Simple Summary
The connection between neurodegenerative diseases (NDs) and cancer has sparked a growing interest in biomedical research. Cancer cells show alterations in proteins linked to ND (tau, amyloid-β, α-synuclein, SOD1, and TDP-43). This review offers an updated summary of the biological role of these proteins in cancer. Specifically, we explore the effects of these proteins on cancer biology and how they affect these processes. Finally, we address the challenges and opportunities of targeting these proteins in the development of new cancer treatments.
Abstract
The link between neurodegenerative diseases (NDs) and cancer has generated greater interest in biomedical research, with decades of global studies investigating neurodegenerative biomarkers in cancer to better understand possible connections. Tau, amyloid-β, α-synuclein, SOD1, TDP-43, and other proteins associated with nervous system diseases have also been identified in various types of solid and malignant tumors, suggesting a potential overlap in pathological processes. In this review, we aim to provide an overview of current evidence on the role of these proteins in cancer, specifically examining their effects on cell proliferation, apoptosis, chemoresistance, and tumor progression. Additionally, we discuss the diagnostic and therapeutic implications of this interconnection, emphasizing the importance of further research to completely comprehend the clinical implications of these proteins in tumors. Finally, we explore the challenges and opportunities in targeting these proteins for the development of new targeted anticancer therapies, providing insight into how to integrate knowledge of NDs in oncology research.
1. Introduction
Neurodegenerative disorders (NDs) and cancer are often considered to be at opposite extremes in terms of disease mechanisms [1]. While NDs, such as Alzheimer’s disease (AD), Parkinson’s (PD), and amyotrophic lateral sclerosis (ALS), are associated with faster rates of spontaneous cell loss than aging, cancer is distinguished by enhanced resistance cell death [2,3]. However, these opposite directions suggest the possibility of shared etiopathogenic mechanisms because changes to these regulatory systems could promote either the death of cells or their proliferation [4,5]. Moreover, aging, defined as the decline of physiological processes essential for survival and reproduction, is believed to be one of the main risk factors for both NDs and cancer, thus highlighting a complex and intriguing interrelation between them [6]. This relationship is further confirmed by several epidemiological studies that show a low cancer rate in patients affected by NDs and vice versa, suggesting that susceptibility to one disease may protect against the other [7,8,9,10,11,12,13]. Similarly, cancer survivors are more susceptible to developing other age-related disorders, such as osteoarthritis, stroke, non-neurodegenerative dementia, and macular degeneration [10,14]. Nevertheless, this inverse association is not consistent across all tumor types, with PD patients having an increased incidence of malignant melanoma and breast and prostate cancers [15,16,17]. This intricate interplay can also be altered by cancer treatment: some research found that chemotherapy-treated individuals who have survived breast cancer may have less connectivity and organization in their white matter compared to healthy controls [18], while other studies link chemotherapy to a decreased risk of developing AD [14].
While the molecular and cellular mechanisms driving NDs and cancer differ, both conditions underscore the vulnerability of normal cellular processes. NDs target neurons and their complex networks, disrupting communication and leading to the gradual degradation of cognitive and motor functions [19,20]. In contrast, cancer disrupts the delicate balance of cell division, apoptosis, and survival, resulting in the uncontrolled growth of aberrant cells [21,22]. It is speculated that a variety of factors and processes, such as oxidative stress, mitochondrial dysfunction, epigenetic modification, inflammation, metabolic dysregulation, and abnormal cell cycle regulation, play a combined role with varying weights on the basis of the inverse comorbidity between NDs and cancer. This could favor the development of either neurodegeneration or cancer, or both, alternatively or in mutual exclusion [23]. Among pathways and proteins dysregulated in both conditions, the expression of the p53 tumor suppressor is increased in AD and PD [24,25,26] while downregulated in the majority of cancer types [27]. Moreover, the PIN1 gene is upregulated in several tumors but downregulated in AD [28]. Recently, it has been suggested that this inverse comorbidity may be regulated by non-coding RNAs, activating specific pathways leading to one of the two clinical phenotypes [29]. Hence, exploring the relationship between NDs and cancer could provide new insights into the etiologies and potential therapeutic targets for both conditions.
Herein, this review aims to discuss recent evidence on the role of the main neurodegenerative-associated proteins in cancer development as potential prognostic and diagnostic biomarkers. The challenges and opportunities in targeting these proteins for developing new targeted anticancer drugs will also be explored.
2. A Brief Overview of the Main Neurodegenerative Diseases
The term NDs refers to a broad range of heterogeneous neurological disorders affecting the central or peripheral nervous systems and characterized by a progressive and selective process of cellular death affecting neurons [30]. Among them, AD, PD, and ALS are three of the major NDs, and their incidence and prevalence increase rapidly with age, posing substantial issues for public health systems worldwide [31,32]. However, certain disorders can be distinguished by the presence of distinctive symptoms that vary according to the region of the brain where the loss of neurons is occurring [31]. Neuronal deterioration leads to irreversible impairment of cerebral functions, which may manifest as cognitive deficits, dementia, motor alterations, and varied degrees of behavioral and psychological disturbances, depending on the kind of disease [33]. For instance, plaques of amyloid-β (Aβ) and tau tangles are linked to memory loss and cognitive deterioration in AD. PD is characterized by bradykinesia, rigidity, and dopamine-producing neuron loss. ALS causes motor neuron degeneration, which results in gradual muscular weakness and paralysis [34]. With the exception of a small number of familiar cases, the majority of NDs arise from a complex interplay of environmental, genetic, and lifestyle factors [34,35]. Collectively, neuroinflammation, oxidative stress, mitochondrial dysfunction, metabolic dysregulation, and abnormalities in protein quality regulation and degradation are key molecular factors that drive neurodegeneration, leading to chronic cellular stress and ion homeostasis imbalances, resulting in axonal and neuronal death [36,37].
