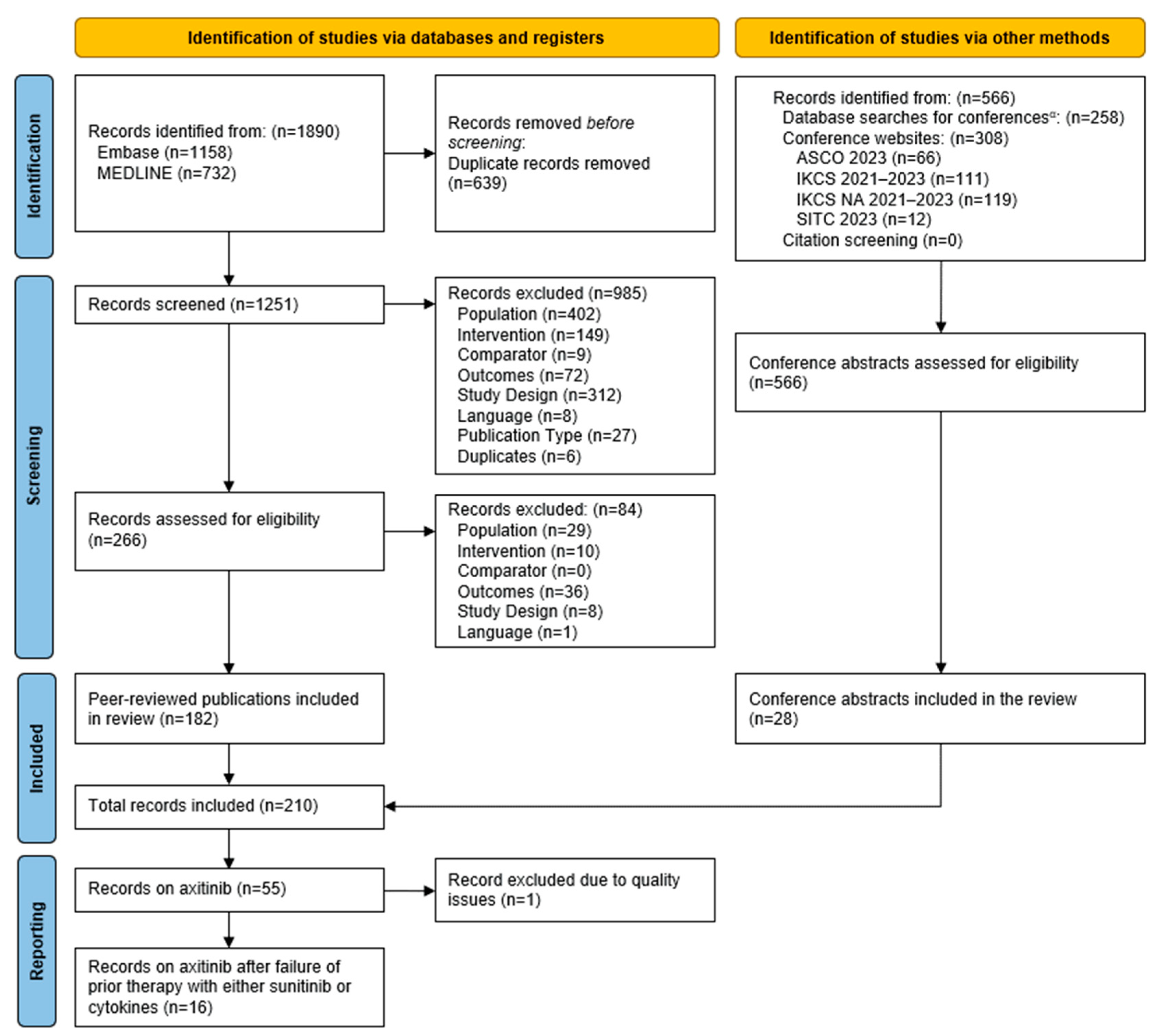
3.1. Literature Search and PRISMA
3.2. Study and Patient Characteristics
3.2.1. RCTs and Single-Arm Clinical Trials
3.2.2. Observational Studies
| Author, Year | Country | Study Design | Study Population (N) | Tx (n) | Tx Line | Prior Tx | Outcomes |
|---|---|---|---|---|---|---|---|
| Randomised controlled trials | |||||||
| Rini, 2011 [24]; Bracarda, 2019 [25]; Cella, 2013 [26]; Motzer, 2013 [28]; Ueda, 2013 [27] AXIS (NCT00678392) | International | Phase 3, open-label | Patients with aRCC who had disease progression after initial systemic therapy (N = 723) | I: Axitinib (n = 361) C: Sorafenib (n = 362) | 2L | 1L: Sunitinib (n = 389) Cytokines (n = 251) Bevacizumab (n = 59) Temsirolimus (n = 24) | Primary outcome: PFS Secondary outcomes: OS, ORR, duration of response, TTD, safety, patient-reported outcomes |
| Kadono, 2023 [29] ESCAPE (UMIN000012522) | Japan | Phase 3, open-label | Patients with favourable and intermediate risk mRCC (N = 35) | I: Axitinib after cytokines (n = 35) C: Axitinib after sunitinib (n = 15) | 2L | 1L: Cytokines (n = 18) Sunitinib (n = 15) | Primary outcome: PFS Secondary outcomes: OS, ORR, DCR, safety |
| Single-arm clinical trials | |||||||
| Eto, 2014 [30] (NCT00569946) | Japan | Phase 2, single-arm | Patients with mRCC with a clear-cell component (N = 64) | Axitinib (n = 64) | ≥2L | 1L or 2L: Cytokines (n = 64) | Primary outcome: ORR Secondary outcomes: OS, PFS, duration of response, safety, pharmacokinetics |
| Rini, 2009 [31] (NCT00282048) | US | Phase 2, single-arm | Patients with refractory mRCC (N = 62) | Axitinib (n = 62) | ≥2L | 1L or 2L: Sorafenib (n = 62) Sunitinib (n = 14) Cytokines (n = 38) Chemotherapy (n = 12) Bevacizumab (n = 5) Temsirolimus (n = 3) | Primary outcome: ORR Secondary outcomes: OS, PFS, duration of response, safety, patient-reported outcomes, pharmacokinetics |
| Rixe, 2007 [32] (NCT00076011) | France, Germany, US | Phase 2, single-arm | Patients with mRCC who had failed previous cytokine-based treatment (N = 52) | Axitinib (n = 52) | 2L | 1L: Cytokines (n = 52) | Primary outcome: ORR Secondary outcomes: OS, PFS, duration of response, time to progression, safety, pharmacokinetics, HRQoL |
