Simple Summary
This study explored the prognostic significance of the pontine-white matter (PW) score in primary central nervous system (CNS) lymphoma patients with post-treatment 18F-FDG PET/CT and PET/MR imaging. Eligible patients were enrolled from January 2014 to December 2022. The PW score, derived from FDG uptake of the pons and white matter, was used to evaluate the metabolic activity of the treated lesion and its prognostic implications. A total of 90 patients across PET/CT and PET/MR modalities were assessed. The PW score demonstrated a robust discriminative ability in identifying patients with worse outcomes. It was also found to be a significant and independent indicator for worse prognosis in both PET/CT and PET/MR groups. The study demonstrated that this novel internal standardization indicator was an effective tool for risk stratification in primary CNS lymphoma post-treatment scenarios.
Abstract
Background: Limited data exist on the significance of PET imaging and quantitative PET parameters in primary central nervous system (CNS) lymphoma due to its relative rarity. This study was conducted to investigate the prognostic value of a novel internal standardization indicator, the pontine-white matter (PW) score, in primary CNS lymphoma patients undergoing post-treatment 18F-FDG PET/CT and PET/MR imaging. Methods: From January 2014 to December 2022, eligible patients with primary CNS lymphoma who underwent post-treatment PET imaging were enrolled. Using the FDG uptake of the pons and white matter as an internal reference, the PW score was graded based on the metabolism of the post-therapeutic lesion for each patient, and its associations with patients’ prognosis were investigated. Results: In total, 41 patients with post-treatment PET/CT and 49 patients with post-treatment PET/MR imaging were enrolled. ROC curve analysis indicated that the PW score possessed robust discriminative ability in distinguishing patients with worse outcomes. Furthermore, a higher PW score was significantly correlated with and identified as an independent prognostic indicator for, worse prognosis in both the PET/CT and PET/MR cohorts. Conclusion: The study demonstrated that the PW score was an effective prognostic indicator for identifying post-treatment primary CNS lymphoma patients with worse outcomes.
1. Introduction
Primary central nervous system (CNS) lymphoma is a rare but aggressive type of extra-nodal non-Hodgkin lymphoma that is confined to the CNS compartment at diagnosis. In immunocompetent patients, it accounts for about 4% of all intracranial neoplasms and 4–6% of all extra-nodal lymphomas [1,2]. Histologically, primary diffuse large B-cell lymphoma (DLBCL) of the CNS is recognized as a distinct entity in the WHO classification of lymphoid neoplasms, accounting for 90% of all primary CNS lymphomas. Occasionally, primary CNS lymphoma may also present as Burkitt lymphoma, low-grade lymphoma, or T-cell lymphoma [3,4,5]. The standard diagnostic procedure for primary CNS lymphoma involves histopathological confirmation through a stereotactic biopsy of the intracranial lesion [6].
Unlike other intracranial neoplasms, primary CNS lymphoma responds favorably to high-dose methotrexate (HD-MTX)-based chemotherapy, which is also the standard treatment recommended by clinical practice guidelines [7,8,9]. However, survival rates are still lower compared to lymphomas not involving the CNS, with only half of the patients achieving durable remissions. The prognosis of the non-responders to the first-line chemotherapy remains poor [10,11].
Although limited data have been established on the value of positron emission tomography (PET) imaging and quantitative PET parameters for CNS lymphoma, a consensus has been reached that 18F-fluorodeoxyglucose (FDG) PET/CT could serve as a practical option for the diagnosis, post-therapeutic response evaluation, and relapse monitoring of CNS lymphoma during follow-up. This utility is due to the extremely high and homogeneous FDG uptake of this type of lesion compared to other intracranial tumors, such as high-grade gliomas and metastases [12,13]. Although the cortical brain exhibits high FDG uptake, most primary CNS lymphomas are actually located in the white matter, which potentially reduces interference in the detection of these FDG-avid intracranial lesions [14,15,16]. Kawai et al. identified that high maximum standard uptake values (SUVmax) were correlated with worse progression-free survival and overall survival in univariate analyses [17]. Additionally, Yamaguchi et al. suggested that the ratio of tumor activity to normal contralateral cortex activity is a superior indicator to SUVmax for detecting primary CNS lymphoma [18]. However, the number of patients with primary CNS lymphoma in these studies was relatively small (n = 17 and n = 19, respectively), and the potentially more suitable PET imaging facility for primary CNS lymphoma [19], the PET/MR, has not yet been investigated.