Most notably, NDs almost universally share common pathophysiological mechanisms, including protein aggregation (proteinopathy) and the formation of visible inclusion bodies on a neuropathological level [38]. Aggregates and inclusion bodies are observed in surviving neurons within the brain regions undergoing neurodegenerative processes, but their composition varies according to the specific disease [39]. Various proteins are identified based on the neuropathological profile, such as Aβ and tau in AD, alpha-synuclein (a-syn) in PD, and SOD1 and TDP-43 in ALS [40] (Figure 1). However, certain proteins intended to serve as diagnostic biomarkers for a particular ND may also serve as prognostic or diagnostic predictors for other NDs. For instance, elevated levels of total tau (t-tau) and the lowered ratio of phosphorylated tau (p-tau)/t-tau in cerebrospinal fluid (CSF) have emerged as potential diagnostic biomarkers for ALS [41]. Furthermore, the correlation between high CSF t-tau levels and poor survival suggests the possible utility of this biomarker for ALS prognosis [42]. In PD patients, CSF tau levels significantly correlated with cognitive deficits: individuals with high p-tau and p-tau/Aβ42 ratios experienced a deterioration in executive and memory abilities [43]. Additionally, core AD biomarkers can also be used to monitor PD progression. In a longitudinal study, decreased levels of t-tau/Aβ42 and increased p-tau and p-tau/t-tau were found in the CSF of PD patients over a 12-year period [44]. According to Dolatshahi et al., p-tau levels in CSF were low initially but dramatically increased in the PD group during the 1-year follow-up [45]. Similarly, a substantial number of studies reported that CSF total α-syn levels tend to increase in AD patients as compared with controls, appearing to be more indicative of general neurodegeneration than of mechanisms exclusive to AD [46].
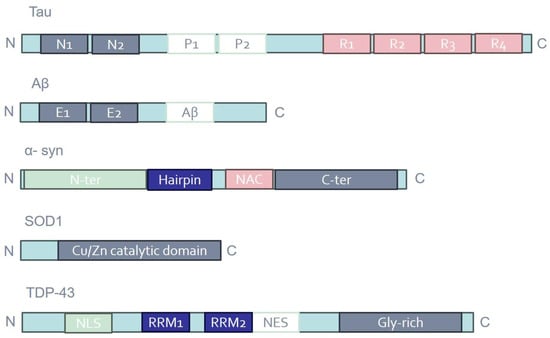
Figure 1.
Schematic representation of the domain structures of tau, Aβ, α-syn, SOD1, and TDP-43. Distinct protein domains are indicated in different colors. E1/2; extracellular domains; Gly-rich, glycine-rich region; N1/2, N-terminal regions; NAC, non-amyloid β component; NES, nuclear export signal; NLS, nuclear localization signal; P1/2, proline-rich regions; R1-4, microtubule-binding repeats; RRM1/2, RNA recognition motifs.
3. Alzheimer’s Disease (AD)
AD is an ND that progresses over time, predominantly impacting individuals in advanced age, leading to profound deterioration in the quality of life for affected patients [47]. As the disease advances, individuals with AD experience a gradual loss of memory, reasoning, and other cognitive abilities essential for daily functioning [48]. The major pathological hallmarks of AD include brain atrophy, the extracellular deposition of senile plaques composed of insoluble Aβ peptide, and the intracellular formation of neurofibrillary tangles (NFTs) constituted by hyperphosphorylated twisted filaments of the microtubule-associated protein tau in the hippocampus [49,50,51]. Additionally, the presence of these abnormal protein aggregates triggers inflammatory responses and oxidative stress, further exacerbating neuronal damage and contributing to disease progression [52]. Overall, AD represents a significant public health challenge with profound implications for affected individuals and their caregivers.
3.1. Tau
Tau is a microtubule-binding protein mainly expressed in neuronal tissue, where it plays a crucial role in cytoskeleton stabilization [53]. Beyond its role in regulating microtubules, tau also participates in other important signaling pathways, including those that control cell proliferation and differentiation, morphogenesis, and motility [54]. In the human central nervous system, six tau isoforms are produced by alternative mRNA splicing of exons 2, 3, and 10 from the microtubule-associated protein tau (MAPT) gene [55]. Among three other repeat domains (R1, R3, and R4), exon 10 encodes the second imperfect-repeat microtubule-binding domain (R2) [56]. Depending on the exclusion or inclusion of exon 10, tau proteins become either 3R or 4R tau, respectively, with an approximately 1:1 ratio in the healthy adult human brain [57]. This balance is tightly regulated developmentally, and dysregulation of 3R:4R tau isoforms is associated with the pathogenesis of several NDs [58]. Moreover, tau undergoes a variety of post-translational modifications (PTMs), such as phosphorylation, truncation, methylation, acetylation, glycosylation, nitration, and SUMOylation [59]. Among these PTMs, phosphorylation is the most common, and hyperphosphorylated tau molecules at certain sites reduce the affinity of this protein to microtubules, resulting in neuronal cytoskeleton destabilization and NFT formation [60].
Tau, initially recognized for its significant involvement in neurodegenerative conditions, has recently garnered attention as a crucial contributor to the development of cancer [61]. Aberrantly high expression of tau has been found in various types of tumors, spanning from brain malignancies such as gliomas [54,61] to solid tumors like breast [62,63,64], ovarian [65], gastric [66,67], and prostate cancer [68,69]. The abnormal expression of tau in these tumors is not necessarily linked to the progression of the pathology. For instance, elevated tau expression in a number of carcinoma types, including gliomas, breast cancer, and prostate cancer, has been associated with better patient outcomes [66,70,71], while it appears to be related to a lower prognosis in other cancer forms, like ovarian cancer [72,73].
Surprisingly, tau protein is not just exclusive to neurons; it has also been found in glial cells. Its expression in gliomas, particularly aggressive and incurable types of glial-derived brain cancer, seems to be associated with a better prognosis [61], suggesting that this protein could potentially serve as a valuable prognostic marker in cancer diagnosis and treatment planning [74]. Given its crucial role in the regulation of microtubule stability and dynamics, tau affects the behavior of tumor cells, altering their ability to proliferate and spread throughout the body [74]. Moreover, this protein appears to participate in signaling pathways that regulate the cell cycle and promote survival [75]. By modulating these pathways, tau may induce cancer cell proliferation, contributing to tumor progression and metastasis [76]. Among gliomas, glioblastoma (GBM) is the most aggressive primary brain tumor in the elderly and remains incurable, with a median survival of about 15 months. Interestingly, PTMs in GBM have been studied, suggesting that their regulation may represent a novel therapeutic approach to GBM. Apart from tau hyperphosphorylation inhibitors, other tau-regulating proteins have been identified and are currently undergoing trials for GBM in order to investigate their beneficial effects on therapy [54]. These include the PP2A protein, which promotes tau dephosphorylation; the HAT/HDAC proteins, which can acetylate or deacetylate tau; the OGT/OGA proteins, which transfer or remove GlcNAc from tau; and the HSP70 chaperone system protein, which mediates ubiquitinylation of abnormal tau species for specific degradation [54,77,78,79,80].