| Observational studies | |||||||
| Cesas, 2023 [33] | Lithuania | Retrospective and prospective study cohorts | Patients with mRCC who had received 1L VEGF-targeted therapy with either sunitinib or pazopanib (N = 143) | Axitinib (n = 59) Cabozantinib (n = 30) Everolimus (n = 8) Nivolumab (n = 46) | ≥2L | 1L: Sunitinib (n = 123) Pazopanib (n = 20) | Outcomes: PFS2 |
| Facchini, 2019 [34]; D’Aniello, 2016 [39] | Italy | Retrospective | Patients with mRCC (N = 148) | Axitinib (n = 148) | 2L | 1L: Sunitinib (n = 148) | Primary outcomes: PFS, OS, ORR, DCR, safety Secondary outcomes: relationship between patients’ demographic and baseline characteristics, AEs, and response |
| Géczi, 2020 [35] | Hungary | Retrospective | Patients with mRCC (N = 512) | Axitinib (n = 128) Everolimus (n = 384) | 2L | 1L: Sunitinib (n = 446) Pazopanib (n = 66) | Outcomes: OS, duration of 1L treatment |
| Iacovelli, 2018 [36] | Italy | Retrospective | Patients with metastatic ccRCC (N = 182) | Axitinib (n = 103) Everolimus (n = 79) | 2L | 1L: Sunitinib (n = 182) | Outcomes: PFS, OS |
| Kang, 2023 [37] | Korea | Retrospective | Patients with mRCC (N = 3247) | Axitinib (n = 773) Everolimus (n = 2198) Cabozantinib (n = 276) | 2L | 1L: Sunitinib (n = 1787) Pazopanib (n = 1460) | Outcome: OS |
| Tamada, 2018 [38] | Japan | Retrospective | Patients with mRCC (N = 83) | Axitinib (n = 52) Everolimus or temsirolimus (n = 31) | 2L | 1L: Sunitinib (n = 83) | Outcomes: OS, PFS, time to treatment failure |
3.3. Summary of Evidence from Clinical and Observational Studies
3.3.1. Progression-Free Survival
3.3.2. Overall Survival
3.3.3. Response Rates
3.3.4. Safety—Treatment Discontinuation Due to Adverse Events
3.3.5. Dose Reductions and Dose Escalations
3.3.6. Health-Related Quality of Life
3.4. Study Quality Appraisal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of renal cell carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- van Laar, S.; Gombert-Handoko, K.; Groenwold, R.; van der Hulle, T.; Visser, L.E.; Houtsma, D.; Guchelaar, H.J.; Zwaveling, J. Real-world metastatic renal cell carcinoma treatment patterns and clinical outcomes in The Netherlands. Front. Pharmacol. 2022, 13, 803935. [Google Scholar] [CrossRef]