The present study investigated the potential prognostic value of post-treatment 18F-FDG PET/CT and PET/MR in patients with primary CNS lymphoma. Using normal intracranial structures, the pons and white matter, as internal references, we proposed a simple visual metabolic score, the Pontine-white matter score (PW Score), as an indicator of lymphoma treatment response.
2. Materials and Methods
2.1. Cohort Selection
The study was examined and approved by the Institutional Review Board and the Medical Ethics Committee of Sun Yat-sen Cancer Center. Patients with primary CNS lymphoma admitted to our institution between January 2014 and December 2022 with end-of-treatment PET/CT or PET/MR examination were enrolled in the study. The inclusion criteria were: (1) biopsy-verified primary CNS lymphoma; (2) exclusion of systemic lymphoma and concomitant malignancy; (3) absence of leptomeningeal lesion or eye involvement; (4) exclusion of HIV infection; (5) age ≥ 15 years; (6) PET/CT or PET/MR conducted within 8 weeks after the last dose of chemotherapy. The patients with incomplete treatment and follow-up data at our institution were excluded.
2.2. PET/CT and PET/MR Imaging
All patients fasted for 6 h before 18F-FDG administration, and their blood glucose level was checked to be stable and below 200 mg/dL (11.1 mmol/L). PET/CT scans were performed with integrated PET/CT scanners (Biograph mCT, Siemens Healthcare, Henkestr, Germany, or uEXPLORER, United Imaging Healthcare, Shanghai, China), and PET/MR scans were conducted with uPMR 790 scanner (United Imaging Healthcare, Shanghai, China). Scans were conducted 60 min after 18F-FDG injection (0.1 mCi/kg or 3.7 MBq/kg body weight).
For the PET/CT imaging, CT scans of the whole body from skull to mid-thigh were obtained without contrast enhancement for attenuation correction and fusion (80–200 mAs, 120 kVp, 3 mm slice thickness for the Biograph mCT scanner, and 2.89 mm slice thickness for the uEXPLORER scanner), and were reconstructed in a 512 × 512 matrix. The subsequent PET scan was conducted and reconstructed with a slice thickness of 2 mm, using the Ordered Subsets Expectation Maximization (OSEM) iterative reconstruction method.
For the PET/MR scan, the images were reconstructed using the OSEM algorithm, which incorporated 20 subsets and 2 iterations, along with point spread function (PSF) and time-of-flight (TOF) modeling. The resulting matrix was 256 × 256 × 113, with each voxel measuring 2.4 × 2.4 × 2.85 mm3. The MR imaging protocols encompassed T1-weighted spin-echo sequence, T2-weighted imaging, fluid-attenuated inversion recovery (FLAIR) imaging, diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) mapping.
2.3. Image Analysis
The image analysis of the PET/CT and PET/MR images was conducted by two experienced nuclear medicine physicians through visual and semiquantitative evaluation, focusing on predefined regions of interest (ROIs). The ROIs were selected to include areas of lesions as well as reference regions including the white matter and pons for comparative purposes. The PW score was graded according to the following principle: if the lesion’s uptake was greater than that of the pons, it was scored as 2 points; if the lesion’s uptake was between that of the pons and the white matter, it was scored as 1 point; and if the lesion’s uptake was less than that of the white matter, it was scored as 0 points.
2.4. Statistical Analysis
Statistical analyses were conducted using SPSS Statistics version 22.0 (IBM Corp., Chicago, IL, USA). Receiver operating characteristic (ROC) analyses were conducted using 2-year disease progression and overall survival as end-points, and the corresponding areas under the curves (AUCs) were calculated. The survival curves were generated using the Kaplan–Meier analysis and evaluated with the log-rank test. Indicators with p < 0.1 in the univariate analysis were included in the multivariate analysis. Univariate and multivariate analyses for progression-free survival (PFS) and overall survival (OS) were performed using the Cox regression model to identify independent prognostic indicators.