Patients who test negative for tau in breast cancer represent just over half of the population, accounting for approximately 49–50% [74,81]. Over the years, multiple studies have established a significant correlation between high tau expression levels and the presence of estrogen (ER)/progesterone (PR) receptors, which play a crucial role in breast cancer pathology. Tumors that exhibit positivity for both ER and tau proteins tend to respond well to hormonal therapy owing to an anomalous estrogen response element [62,82]. Furthermore, studies have shown that the administration of ER inhibitors affects tau expression primarily in cells with elevated tau levels [83,84]. Despite the fact that tau is more common in metastatic breast tumors than in other cancer types, emerging evidence suggests its critical role in enabling tumor cell reattachment through the formation of microtentacles (McTNs), which are dynamic microtubule-based extensions of the plasma membrane [85]. Significant changes in McTN frequency, morphology, and halted cell reattachment were observed with the 3R tau isoform, but future research will examine the impact of specific splice or phosphorylation variants on McTN formation [85]. Notably, breast and ovarian cancer patients receiving paclitaxel display higher tau protein levels than untreated individuals. Therefore, researchers employ elevated tau concentrations as a diagnostic marker to identify hormone-sensitive tumors that are resistant to chemotherapy [86,87,88,89].
Tau has also been found to be overexpressed in gastrointestinal stromal tumors, specifically in Auerbach’s plexus of the small intestine [66,90]. Moreover, short DNA sequences containing an unusually high abundance of CpG dinucleotides, known as CpG islands, have been recognized as crucial functional elements within the genome, governing promoter regions in vertebrates. The progression of colorectal cancer has been linked to the CpG island methylator phenotype (CIMP). In stage II colorectal cancer patients, hypermethylation of tau promoter CpG islands indicates an unfavorable prognosis and serves as a pivotal diagnostic marker for disease progression [91]. Additionally, increased phosphorylation of tau protein, which disrupts microtubule stabilization, has been documented in the SW480 and HCT 116 colorectal cancer cell lines [92]. Enhanced tau expression and phosphorylation, similar to what occurs in some NDs, are associated with the progression and drug resistance reported in various cancers [92,93].
Prostate cancer is the second most frequent cancer in males worldwide, resulting in significant morbidity and mortality. It typically progresses from normal prostate tissue to prostatic intraepithelial neoplasia before advancing to malignancy [94]. While surgical resection and radiotherapy are common treatments, metastasis often occurs upon disease recurrence. Androgen deficiency therapy is frequently employed for metastatic cases due to the crucial role of androgen signaling in tumor growth, given the expression of androgen receptors in prostate tumors [95]. Some authors found that cancerous prostate cells express hyperphosphorylated tau and multiple protein isoforms [68]. In another study, it was demonstrated that the phosphorylated tau form at residue Thr231 is restricted to the G2/M cell cycle phase [69]. According to this finding, the phosphorylation status of tau protein is a crucial marker of the G2/M phase in prostate cancer cells, and altering tau phosphorylation may impair the ability of the cell to proceed through the phase more quickly [69].
The association between tau and chemoresistance, particularly to drugs like taxanes, adds another layer of complexity. Taxanes, which are extensively used in the treatment of various tumor types such as breast, ovarian, and gastric carcinomas, exert their effects by targeting microtubules [65,93]. However, the presence of tau, which shares the same binding site on microtubules as taxanes, can confer resistance to these drugs, making treatment more challenging [96]. Overall, the emerging understanding of the multifaceted roles of tau in cancer highlights its importance as a possible target for therapeutic intervention. Further research into the precise mechanisms underpinning the involvement of tau in tumorigenesis holds promise for developing novel strategies to treat cancer and improve patient outcomes.
3.2. Aβ
As mentioned above, the Aβ protein has been associated with the formation of senile plaques in the brain, a hallmark of AD pathology [49,97]. However, the exact role of Aβ and its mechanisms of action are not yet fully understood. The Aβ derives from the sequential cleavage of the amyloid precursor protein (APP) and can be a peptide of 40 (Aβ40) or 42 (Aβ42) amino acids [98,99]. Besides differences in size, Aβ can undergo several PTMs, such as phosphorylation, oxidation, glycosylation, nitration, racemization, isomerization, and pyroglutamylation, resulting in a variety of peptides with different physiological or pathological properties [100]. Similarly, APP is also subjected to a variety of PTMs that regulate its location and trafficking throughout the cell, including phosphorylation, proteolytic processing, glycosylation, and sulfation [101].
Compared to tau protein, the exact role of Aβ in cancer is still being investigated and debated by scientists. The majority of studies have shown an elevated expression of APP in several types of tumors, including breast, pancreatic, prostate, and colon cancer, promoting cellular growth and proliferation [102,103,104,105]. Additionally, high levels of Aβ have been observed in patients affected by primary brain tumors [106,107,108]. Conversely, it has been demonstrated that non-toxic oligomers of Aβ can promote the death of tumor cells in human hematological (NB4) and solid (A549, lung, and MCF-7, breast) cancer cell lines, suggesting its potential protective role against tumors. In particular, the authors demonstrated that peptides of Aβ, particularly those with a high antiparallel β-sheet organization but a relatively low amount of β-sheet structures, are able to inhibit the growth of hematological and solid cancer cells, highlighting the importance of the structural features of amyloid species [109].
In breast cancer, it has been discovered that APP promotes tumor growth and metastasis [110,111,112]. Indeed, its overexpression is associated with increased invasiveness and the recurrence of breast cancer [113]. According to studies, APP interacts with a variety of signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway, which contributes to cancer progression [110,114]. Moreover, APP expression is influenced by androgen receptor activity and is associated with a poor prognosis in some breast cancer subtypes [115]. Inhibiting APP expression or its processing products, such as soluble amyloid precursor protein alpha (sAPPα), can reduce breast cancer cell migration and proliferation. These findings highlight the potential advantages of targeting APP-related pathways in breast cancer therapy [116].