- McDermott, D.F.; Regan, M.M.; Clark, J.I.; Flaherty, L.E.; Weiss, G.R.; Logan, T.F.; Kirkwood, J.M.; Gordon, M.S.; Sosman, J.A.; Ernstoff, M.S.; et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2005, 23, 133–141. [Google Scholar] [CrossRef]
- Negrier, S.; Escudier, B.; Lasset, C.; Douillard, J.Y.; Savary, J.; Chevreau, C.; Ravaud, A.; Mercatello, A.; Peny, J.; Mousseau, M.; et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N. Engl. J. Med. 1998, 338, 1272–1278. [Google Scholar] [CrossRef]
- Powles, T.; Albiges, L.; Bex, A.; Grünwald, V.; Porta, C.; Procopio, G.; Schmidinger, M.; Suárez, C.; De Velasco, G.; ESMO Guidelines Committee. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann. Oncol. 2021, 32, 1511–1519. [Google Scholar] [CrossRef]
- Gross-Goupil, M.; François, L.; Quivy, A.; Ravaud, A. Axitinib: A review of its safety and efficacy in the treatment of adults with advanced renal cell carcinoma. Clin. Med. Insights Oncol. 2013, 7, 269–277. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Inlyta. Summary of Product Characteristics. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/inlyta-epar-product-information_en.pdf (accessed on 6 May 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Published September 2020. Available online: https://training.cochrane.org/handbook/current (accessed on 6 May 2024).
- National Institute for Health Research (NIHR). PROSPERO: International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 6 May 2024).
- American Society of Clinical Oncology. ASCO Meetings. Available online: https://conferences.asco.org/ (accessed on 6 May 2024).
- American Urological Association. American Urological Association—Annual Meeting. Available online: https://www.auanet.org/ (accessed on 6 May 2024).
- European Association of Urology. Education and Events. Available online: https://uroweb.org/education-events/events (accessed on 6 May 2024).
- European Society for Medical Oncology. Past Meetings. Available online: https://www.esmo.org/meeting-calendar/past-meetings?page=2 (accessed on 6 May 2024).
- Kidney Cancer Association. International Kidney Cancer Symposium. Available online: https://www.kidneycancer.org/ikcs/ (accessed on 6 May 2024).