3. Results
3.1. Patient Characteristics
A total of 41 patients were enrolled in the PET/CT cohort and 49 patients were enrolled in the PET/MR cohort. The characteristics of the patients are summarized in Table 1. The PET/CT cohort had 21 male patients (51.2%), and 19 patients (46.3%) with age over 60. The PET/MR had 26 male patients (53.1%), and 29 patients (59.2%) with age over 60. Multiple lesions were presented in 22 patients (53.7%) of the PET/CT cohort, and 31 patients (63.3%) of the PET/MR cohort. The ECGO score was 0–1 point for 20 patients (48.8%) in the PET/CT cohort and 27 patients (55.1%) in the PET/MR cohort. All the patients received high-dose methotrexate based chemotherapy, whole-brain radiotherapy was given in 19 patients (46.3%) of the PET/CT cohort and 7 patients (14.3%) of the PET/MR cohort, and autologous stem cell transplantation (ASCT) was given in 7 patients (17.1%) of the PET/CT cohort and 10 patients (20.4%) of the PET/MR cohort. In total, 28 patients (68.3%), 8 patients (19.5%) and 5 patients (12.2%) had the PW score of 0, 1 and 2 points, respectively; while 30 patients (61.2%), 15 patients (30.6%) and 4 patients (8.2%) had the PW score of 0, 1 and 2 points, respectively.

Table 1.
Patient characteristics.
3.2. ROC Curve Analysis
ROC curves were generated to evaluate the discriminatory ability of the PW score for 2-year disease progression and overall survival. As shown in Figure 1, the AUC values for 2-year disease progression were 0.88 (95% confidence interval (CI) 0.74–0.96; p < 0.001) in the PET/CT cohort and 0.86 (95% CI 0.73–0.94; p = 0.001) in the PET/MR cohort. For 2-year overall survival, the AUC values were 0.90 (95% CI 0.76–0.97; p < 0.001) in the PET/CT cohort and 0.69 (95% CI 0.54–0.81; p = 0.338) in the PET/MR cohort.
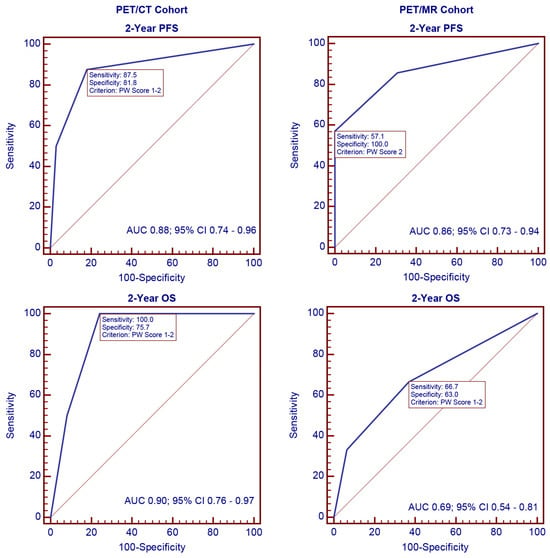
Figure 1.
ROC analysis of the PW score for 2-year disease progression and overall survival.
3.3. Survival Curve Analysis for PFS in PET/CT and PET/MR Cohorts
During the follow-up period, disease progression occurred in 12 patients (29.3%) and 8 patients (16.3%) of the PET/CT and PET/MR cohorts, respectively. The survival curves for PFS according to the PW scores are shown in Figure 2. In general, worse PFS was correlated with higher PW scores (Figure 2a,b, p < 0.001). When the 3-point scale was categorized into binary subgroups, PW score 2 was significantly associated with worse PFS in the PET/CT cohort (Figure 2c, p < 0.001) and PET/MR cohort (Figure 2d, p < 0.001) compared with PW score 0–1. Similarly, patients with PW score 1–2 had significantly worse PFS in the PET/CT cohort (Figure 2e, p < 0.001) and PET/MR cohort (Figure 2f, p = 0.004) than those with PW score 0.