In glioma and GBM cell lines, inflammatory markers like cyclooxygenase-2 (COX-2), cytosolic phospholipase, interleukin-1β (IL-1β), and APP have been found to be overexpressed, suggesting a possible link between inflammation and disease progression [117]. Additionally, GBM has been associated with increased mortality in individuals with AD [6,118,119]. Immunostaining studies in animal models found Aβ42 deposits in glioma tumors and blood vessels. The use of thioflavin has improved the detection of aggregated Aβ in glioma tumors [107]. Furthermore, amyloid precursor-like protein 2 (APLP2) has emerged as a relevant factor in GBM, with its involvement documented in a variety of cellular processes and its association observed with metastasis, cell proliferation, and invasion across multiple cancer types, including breast, pancreatic, lung, and colon cancer [120,121]. APLP2 was also reported to be highly expressed in pancreatic cancer cell lines, especially the APLP2 C-terminal fragment and the APLP2-modified glycosaminoglycan in relation to APP full-length and C-terminal fragments [122]. APLP2 is able to alter the actin cytoskeleton and promote pancreatic cancer growth and metastasis [121]. Studies have found a relationship between APP and androgen-responsive genes in prostate cancer [103], implicating APP in malignancy [123]. Research has shown that APP affects prostate cancer cell proliferation and migration, with higher APP levels associated with increased migratory activity and the expression of genes linked to metastasis [123].
Regarding other types of tumors, studies have been conducted on colorectal cancer, nasopharyngeal carcinoma (NPC), hepatocellular carcinoma (HCC), and non-small cell lung carcinoma (NSCLC) [113]. In colon cancer, carbamazepine (CBZ), a drug with antiepileptic properties, and valproic acid (VPA) have emerged as promising therapeutic options. Recent studies indicate that both CBZ and VPA exhibit anticancer effects by reducing APP levels in human colon cancer cells [113,124]. Concerning NPC, the expression of APP is elevated in NPC tissues, and patients treated with radiotherapy exhibit higher APP levels, raising the possibility that APP could serve as a helpful biomarker for prognosis and diagnosis in NPC patients [22]. Additionally, APP contributes to the invasion and migration of NPC cells. An in vitro study demonstrated that APP silencing significantly inhibits the epithelial–mesenchymal transition (EMT) of NPC cells through the downregulation of the MAPK signaling pathway [125]. Interestingly, the regulation of APP is mediated by histone deacetylase in HCC, suggesting the importance of epigenetic modifications in the development of cancer [126]. Finally, it has been found that APP and, in particular, its phosphorylated form are potent prognostic factors in NSCLC [127].
4. Parkinson’s Disease (PD)
PD is a chronic progressive ND that primarily affects the nervous system, leading to motor and non-motor symptoms that significantly impair patients’ quality of life [128,129,130]. The etiology of PD is complex and multifactorial, involving a combination of genetic, environmental, and neurochemical factors [129]. One of the key hallmarks of PD is the progressive degeneration of dopaminergic nerve cells in the substantia nigra of the brain, which results in dopamine loss in the striatum, a brain region involved in the control of voluntary movement [131,132]. Despite advances in understanding the pathogenic mechanisms of PD and in the development of symptomatic treatments to alleviate motor symptoms, the long-term management of the disease remains challenging, with limited therapeutic options available to slow or halt disease progression.
Alpha-syn has garnered considerable interest in PD research due to its central role in disease pathogenesis [133]. It is a protein rich in alpha-helical repeat sequences that are widely expressed in the nervous system, particularly in presynaptic neurons. Although the exact physiological function of α-syn is not fully understood, it is believed to play a role in regulating synaptic vesicle release and reuptake. However, it has become increasingly evident that α-syn is also involved in the formation of insoluble protein aggregates, known as Lewy bodies, which are a hallmark clinical feature of PD [134] (Figure 2). These protein aggregates accumulate inside nerve cells, impairing their function and causing progressive deterioration of the nervous system [135]. Numerous studies have investigated the mechanisms underlying the formation of α-syn aggregates and their role in causing neuronal death in PD [136]. A deeper understanding of these processes may lead to new therapeutic strategies aimed at preventing or slowing the progression of PD [137]. The aggregation and stability of α-syn may be influenced by several PTMs, including phosphorylation, ubiquitination, truncation, acetylation, SUMOylation nitration, O-GlcNAcylation, and C-terminal cleavage [138].
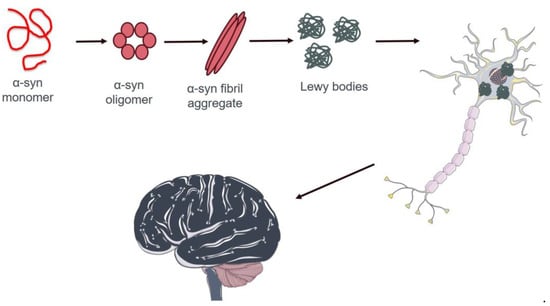
Figure 2.
Schematic representation of pathological protein aggregation of α-syn in neuron cells in the brain of patients affected by PD. Proper α-syn formation is essential for neuronal function and tissue health. In patients with PD, this protein aggregates into protein fibers known as Lewy bodies. These aberrant structures occur in the cytoplasm of neurons, causing a pathological condition that leads to severe nervous system degeneration and, ultimately, cell death. The image was created using Servier Medical Art modified templates, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com, accessed on 10 April 2024).
4.1. Alpha-Syn
In recent years, new research avenues have been pursued concerning this protein, which is typically studied in the neurological field, particularly with several studies investigating its relevance in oncological diseases. The syn family has been shown to be involved in oncogenic pathways, which accelerate cellular processes leading to cancer onset. A study demonstrated that α-syn has a role in the development of pancreatic ductal adenocarcinoma (PDAC). Researchers found that α-syn is overexpressed in 20 cases of cancer patients with two types of this tumor. However, this was more prominent in PDAC samples with perineural infiltration than in tumors without perineural infiltration [139].