- Society of Immunotherapy of Cancer. Society of Immunotherapy of Cancer—Abstracts. Available online: https://www.sitcancer.org/2023/home (accessed on 6 May 2024).
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare. 2009. Available online: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed on 6 May 2024).
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Trac, M.H.; McArthur, E.; Jandoc, R.; Dixon, S.N.; Nash, D.M.; Hackam, D.G.; Garg, A.X. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ 2016, 188, E120–E129. [Google Scholar] [CrossRef] [PubMed]
- van Raath, M.I.; Chohan, S.; Wolkerstorfer, A.; van der Horst, C.M.; Limpens, J.; Huang, X.; Ding, B.; Storm, G.; van der Hulst, R.R.; Heger, M. Clinical outcome measures and scoring systems used in prospective studies of port wine stains: A systematic review. PLoS ONE 2020, 15, e0235657. [Google Scholar] [CrossRef]
- Farley, J.B.; Barrett, L.M.; Keogh, J.W.; Woods, C.T.; Milne, N. The relationship between physical fitness attributes and sports injury in female, team ball sport players: A systematic review. Sports Med. Open 2020, 6, 45. [Google Scholar] [CrossRef]
- Eng, J.; Teasell, R.; Miller, W.; Wolfe, D.; Townson, A.; Aubut, J.A.; Abramson, C.; Hsieh, J.; Connolly, S.; Konnyu, K. Spinal cord injury rehabilitation evidence: Method of the SCIRE systematic review. Top. Spinal Cord Inj. Rehabil. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Escudier, B.; Tomczak, P.; Kaprin, A.; Szczylik, C.; Hutson, T.E.; Michaelson, M.D.; Gorbunova, V.A.; Gore, M.E.; Rusakov, I.G.; et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011, 378, 1931–1939. [Google Scholar] [CrossRef]
- Bracarda, S.; Bamias, A.; Casper, J.; Negrier, S.; Sella, A.; Staehler, M.; Tarazi, J.; Felici, A.; Rosbrook, B.; Jardinaud-Lopez, M.; et al. Is axitinib still a valid option for mRCC in the second-line setting? Prognostic factor analyses from the AXIS trial. Clin. Genitourin. Cancer 2019, 17, e689–e703. [Google Scholar] [CrossRef]
- Cella, D.; Escudier, B.; Rini, B.; Chen, C.; Bhattacharyya, H.; Tarazi, J.; Rosbrook, B.; Kim, S.; Motzer, R. Patient-reported outcomes for axitinib vs sorafenib in metastatic renal cell carcinoma: Phase III (AXIS) trial. Br. J. Cancer 2013, 108, 1571–1578. [Google Scholar] [CrossRef]
- Ueda, T.; Uemura, H.; Tomita, Y.; Tsukamoto, T.; Kanayama, H.; Shinohara, N.; Tarazi, J.; Chen, C.; Kim, S.; Ozono, S.; et al. Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: Subgroup analysis of Japanese patients from the global randomized phase 3 AXIS trial. Jpn. J. Clin. Oncol. 2013, 43, 616–628. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; Tomczak, P.; Hutson, T.E.; Michaelson, M.D.; Negrier, S.; Oudard, S.; Gore, M.E.; Tarazi, J.; Hariharan, S.; et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013, 14, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Kadono, Y.; Konaka, H.; Nohara, T.; Izumi, K.; Anai, S.; Fujimoto, K.; Koguchi, T.; Ishibashi, K.; Kawai, N.; Nakane, K.; et al. Efficacy and safety of first-line cytokines versus sunitinib and second-line axitinib for patients with metastatic renal cell carcinoma (ESCAPE study): A phase III, randomized, sequential open-label study. Cancers 2023, 15, 2745. [Google Scholar] [CrossRef]