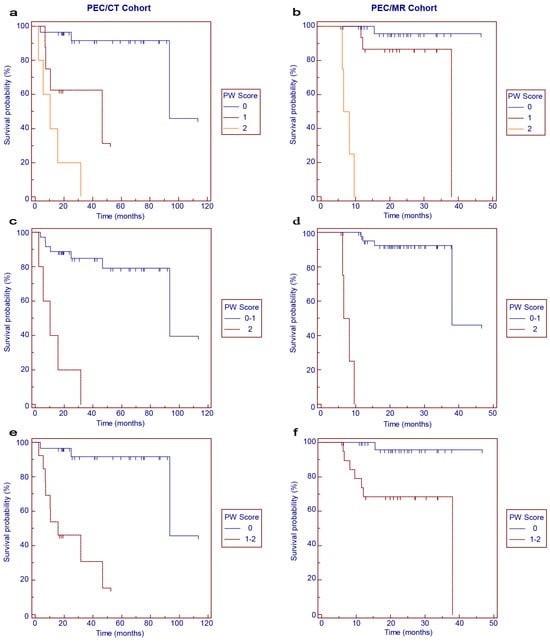
Figure 2.
Kaplan–Meier survival curves for progression-free survival in the PET/CT (a,c,e) and PET/MR (b,d,f) cohorts according to PW scores.
3.4. Survival Curve Analysis for OS in PET/CT and PET/MR Cohorts
The survival curves for OS according to the PW scores are shown in Figure 3. In the PET/CT cohort, worse OS was correlated with higher PW scores (Figure 3a, p < 0.001). When the 3-point scale was categorized into binary subgroups, PW score 2 was significantly associated with worse OS than PW score 0–1 (Figure 3c, p < 0.001), and PW score 1–2 was significantly associated with worse OS than PW score 0 (Figure 3e, p < 0.001). In the PET/MR cohort, a trend of worse OS associated with higher PW scores could be observed, but the significance was not reached (Figure 3b,d,f).
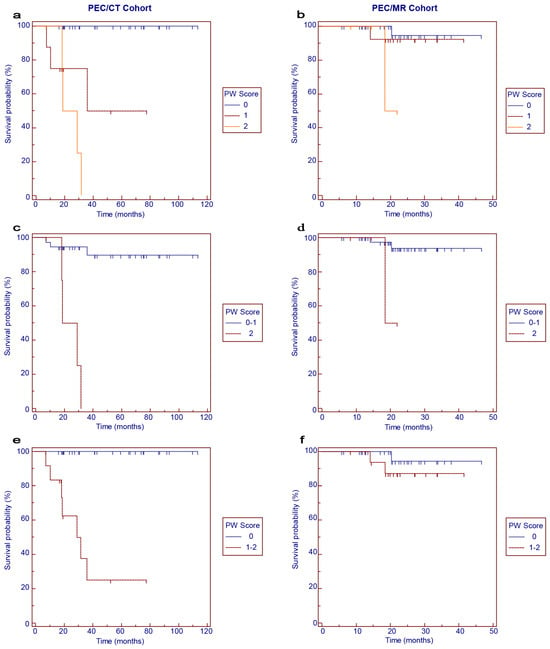
Figure 3.
Kaplan–Meier survival curves for overall survival in the PET/CT (a,c,e) and PET/MR (b,d,f) cohorts according to PW scores.
3.5. Univariate and Multivariate Analysis for PFS and OS in PET/CT and PET/MR Cohorts
The univariate analysis for PFS in the PET/CT and PET/MR cohorts was presented in Table 2. The PW score was significantly correlated with worse PFS in the PET/CT cohort (hazard ratio (HR) 5.34, p < 0.001), while radiotherapy (HR 5.53, p = 0.026) and the PW score (HR 32.80, p = 0.001) was significantly associated with worse PFS in the PET/MR cohort.

Table 2.
Univariate analysis for progression-free survival in PET/CT and PET/MR cohorts.
The univariate analysis for OS in the PET/CT and PET/MR cohorts was presented in Table 3. The PW score was significantly correlated with worse OS in the PET/CT cohort (HR 10.20, p = 0.001), and no indicator was found to be significantly associated with worse OS in the PET/MR cohort.

Table 3.
Univariate analysis for overall survival in PET/CT and PET/MR cohorts.
The results of the multivariate analysis are shown in Table 4. For PFS, the PW score was identified as the independent prognostic indicator in the PET/CT cohort (HR 5.34, p < 0.001) and PET/MR cohort (HR 25.66, p = 0.009). For OS, the PW score was identified as the independent prognostic indicator in the PET/CT cohort (HR 9.01, p = 0.001), and no significant indicator was identified in the PET/MR cohort.