The involvement of α-syn in the oncological field is not limited solely to the aforementioned tumor. Melanoma has also been found to express this protein [140,141]. In particular, researchers discovered overexpression of this abnormal protein through numerous preclinical studies. Shekoohi and colleagues demonstrated that inhibiting α-syn expression reduces the development of melanoma tumor cells [141]. This evidence suggests that α-syn may have an active role in oncogenesis rather than just being an anomalous biological phenomenon [141]. Another potential role of this marker has been elucidated: the loss of α-syn expression in skin cancer cells altered iron metabolism regulation, resulting in cells with reduced TfR1 and FPN1 and increased ferritin and DMT1 levels [141]. Overexpression of α-syn in melanomas is thought to be associated with significant alterations in autophagy. However, further investigations will be needed in this regard [142]. This protein is highly expressed in both primary and metastatic melanomas, indicating its association with these types of skin cancer. On the other hand, it is notably absent in non-melanocytic cutaneous carcinoma and healthy skin samples, suggesting a specific role for α-syn in melanoma development and progression [143]. Importantly, the Ser129 phosphorylated form of α-syn plays a role in the transition of this protein to pathogenic species not only in PD but also in melanoma, where it localizes in the extracellular space of melanoma cells [143,144].
The increase in α-syn levels plays a significant role in promoting the aggressive characteristics of meningiomas by activating the Akt/mTOR pathway. This finding suggests that targeting α-syn could offer a promising therapeutic strategy for treating malignant meningiomas [145].
4.2. Other PD-Related Proteins
In addition to α-syn, other PD-associated proteins are also involved in some types of cancer. Among them, ubiquitin carboxyl-terminal hydrolase L1 (UCHL1), also called PARK5, is expressed widely throughout the brain and plays an important role in the ubiquitin–proteasome system (UPS), functioning as a hydrolase or de-ubiquitinase enzyme [146]. Although this protein is normally expressed primarily in neurons, it not only causes NDs but also displays a complex involvement in the development and progression of cancer by acting as a tumor suppressor or oncogenic factor depending on different cancer types [147,148]. UCHL1 undergoes promoter methylation, which in turn causes the progression of many malignancies, including HCC, NPC, head and neck squamous cell, gastric, ovarian, breast, and pancreatic neuroendocrine cancers [147]. When UCHL1 expression is restored, it controls important cyclin levels (like p53), prevents cancer cells from proliferating, and promotes their apoptosis [149,150]. On the other hand, UCHL1 acts as an oncogenic factor via PI3K/Akt, MAPK/Erk, and other signaling pathways to promote the development, invasion, and metastasis of breast cancer as well as NSCLC, lymphoma, osteosarcoma, and neuroblastoma [147].
Other proteins that are involved in PD pathogenesis are DNAJ/HSP40 molecular chaperones, as evinced by genetic studies in rare familial forms of PD [151]. Through the J domain, DNAJ proteins bind to Hsp70 and promote its ATPase activity [152]. Among members of the DNAJ family, a whole transcriptomic analysis revealed an upregulation of DNAJC14 in osteosarcoma, suggesting its potential role also in cancer [148].
5. Amyotrophic Lateral Sclerosis (ALS)
ALS, also known as Lou Gehrig’s disease, is a progressive ND that primarily affects the motor neurons in the brain and spinal cord. This devastating condition leads to the gradual degeneration and death of these motor neurons, causing a progressive loss of voluntary muscle control [153,154]. As a result, individuals with ALS experience muscle weakness, twitching, and eventually paralysis. To date, more than 50 modified genes have been examined in ALS, but they are primarily linked to mutations in the SOD1, TARDBP, and FUS/TLS genes, encoding Cu/Zn superoxide dismutase-1, transactive response DNA-binding protein 43 (TDP-43), fused in sarcoma/translocated in liposarcoma, respectively [39,153]. These proteins are involved in a broad range of cellular pathways, including mitochondrial dysfunction, excitotoxicity, autophagy with protein homeostasis loss, inflammation, DNA damage repair, aberrant RNA metabolism, and impaired intracellular trafficking [155].
5.1. SOD1
In 1993, research identified ALS-associated mutations in the SOD1 gene located on chromosome 21. This gene encodes the Cu/Zn superoxide dismutase protein, a vital enzyme within the cytoplasm responsible for neutralizing superoxide radicals into hydrogen peroxide and molecular oxygen [39,156,157]. SOD1 is ubiquitously expressed, highly conserved, and accounts for approximately 1% of all proteins in the cytoplasm of cells [158]. Protein function and stability are regulated by several PTMs on key amino acid residue side chains, including phosphorylation, lysine modifications, redox modifications, and nitration [159]. Regarding the involvement of this protein in oncological pathogenesis, recent studies have shown that SOD1 is altered and overexpressed in tumors, and it appears to be able to promote the metastasis of tumor masses. Many studies have been published in the search for inhibitors in lung cancer, in particular NSCLC [160,161,162]. Somwar et al. made a significant discovery when investigating inhibitors that target molecules commonly found in tumor cells. Among them, LCS-1 was distinguished for its ability to inhibit SOD1, successfully blocking apoptosis while promoting cell growth. This finding underscored LCS-1 as a potential therapeutic candidate, particularly in counteracting tumor progression [160] (Figure 3). Furthermore, their research demonstrated that the administration of CSF-1 could effectively suppress the overexpression of SOD1, slowing tumor growth in lung cancer. Notably, their study extended beyond lung cancer to highlight LCS efficacy in combating breast cancer [160].
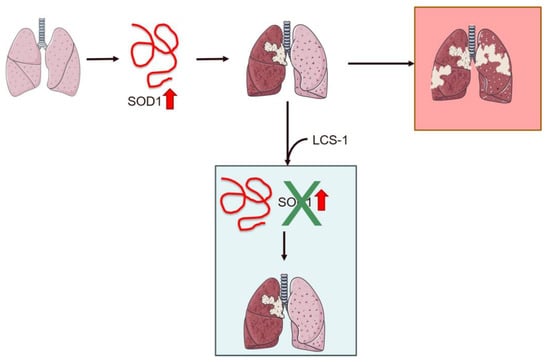
Figure 3.