- Eto, M.; Uemura, H.; Tomita, Y.; Kanayama, H.; Shinohara, N.; Kamei, Y.; Fujii, Y.; Umeyama, Y.; Ozono, S.; Naito, S.; et al. Overall survival and final efficacy and safety results from a Japanese phase II study of axitinib in cytokine-refractory metastatic renal cell carcinoma. Cancer Sci. 2014, 105, 1576–1583. [Google Scholar] [CrossRef]
- Rini, B.I.; Wilding, G.; Hudes, G.; Stadler, W.M.; Kim, S.; Tarazi, J.; Rosbrook, B.; Trask, P.C.; Wood, L.; Dutcher, J.P. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J. Clin. Oncol. 2009, 27, 4462–4468. [Google Scholar] [CrossRef]
- Rixe, O.; Bukowski, R.M.; Michaelson, M.D.; Wilding, G.; Hudes, G.R.; Bolte, O.; Motzer, R.J.; Bycott, P.; Liau, K.F.; Freddo, J.; et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: A phase II study. Lancet Oncol. 2007, 8, 975–984. [Google Scholar] [CrossRef]
- Cesas, A.; Urbonas, V.; Tulyte, S.; Janciauskiene, R.; Liutkauskiene, S.; Grabauskyte, I.; Gaidamavicius, I. Sequential treatment of metastatic renal cell carcinoma patients after first-line vascular endothelial growth factor targeted therapy in a real-world setting: Epidemiologic, noninterventional, retrospective–prospective cohort multicentre study. J. Cancer Res. Clin. Oncol. 2023, 149, 6979–6988. [Google Scholar] [CrossRef]
- Facchini, G.; Rossetti, S.; Berretta, M.; Cavaliere, C.; Scagliarini, S.; Vitale, M.G.; Ciccarese, C.; Di Lorenzo, G.; Palesandro, E.; Conteduca, V.; et al. Second line therapy with axitinib after only prior sunitinib in metastatic renal cell cancer: Italian multicenter real world SAX study final results. J. Transl. Med. 2019, 17, 296. [Google Scholar] [CrossRef]
- Géczi, L.; Bodoky, G.; Rokszin, G.; Fábián, I.; Torday, L. Survival benefits of second-line axitinib versus everolimus after first line sunitinib treatment in metastatic renal cell carcinoma. Pathol. Oncol. Res. 2020, 26, 2201–2207. [Google Scholar] [CrossRef]
- Iacovelli, R.; Rocca, M.C.; Galli, L.; Sabbatini, R.; De Giorgi, U.; Santini, D.; Facchini, G.; Mosca, A.; Atzori, F.; Zucali, P.; et al. The outcome to axitinib or everolimus after sunitinib in metastatic renal cell carcinoma. Anticancer Drugs 2018, 29, 705–709. [Google Scholar] [CrossRef]
- Kang, D.H.; Lee, J.Y.; Lee, Y.; Ha, U.S. Optimal sequencing of the first- and second-line target therapies in metastatic renal cell carcinoma: Based on nationally representative data analysis from the Korean National Health Insurance System. BMC Cancer 2023, 23, 483. [Google Scholar] [CrossRef]