Table 4.
Multivariate analysis for progression-free survival and overall survival in PET/CT and PET/MR cohorts.
4. Discussion
In this study, we first introduced the PW score as a novel and effective prognostic indicator in patients with primary CNS lymphoma receiving post-treatment PET/CT imaging. Additionally, we validated its effectiveness in predicting disease progression within the PET/MR cohort.
Due to the enhanced soft tissue resolution and multiple imaging sequences of MR, the International Primary CNS Lymphoma Collaborative Group (IPCG) has recommended MR as an essential imaging modality for the treatment of primary CNS lymphoma [20]. Although 18F-FDG PET/MR use in primary CNS lymphoma has yet to be systematically investigated, it is reasonable to infer that PET/MR imaging, which integrates the strengths of both PET and MR, will significantly impact the management of these patients. The imaging analysis focused on white matter, providing detailed insights into uptake patterns relevant to primary CNS lymphoma. Since MR scans generally take longer to complete than CT scans, in clinical practice, PET/MR is often used for localized scans rather than the whole-body scans typically performed in PET/CT. The PW score, instead of referencing extracranial structures requiring whole-body PET imaging, relies on FDG uptake in the pons and white matter. These regions can be captured simultaneously in a regional PET scan of the head, making this method particularly suitable for PET/MR imaging that targets specific areas.
The utility of the PW score necessitates a consistent physiological metabolism in normal intracranial structures to ensure comparability among individuals. Sprinz et al. demonstrated that higher blood glucose levels significantly reduce FDG uptake in the brain [21], and that the time interval can also impact FDG uptake in normal organs [22]. Moreover, cerebral glucose metabolism in normal individuals can be affected by recent administration of chemotherapy, caffeine, alcohol, amphetamines, cocaine, anesthetics, benzodiazepines, and other psychotropic drugs [23,24,25]. In our cohorts, blood glucose levels were strictly monitored before each scan, excluding patients with unsuitable glucose levels from PET imaging. Additionally, other factors such as the time interval and resting period before the PET scan were controlled according to clinical routine, and recent use of specific drugs was checked, ensuring that the FDG uptake of the referenced structures was minimally influenced by these physiological and pharmacological factors.
Although SUVs and metabolic tumor burden measured on 18F-FDG PET/CT have been recognized as prognostic indicators in patients with systemic lymphoma, data on primary CNS lymphoma remain limited and primarily explored retrospectively in a few studies. Albano et al. found an inverse correlation between higher metabolic tumor burden on pre-treatment 18F-FDG PET/CT and prognosis in a cohort of 52 primary CNS lymphoma patients [15]. In another study involving 53 primary CNS lymphoma patients receiving HD-MTX and ibrutinib combination therapy, Kerbs et al. observed that higher metabolic parameters significantly correlated with worse prognosis, with the sum of SUVmax emerging as a robust independent indicator [13]. On the other hand, Kasenda et al. suggested that measurement through internal standardization (using a reference region) would be a more robust approach and better suited for inter-individual comparison in primary CNS lymphoma patients than using SUVs [26]. This is because SUV calculations can be cumbersome and potentially error-prone due to factors such as variations in injected dose calculations, body weight assessments, and definitions of ROIs.
The ratio of tumor to normal contralateral cortex (T/N ratio) has been implicated as a method of internal standardization in the diagnosis of CNS lymphoma compared to other malignant brain tumors, which is more reliable than SUVmax for differential diagnosis [18]. Additionally, this ratio is minimally influenced by individual factors such as plasma glucose level, age, body weight, and dosage level. Similarly, another semiquantitative visual rating scale, which uses the physiological FDG uptake of the cerebellum as the baseline reference region and rates the metabolism of CNS lymphoma lesions on a 10-point scale linearly related to SUVmax, showed a significant inverse correlation between a rating over 3 points and patient prognosis [26]. In our study, the PW score, based on the FDG uptake of the pontine and white matter, was significantly associated with a worse prognosis in both PET/CT and PET/MR cohorts. Furthermore, whether the 3-point PW score was categorized by a cutoff of PW0–1/2 or PW0/1–2, it demonstrated favorable discriminative ability among groups of primary CNS lymphoma patients, indicating its potential usability in clinical applications.