Schematic representation of the role of SOD1 overexpression in lung cancer and the effect of treatment with the inhibitor LCS-1 on cancer progression. Overexpression of SOD1 promotes metastasis in NSCLC patients. Treatment with LCS-1 blocks SOD1 expression, decreasing tumor growth. The red arrow indicates SOD1 overexpression. The image was created using Servier Medical Art modified templates, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com, accessed on 10 April 2024).
Intriguingly, the role of SOD1 in oncological biology is not limited to lung cancer alone. Indeed, breast tumors also exhibit high levels of SOD1, encouraging studies into pharmacological interventions aimed at reducing its overexpression [160,163,164]. Even with this type of tumor, research has focused on the study of pharmacological molecules aimed at inhibiting the overexpression of SOD1 in breast cancer cells. In-depth investigations of erbB2 (MMTV-iErbB2) and Wnt (MMTV-Wnt) inducible transgenic breast cancer mouse models revealed a strong correlation. Inhibiting SOD1 resulted in a significant reduction in tumor proliferation, particularly evident in samples treated with LCS-1. These promising findings were further confirmed in cell line studies, highlighting the therapeutic potential of targeting SOD1 in breast cancer management [165]. The mentioned research confirms that SOD1 plays a role in tumor cell proliferation and hence represents a promising target for anti-tumor chemical compounds. The discovery that SOD1 plays a crucial role in the initiation of tumors correlates with a subsequent report that mTORC1 controls SOD1 via phosphorylation, suggesting that increased SOD1 under starvation conditions is required for enhancing cancer cell survival and contributing to chemoresistant mechanisms [166].
Along with LCS-1, clinical and preclinical studies are also investigating other inhibitory compounds, such as ATN-224, a copper chelator compound that has been examined in the field of oncology. In particular, there are clinical trials involving patients with prostate cancer. However, no clear and definitive results have yet been obtained in these studies, so further research is required to fully understand the role of SOD1 in oncological diseases. Additionally, future studies on this protein will be essential for developing new drugs useful for cancer research [161,167,168,169].
5.2. TDP-43
TDP-43 was first identified in 1995 when it was found to bind the transactivation response region (TAR) of HIV DNA, hence the name TAR DNA binding protein. Soon later, it was discovered in the human brain and various cell culture systems [39,170]. TDP-43 has proven to be a protein of significant interest due to its highly conserved nature across species and widespread presence in both human and rodent cells, where it is predominantly found in the nucleus. TDP-43, consisting of 414 amino acids and weighing 43 kDa, is the protein product of the TARDBP gene located on chromosome 1. It belongs to the heterogeneous ribonucleoprotein (hnRNP) family, which is a varied group of RNA-binding proteins [171,172,173]. Despite its ubiquitous presence, the exact cellular function of TDP-43 is unknown, although numerous studies have revealed its involvement in biological processes, including the regulation of gene transcription, modulation of splicing events, and preservation of mRNA stability [174]. Aberrant aggregation and mislocalization of TDP-43 are associated with different classes of PTMs that can influence its functionality and cellular status. Among them, phosphorylation, formation of C-terminal fragments, disulfide bridge formation, acetylation, ubiquitination, and SUMOylation are extensively investigated [175].
Delving into some research conducted studying TDP-43 in certain types of tumors, Lin and colleagues focused their study on GBM. In particular, it was found first that exogenous overexpression of TDP-43 activated autophagy and prevented stress-induced apoptosis, and that histone deacetylase 6 (HDAC6) was involved in the activation of autophagy in these cells. Following these results, an HDAC6 inhibitor, SAHA, was used, which demonstrated a significant reduction in the phenomenon activated by TDP-43 and HDAC6. Also, in this intriguing study, it emerged from experiments performed on patient biopsies that TDP-43 and HDAC6 were negatively correlated and colocalized in tumor lesion sites [176].
In addition to GBM, TDP-43 is also implicated in HCC, although its role is not yet fully understood. In one study, it was shown that TDP-43 is overexpressed in both clinical samples of patients with this condition and in HCC cell lines, resulting in changes in cell proliferation and metastasis formation. XAV939, an inhibitor of the Wnt/β-catenin signaling pathway, effectively blocked TDP-43 overexpression. This study revealed how TDP-43 may act and the signaling pathways it uses, which makes it a possible future treatment target [177].
Recently, the importance of studying TDP-43 has also emerged in skin tumors, particularly melanoma. A study revealed that TDP-43 is overexpressed in samples from patients with melanoma. This significant increase in TDP-43 has been found to be correlated with patient mortality. This study demonstrated that silencing this protein significantly inhibited cell proliferation and metastasis in the A375 and WM451 cell lines. The results confirm prior studies on different tumors, suggesting that blocking the overexpression of TDP-43 lowers cell proliferation and metastasis formation [178].
Another notable example of the involvement of this important neurological biomarker in tumors is breast cancer research. In this context, aberrant alternative splicing is recognized as a hallmark of malignancy. In 2018, TDP-43 was identified as a splicing regulator responsible for triple-negative breast cancer (TNBC). Researchers discovered an overexpression of TDP-43 in this tumor type, which leads to a poorer prognosis for TNBC. This study also demonstrated how silencing TDP43 can halt tumor progression and metastasis, whereas overexpression of TDP43 has the opposite effect, enhancing the malignancy of mammary epithelial cells [179]. Furthermore, it has been discovered that the role of TDP-43 in splicing also involves CD44. This is a hallmark of breast cancer stem cells (BCSCs), and the influence of their alternative splicing is believed to play an important role in the rapid progression of the oncological pathology [179,180]. Kim and colleagues also found that in some cases of breast cancer, the overexpression of TDP-43 is important for the apoptosis induced by TRIM16, a member of the tripartite motif protein family that acts as a potential tumor suppressor [181].
However, despite the very interesting results about the involvement of TDP-43 in tumors, there is a significant need for future research to fully understand how this typically neurological marker works in cancer.
6. Other Neurological Biomarkers in Cancer Disease
In this chapter, we will discuss additional biomarkers associated with neurological disorders that are not conventional NDs. However, some other conditions show markers that have emerged to play a significant role in the field of tumors and have been the topic of recent oncological research.