- Tamada, S.; Iguchi, T.; Kato, M.; Yasuda, S.; Yamasaki, T.; Nakatani, T. Second-line treatment after sunitinib therapy in patients with renal cell carcinoma: A comparison of axitinib and mammalian target of rapamycin inhibitors. Oncotarget 2018, 9, 37017–37025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Aniello, C.; Vitale, M.G.; Farnesi, A.; Calvetti, L.; Laterza, M.M.; Cavaliere, C.; Della Pepa, C.; Conteduca, V.; Crispo, A.; De Vita, F.; et al. Axitinib after sunitinib in metastatic renal cancer: Preliminary results from Italian “real-world” SAX study. Front. Pharmacol. 2016, 7, 331. [Google Scholar] [CrossRef] [PubMed]
| PICOS | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Adult patients (≥18 years of age) with aRCC who have failed prior treatment with cytokines, TKI monotherapy, TKI-ICI combination, ICI monotherapy, or ICI combination therapy. Patients receiving second-line, third-line, or later lines of therapy. | Adult patients with aRCC receiving first-line treatment |
| Interventions/ Comparators | Axitinib Cabozantinib Everolimus Lenvatinib + everolimus Nivolumab Pazopanib Sunitinib Tivozanib Belzutifan | Interventions not listed in the inclusion criteria |
| Outcomes | Efficacy: OS (median, landmark time-point analysis) PFS, PFS2 (median, landmark time-point analysis) Response: OR, CR, PR, duration of response Duration of treatment Time to next therapy Safety: Incidence of any grade AE Incidence of grade 3–4 AE Incidence of specific AE Incidence of discontinuation due to AE Dose intensity Incidence of dose reduction HRQoL: Disease-specific and general | Outcomes not listed in the inclusion criteria |
| Study design | Randomised controlled trials Non-randomised interventional trials Pragmatic clinical trials (randomised or non-randomised) Observational or any RWE study design | Narrative reviews Prognostic studies Case reports Commentaries and letters Consensus reports Pooled analyses, SLRs, and meta-analyses 1 |
| Other criteria | English language only Sample size > 20 participants for non-randomised interventional trials and observational studies |
| Author, Year | Median Follow-Up | Prior Treatment | Study Treatment | N | Median PFS (95% CI) |
|---|---|---|---|---|---|
| Randomised controlled trials | |||||
| Motzer, 2013 [28] AXIS (NCT00678392) | NR | 1L sunitinib | 2L axitinib | 194 | 6.5 months (5.7–7.9) |
| 2L sorafenib | 195 | 4.4 months (2.9–4.7) | |||
| 1L cytokines | 2L axitinib | 126 | 12.2 months (10.2–15.5) | ||
| 2L sorafenib | 125 | 8.2 months (6.6–9.5) | |||
| Kadono, 2023 [29] ESCAPE (UMIN000012522) | 1L sunitinib | 2L axitinib | 5 | 3.7 months (0.0–8.2) | |
| 3 years | 1L cytokines | 2L axitinib | 13 | 14.7 months (2.5–26.9) | |
| Single-arm clinical trials | |||||
| Eto, 2014 [30] (NCT00569946) | NR | 1L or 2L cytokines | ≥2L axitinib | 64 | 11 months (9.2–12.0) |
| Rini, 2009 [31] (NCT00282048) | 22.7 months | Sunitinib + sorafenib | 3L axitinib | 14 | 7.1 months (3.9–7.6) |
| Cytokines + sorafenib | 3L axitinib | 29 | 9.1 months (7.1–21.4) | ||
| Rixe, 2007 [32] | 31 months | 1L cytokines | 2L axitinib | 52 | 15.7 months (8.4–23.4) |
| Observational studies | |||||
| Cesas, 2023 [33] | 29.26 months | 1L sunitinib | IMCD risk favourable: | PFS2 | |
| 2L cabozantinib | 4 | 27.15 months | |||