Our study had some limitations. Firstly, it included only primary CNS patients with the histological type of aggressive B-cell lymphoma, which constitutes the majority of all primary CNS lymphomas. The applicability of the PW score to other histological types of CNS lymphoma remains to be further investigated. Secondly, the use of PET/CT or PET/MR scans may lead to the preferential inclusion of patients who are more compliant and financially stable. Thirdly, although this is currently the largest study investigating the role of 18F-FDG PET imaging in primary CNS lymphoma, the sample size remains small due to the rarity of the disease. The results should be interpreted with caution and require further validation.
5. Conclusions
In conclusion, the study demonstrated that the PW score was a novel and effective prognostic indicator for patients with primary CNS lymphoma receiving post-treatment PET/CT, which also exhibited satisfactory discriminatory ability in predicting disease progression in those undergoing post-treatment PET/MR.
Author Contributions
Y.L., Y.M. and X.Z. contributed to manuscript conceptualization. Y.L., Y.M., M.C. and S.L. contributed to data gathering, methodology and statistical analysis. Y.L., Y.M., M.C. and W.Z. drafted and made revisions of the manuscript. X.Z. guided and supervised the implementation of this study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethics Committee approval was also obtained from the Ethics Committee of Sun Yat-sen University Cancer Center (approval number: SL-B2023-691-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carrabba, M.G.; Reni, M.; Foppoli, M.; Chiara, A.; Franzin, A.; Politi, L.S.; Villa, E.; Ciceri, F.; Ferreri, A.J. Treatment approaches for primary CNS lymphomas. Expert Opin. Pharmacother. 2010, 11, 1263–1276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.S.; Ostrom, Q.T.; Gittleman, H.; Kruchko, C.; DeAngelis, L.M.; Barnholtz-Sloan, J.S.; Grommes, C. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro-Oncology 2018, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Verdin, I.; Kirasic, E.; Wienand, K.; Mokhtari, K.; Eimer, S.; Loiseau, H.; Rousseau, A.; Paillassa, J.; Ahle, G.; Lerintiu, F.; et al. Molecular and clinical diversity in primary central nervous system lymphoma. Ann. Oncol. 2023, 34, 186–199. [Google Scholar] [CrossRef]
- Alcantara, M.; Fuentealba, J.; Soussain, C. Emerging Landscape of Immunotherapy for Primary Central Nervous System Lymphoma. Cancers 2021, 13, 5061. [Google Scholar] [CrossRef]
- Calimeri, T.; Steffanoni, S.; Gagliardi, F.; Chiara, A.; Ferreri, A.J.M. How we treat primary central nervous system lymphoma. ESMO Open 2021, 6, 100213. [Google Scholar] [CrossRef]
- Schaff, L.R.; Grommes, C. Primary central nervous system lymphoma. Blood 2022, 140, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Wang, Y.; Chang, Q.; Wu, J.; Wang, Z.; Geng, D.; Yu, J.T.; Li, Y.; Li, X.Q.; et al. Evidence-based expert consensus on the management of primary central nervous system lymphoma in China. J. Hematol. Oncol. 2022, 15, 136. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Rudà, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef]
- Tateishi, K.; Miyake, Y.; Nakamura, T.; Yamamoto, T. Primary central nervous system lymphoma: Clinicopathological and genomic insights for therapeutic development. Brain Tumor Pathol. 2021, 38, 173–182. [Google Scholar] [CrossRef]
- Grommes, C.; DeAngelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Rozenblum, L.; Houillier, C.; Soussain, C.; Bertaux, M.; Choquet, S.; Galanaud, D.; Hoang-Xuan, K.; Kas, A. Role of Positron Emission Tomography in Primary Central Nervous System Lymphoma. Cancers 2022, 14, 4071. [Google Scholar] [CrossRef] [PubMed]