6.1. Double Homeobox 4 (DUX4)
DUX4 is the biomarker typically associated with a form of muscular dystrophy, namely facioscapulohumeral muscular dystrophy (FSHD). DUX4 is expressed in the early stages of development in stem cells and germ cell lines, while it is repressed through a methylation mechanism mediated by repeats [182,183] occurring during cellular differentiation [184] and in most somatic tissues, including muscles [182,185,186], except for the thymus [187] and keratinocytes [188]. DUX4 toxicity is related to different PTMs, such as phosphorylation, methylation, and acetylation [189].
Its overexpression has historically been linked to muscular dystrophy and other muscle changes [182,190]. However, in recent years, a number of studies have linked this marker to oncological pathologies. In 2019, a study showed alterations in blood tumor samples [191]. This was confirmed by several other reports, which found that altered DUX4 with genetic mutations could serve as a marker for the development of acute lymphoblastic leukemia [192,193,194]. It has also been demonstrated that this marker is altered in round-cell sarcoma or Ewing-like sarcomas [195]. However, in an aggressive type of sarcoma that primarily affects pediatric and young patients, the causal event is a fusion between the high mobility group (HMG) box, which contains the capicua protein (CIC), and DUX4. In a non-pathological state, CIC acts as a transcription repressor, but when fused with DUX4, it becomes a transcription activator [196,197,198]. CIC-DUX4 induces small round-cell sarcomas distinct from Ewing sarcoma. All this has led to the understanding that CIC-DUX4 can become an oncogene. However, altered DUX4 also appears to function as a tumor suppressor in synovial sarcoma and colon cancer [199,200]. However, the role of altered DUX4 expression in various types of tumors requires further specific and in-depth studies, as all studies so far are relatively recent. In the future, new evidence may emerge on how this marker acts in cancer, and these findings may also be useful in neurobiological research for the development of definitive therapies for FSHD.
6.2. Neurofilament Light Chain (NfL)
The neurofilament light chain (NfL) is an essential structural component of neurons belonging to the neurofilament family, along with the neurofilament medium chain (NfM) and the neurofilament heavy chain (NfH). These proteins are crucial for maintaining the structural integrity of axons and facilitating intracellular transport within the nervous system [201]. NfL undergoes different PTMs, including phosphorylation, O-linked glycosylation, nitration, and ubiquitination [202]. In recent years, NfL has garnered increasing attention as a potential biomarker for a wide range of neurological diseases, including AD, PD, and ALS [203]. Its relevance stems from the fact that, following neuronal injury or degeneration, NfL is released into CSF and blood, allowing its measurement as an indicator of neuronal damage [204,205]. NfL has been extensively studied in the context of neurological diseases, in particular, multiple sclerosis (MS). In patients with these conditions, elevated levels of NfL in CSF and blood have been correlated with disease severity, symptom progression, and neurological deterioration [205]. In MS, elevated levels of NfL have been associated with increased disease activity and greater brain volume loss, making it a promising biomarker for monitoring treatment response and disease progression over time [205]. Despite the potential of NfL as a biomarker, there are some challenges and limitations to consider. For example, NfL levels can vary significantly between healthy individuals and patients with neurological diseases, making it difficult to establish a universal diagnostic threshold [206,207]. Additionally, factors such as age, sex, and the presence of comorbidities can influence NfL levels and complicate the interpretation of the results. NfL continues to be the focus of intense research for its potential in early diagnosis, monitoring disease progression, and assessing treatment response in neurological diseases. Further studies are needed to refine measurement methods, better understand individual variations, and identify complementary biomarkers that can improve the specificity and accuracy of disease diagnosis and monitoring [208]. While historically studied in the context of neurological diseases, recent research has shed light on the potential involvement of NfL in cancer [209]. NfL has been identified in various types of solid tumors, suggesting a broader role beyond its traditional neurological context [210,211,212]. Efforts are focused on assessing NfL expression in tumors and its correlation with clinical and prognostic parameters [209,213]. Additionally, research is focused on identifying the molecular mechanisms underlying the regulation of NfL expression in tumors and developing new targeted therapeutic strategies that target NfL and its pro-tumorigenic effects.
Recently, a study examined the expression levels of this marker in the blood of NSCLC patients. Specifically, researchers evaluated NfL in serum from subjects with and without brain metastases to determine whether its levels were associated with metastasis development. The most interesting finding of this study was the presence of a high level of NfL in the serum prior to the patient’s diagnosis of brain metastases. Furthermore, it emerged that the level of NfL at the time of diagnosis of brain metastases was correlated with survival [214]. These results are consistent with previous studies [204,215].
NfL was also tested as a biomarker for axonal damage severity in chemotherapy-induced peripheral neuropathies. Peripheral neuropathy is a common complication of chemotherapy, with symptoms ranging from minor to severe, potentially affecting patients’ quality of life and ability to finish treatment cycles. In this context, identifying reliable biomarkers for peripheral neuropathy could be crucial for better predicting and managing this complication. Research has progressed with several studies investigating NfL levels in the blood of patients with gynecological tumors treated with paclitaxel, a routinely used chemotherapeutic drug for this type of tumor [216]. This study revealed an association between serum NfL levels and the severity of paclitaxel-induced peripheral neuropathy in patients with gynecological tumors. Higher levels of serum NfL were observed in patients who developed more severe peripheral neuropathy while receiving paclitaxel. These findings suggest that serum NfL levels could be used as a biomarker to assess the risk and severity of peripheral neuropathy in patients with gynecological tumors undergoing chemotherapy [211,212,216,217].
7. Blood-Based Biomarkers for Early Diagnosis and Surveillance of Cancer
Early diagnosis and effective monitoring of cancer have long been a priority in the field of oncology [218,219]. Traditional diagnostic methods, such as imaging and tissue biopsies, while essential, have significant limitations: they often detect cancer only after it has reached a specific size or stage, potentially delaying diagnosis [220]. Additionally, these procedures can be costly, require specialized equipment, and may not always be accessible in resource-limited settings. Tissue biopsies, while providing definitive pathological confirmation, are invasive, might cause discomfort or complications, and may not always be feasible depending on the tumor’s location or the patient’s health status [221]. In recent years, advancements in biomarker research have highlighted the potential of blood-based biomarkers as an essential resource for both early cancer detection and on-going surveillance [222]. These biomarkers offer a less invasive, more cost-effective, and potentially earlier detection method compared to traditional techniques. Blood-based biomarkers are measurable indicators found in the blood that may suggest the existence of cancer. They include proteins, nucleic acids, exosomes, and metabolites. The measurement of these biomarkers in blood samples provides a non-invasive, easily accessible approach to monitoring disease occurrence and progression, potentially offering real-time insights into the biological behavior of malignancies [223,224].