| 2L nivolumab | 5 | 24.5 months | |||
| 2L axitinib | 17 | 33.18 months | |||
| IMCD risk intermediate: | |||||
| 2L cabozantinib | 20 | 21.55 months | |||
| 2L nivolumab | 25 | 27.76 months | |||
| 2L axitinib | 30 | 23.19 months | |||
| IMDC risk poor: | |||||
| 2L cabozantinib | 1 | Not reached | |||
| 2L nivolumab | 7 | 11.26 months | |||
| 2L axitinib | 5 | 12.05 months | |||
| Facchini, 2019 [34] | NR | 1L sunitinib | 2L axitinib | 148 | 7.14 months (5.78–8.5) |
| 2L axitinib—patients with dose titration to 7 mg or 10 mg twice daily | 35 | 9.9 months (6.2–13.5) | |||
| 2L axitinib—patients without dose titration | 113 | 6.4 months (5.2–7.6) | |||
| Iacovelli, 2018 [36] | 50.2 months | 1L sunitinib | 2L axitinib | 103 | 5.5 months (4.3–6.7) |
| 2L everolimus | 79 | 4.6 months (2.6–6.5), p = 0.7 | |||
| Tamada, 2018 [38] | NR | 1L sunitinib | 2L axitinib | 51 | 8.7 months |
| 2L everolimus or temsirolimus | 31 | 3.4 months, p = 0.001 | |||
| Author, Year | Median Follow-Up | Prior Treatment | Study Treatment | N | Median OS (95% CI) | Landmark OS |
|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||
| Motzer 2013 [28] AXIS (NCT00678392) | NR | 1L sunitinib | 2L axitinib | 194 | 15.2 months (12.8–18.3) | NR |
| 2L sorafenib | 195 | 16.5 months (13.7–19.2) | NR | |||
| 1L cytokines | 2L axitinib | 126 | 29.4 months (24.5-NR) | NR | ||
| 2L sorafenib | 125 | 27.8 months (23.1–34.5) | NR | |||
| Single-arm clinical trials | ||||||
| Eto, 2014 [30] (NCT00569946) | NR | 1L or 2L cytokines | ≥2L axitinib | 64 | 11 months (9.2–12.0) | NR |
| Rini, 2009 [31] (NCT00282048) | 22.7 months | Sunitinib + sorafenib | ≥2L axitinib | 14 | 11.5 months (7.1–15.9) | NR |
| Cytokines + sorafenib | ≥2L axitinib | 29 | 18.5 months (8.4-NR) | NR | ||
| Rixe, 2007 [32] (NCT00076011) | 31 months | 1L cytokines | 2L axitinib | 52 | 29.9 months (20.3-NR) | 1-year: 78.8% |
| Observational studies | ||||||
| Facchini, 2019 [34,39] | NR | 1L sunitinib | 2L axitinib | 148 | 15.5 months (11–20) | NR |
| 2L axitinib with titrated dose | 35 | 19 months (15.3–22.7), p = 0.115 | NR | |||
| 2L axitinib with standard dose | 113 | 14.1 months (9.8–18.3) | NR | |||
| Géczi, 2020 [35] | NR | 1L sunitinib | 2L axitinib | 128 | 41 months | NR |
| 2L everolimus | 318 | 21.7 months, p < 0.0001 | NR | |||
| Iacovelli, 2018 [36] | 50.2 months | 1L sunitinib | 2L axitinib | 103 | 12 months (7.9–16.2) | NR |
| 2L everolimus | 79 | 13.9 months (10.4–17.4), p = 0.3 | NR | |||
| Kang, 2023 [37] | 25 months | 1L sunitinib | 2L axitinib | 300 | HR: 0.795 (0.569–1.110), p = 0.1773 | 5-year: 51.44% |
| 2L cabozantinib | 124 | Reference | 5-year: 43.59% | |||
| Tamada, 2018 [38] | NR | 1L sunitinib | 2L axitinib | 51 | 69.5 months, p = 0.034 | NR |
| 2L everolimus or temsirolimus | 31 | 33.5 months | NR | |||
| Author, Year | Median Follow-Up | Prior Treatment | Study Treatment | N | Definition of Response | Response Outcome |
|---|---|---|---|---|---|---|
| Randomised controlled trials | ||||||