- Krebs, S.; Mauguen, A.; Yildirim, O.; Hatzoglou, V.; Francis, J.H.; Schaff, L.R.; Mellinghoff, I.K.; Schöder, H.; Grommes, C. Prognostic value of [18F]FDG PET/CT in patients with CNS lymphoma receiving ibrutinib-based therapies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3940–3950. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Bosio, G.; Bertoli, M.; Giubbini, R.; Bertagna, F. 18F-FDG PET/CT in primary brain lymphoma. J. Neurooncol. 2018, 136, 577–583. [Google Scholar] [CrossRef]
- Albano, D.; Bertoli, M.; Battistotti, M.; Rodella, C.; Statuto, M.; Giubbini, R.; Bertagna, F. Prognostic role of pretreatment 18F-FDG PET/CT in primary brain lymphoma. Ann. Nucl. Med. 2018, 32, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Bertaux, M.; Houillier, C.; Edeline, V.; Habert, M.O.; Mokhtari, K.; Giron, A.; Bergeret, S.; Hoang-Xuan, K.; Cassoux, N.; Touitou, V.; et al. Use of FDG-PET/CT for systemic assessment of suspected primary central nervous system lymphoma: A LOC study. J. Neurooncol. 2020, 148, 343–352. [Google Scholar] [CrossRef]
- Kawai, N.; Zhen, H.N.; Miyake, K.; Yamamaoto, Y.; Nishiyama, Y.; Tamiya, T. Prognostic value of pretreatment 18F-FDG PET in patients with primary central nervous system lymphoma: SUV-based assessment. J. Neurooncol. 2010, 100, 225–232. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Hirata, K.; Kobayashi, H.; Shiga, T.; Manabe, O.; Kobayashi, K.; Motegi, H.; Terasaka, S.; Houkin, K. The diagnostic role of (18)F-FDG PET for primary central nervous system lymphoma. Ann. Nucl. Med. 2014, 28, 603–609. [Google Scholar] [CrossRef]
- Krebs, S.; Barasch, J.G.; Young, R.J.; Grommes, C.; Schöder, H. Positron emission tomography and magnetic resonance imaging in primary central nervous system lymphoma-a narrative review. Ann. Lymphoma 2021, 5, 15. [Google Scholar] [CrossRef]
- Barajas, R.F.; Politi, L.S.; Anzalone, N.; Schöder, H.; Fox, C.P.; Boxerman, J.L.; Kaufmann, T.J.; Quarles, C.C.; Ellingson, B.M.; Auer, D.; et al. Consensus recommendations for MRI and PET imaging of primary central nervous system lymphoma: Guideline statement from the International Primary CNS Lymphoma Collaborative Group (IPCG). Neuro-Oncology 2021, 23, 1056–1071. [Google Scholar] [CrossRef]
- Sprinz, C.; Zanon, M.; Altmayer, S.; Watte, G.; Irion, K.; Marchiori, E.; Hochhegger, B. Effects of blood glucose level on 18F fluorodeoxyglucose (18F-FDG) uptake for PET/CT in normal organs: An analysis on 5623 patients. Sci. Rep. 2018, 8, 2126. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, H.; Fan, C. Impacts of time interval on 18F-FDG uptake for PET/CT in normal organs: A systematic review. Medicine 2018, 97, e13122. [Google Scholar] [CrossRef] [PubMed]
- Berti, V.; Mosconi, L.; Pupi, A. Brain: Normal variations and benign findings in fluorodeoxyglucose-PET/computed tomography imaging. PET Clin. 2014, 9, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Aiello, M.; Cavaliere, C.; Salvatore, M. Hybrid PET/MR Imaging and Brain Connectivity. Front. Neurosci. 2016, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, D.; Palombit, A.; Castellaro, M.; Silvestri, E.; Bui, F.; Barthel, H.; Sabri, O.; Corbetta, M.; Bertoldo, A. Brain PET and functional MRI: Why simultaneously using hybrid PET/MR systems? Q. J. Nucl. Med. Mol. Imaging 2017, 61, 345–359. [Google Scholar] [CrossRef]
- Kasenda, B.; Haug, V.; Schorb, E.; Fritsch, K.; Finke, J.; Mix, M.; Hader, C.; Weber, W.A.; Illerhaus, G.; Meyer, P.T. 18F-FDG PET is an independent outcome predictor in primary central nervous system lymphoma. J. Nucl. Med. 2013, 54, 184–191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).