| Ueda 2013 [27] AXIS (NCT00678392) | NR | 1L sunitinib | 2L axitinib | 194 | ORR | 11.3% |
| 2L sorafenib | 195 | ORR | 7.7%, p = 0.1085 | |||
| 2L axitinib | 194 | CR | 0% | |||
| 2L sorafenib | 195 | CR | 0% | |||
| 2L axitinib | 194 | PR | 11.3% | |||
| 2L sorafenib | 195 | PR | 7.7% | |||
| 1L cytokines | 2L axitinib | 126 | ORR | 32.5%, p = 0.0002 | ||
| 2L sorafenib | 125 | ORR | 13.6% | |||
| 2L axitinib | 126 | CR | 0% | |||
| 2L sorafenib | 125 | CR | 0% | |||
| 2L axitinib | 126 | PR | 32.5% | |||
| 2L sorafenib | 125 | PR | 13.6% | |||
| Kadono, 2023 [29] ESCAPE (UMIN000012522) | 3 years | 1L cytokines | 2L axitinib | 13 | ORR | 62% |
| CR | 8% | |||||
| PR | 54% | |||||
| 1L sunitinib | 2L axitinib | 5 | ORR | 0% | ||
| CR | 0% | |||||
| PR | 0% | |||||
| Single-arm clinical trials | ||||||
| Eto, 2014 [30] (NCT00569946) | NR | 1L or 2L cytokines | ≥2L axitinib | 64 | ORR | 51.6% |
| CR | 0% | |||||
| PR | 51.6% | |||||
| SD | 43.8% | |||||
| Rini, 2009 [31] (NCT00282048) | 22.7 months | Sunitinib + sorafenib | ≥2L axitinib | 14 | ORR | 7.1% |
| Cytokines + sorafenib | ≥2L axitinib | 29 | ORR | 27.6 | ||
| Rixe, 2007 [32] (NCT00076011) | 31 months | 1L cytokines | 2L axitinib | 52 | ORR | 52.6% |
| CR | 4% | |||||
| PR | 28% | |||||
| SD | 42% | |||||
| Observational studies | ||||||
| Facchini, 2019 [34,39] | NR | 1L sunitinib | 2L axitinib | 148 | ORR | 16.6% |
| CR | 0.6% | |||||
| PR | 16% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Bahl, A.; Frazer, R.; Godhania, E.; Halfpenny, N.; Hartl, K.; Heldt, D.; McGrane, J.; Şahbaz Gülser, S.; Venugopal, B.; et al. Axitinib after Treatment Failure with Sunitinib or Cytokines in Advanced Renal Cell Carcinoma—Systematic Literature Review of Clinical and Real-World Evidence. Cancers 2024, 16, 2706. https://doi.org/10.3390/cancers16152706
Sharma A, Bahl A, Frazer R, Godhania E, Halfpenny N, Hartl K, Heldt D, McGrane J, Şahbaz Gülser S, Venugopal B, et al. Axitinib after Treatment Failure with Sunitinib or Cytokines in Advanced Renal Cell Carcinoma—Systematic Literature Review of Clinical and Real-World Evidence. Cancers. 2024; 16(15):2706. https://doi.org/10.3390/cancers16152706
Chicago/Turabian StyleSharma, Anand, Amit Bahl, Ricky Frazer, Esha Godhania, Nicholas Halfpenny, Kristina Hartl, Dorothea Heldt, John McGrane, Sera Şahbaz Gülser, Balaji Venugopal, and et al. 2024. "Axitinib after Treatment Failure with Sunitinib or Cytokines in Advanced Renal Cell Carcinoma—Systematic Literature Review of Clinical and Real-World Evidence" Cancers 16, no. 15: 2706. https://doi.org/10.3390/cancers16152706
APA StyleSharma, A., Bahl, A., Frazer, R., Godhania, E., Halfpenny, N., Hartl, K., Heldt, D., McGrane, J., Şahbaz Gülser, S., Venugopal, B., Ritchie, A., & Crichton, K. (2024). Axitinib after Treatment Failure with Sunitinib or Cytokines in Advanced Renal Cell Carcinoma—Systematic Literature Review of Clinical and Real-World Evidence. Cancers, 16(15), 2706. https://doi.org/10.3390/cancers16152706