As previously stated, Aβ, tau, α-syn, SOD1, and TDP-43 are key biomarkers in NDs and have been associated with cancer. Aβ peptides can influence cellular processes such as apoptosis and inflammation [109]. Elevated levels of Aβ in the blood have been observed in some cancer patients, suggesting that this protein may serve as a biomarker for cancer diagnosis and monitoring [225]. Tau is also expressed in various malignancies, where it may impact cell structure and division. Blood-based assays for tau could thus be a useful tool for early cancer detection as well as evaluating disease progression and therapy response [61]. Alpha-syn is also found in several cancer types, where it may influence cell proliferation, metastasis, and chemoresistance [226]. The detection of α-syn in blood samples could be utilized for early cancer detection and to monitor the effectiveness of therapeutic interventions [226,227]. Concerning SOD1, measuring its levels in the blood could provide insights into the oxidative stress status of cancer cells, making this protein a possible biomarker for cancer diagnosis and monitoring [228]. Altered SOD1 expression has also been observed in various cancers, possibly contributing to tumor growth and resistance to therapy [229]. As already mentioned, studies have identified abnormal TDP-43 expression in various cancers, suggesting it may play a role in tumorigenesis. TDP-43 has the ability to bind RNA and regulate gene expression, implicating it in cellular processes such as proliferation and apoptosis [230]. The presence of TDP-43 in the blood of cancer patients could serve as a biomarker for early cancer detection and monitoring disease progression.
Future research should focus on integrating these biomarkers into clinical practice and combining them with other diagnostic tools to create a comprehensive, multi-modal approach to cancer diagnosis and surveillance. The development of standardized protocols for biomarker detection and quantification will be essential for their widespread implementation in oncology.
8. Conclusions and Future Directions
The growing evidence of the involvement of typical biomarkers of NDs in the oncological context (summarized in Table 1) opens new avenues in research and clinical practice. These proteins also play a role in many tumor-related signaling pathways leading to cell cycle progression, proliferation, invasion, epithelial–mesenchymal transition (EMT) activation, tumor growth, metastasis formation, and chemoresistance. From a translational perspective, these results offer several pertinent implications for preventive and treatment strategies for both conditions. For instance, the risk of the development of NDs and cancer will be reduced with strategies that slow aging. A healthy lifestyle and metabolism have been demonstrated to reduce the risk of cognitive impairment and cancer [231,232,233]. Related therapies are also known to decrease oxidative stress, enhance mitochondrial function, and lower inflammatory marker levels [234]. Moreover, medications that target different signs of aging are also being investigated in both fields. Metformin, a biguanide used to treat type 2 diabetes, was found to reduce the risk of dementia, cancer, and other age-related disorders [235,236] by reducing insulin and IGF-1 levels, inhibiting the mTOR pathway, blocking mitochondrial function, reducing oxidative damage, and activating the AMP kinase [237,238,239].

Table 1.
A summary of altered ND-related protein in each cancer type.
Different therapeutic strategies targeting ND biomarkers may also be used for cancer. These approaches can consist of targeting these proteins indirectly, through the regulation of post-translational modifications, or directly by inhibiting their aggregation, using active and passive immunotherapies, reducing their cell levels with antisense oligonucleotides, or inducing their clearance via the autophagy–lysosomal system [54,240].
However, it is important to note that the specific role of these biomarkers in tumors and their mechanisms of action remain subjects of ongoing study. Some connections between neurodegeneration and cancer may suggest that they should co-occur, while others could help explain the pattern of inverse comorbidity shown in epidemiologic research. This contradictory picture could potentially shed light on why certain tumors have an inverse relationship with neurodegeneration while others do not. Further research is needed to fully understand the clinical implications of this interconnection and to develop targeted diagnostic and therapeutic strategies that can harness the potential of these biomarkers in the oncological context. Additionally, considering the heterogeneity of tumors and the importance of larger and longitudinal studies to confirm and further explore present findings is essential. Ultimately, integrating knowledge of NDs into oncological research could provide important insights toward improving the management and treatment of cancer patients.
Author Contributions
E.D. and C.V. carried out the literature review, conceptualization, and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Plun-Favreau, H.; Lewis, P.A.; Hardy, J.; Martins, L.M.; Wood, N.W. Cancer and neurodegeneration: Between the devil and the deep blue sea. PLoS Genet. 2010, 6, e1001257. [Google Scholar] [CrossRef] [PubMed]
- Robles Bayón, A.; Gude Sampedro, F. New evidence of the relative protective effects of neurodegenerative diseases and cancer against each other. Neurologia 2019, 34, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Duranti, E.; Cordani, N.; Villa, C. Edaravone: A Novel Possible Drug for Cancer Treatment? Int. J. Mol. Sci. 2024, 25, 1633. [Google Scholar] [CrossRef] [PubMed]
- Staropoli, J.F. Tumorigenesis and neurodegeneration: Two sides of the same coin? Bioessays 2008, 30, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Houck, A.L.; Seddighi, S.; Driver, J.A. At the Crossroads Between Neurodegeneration and Cancer: A Review of Overlapping Biology and Its Implications. Curr. Aging Sci. 2018, 11, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Park, M. Molecular crosstalk between cancer and neurodegenerative diseases. Cell. Mol. Life Sci. 2020, 77, 2659–2680. [Google Scholar] [CrossRef] [PubMed]
- Catalá-López, F.; Crespo-Facorro, B.; Vieta, E.; Valderas, J.M.; Valencia, A.; Tabarés-Seisdedos, R. Alzheimer’s disease and cancer: Current epidemiological evidence for a mutual protection. Neuroepidemiology 2014, 42, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.A.; Beiser, A.; Au, R.; Kreger, B.E.; Splansky, G.L.; Kurth, T.; Kiel, D.P.; Lu, K.P.; Seshadri, S.; Wolf, P.A. Inverse association between cancer and Alzheimer’s disease: Results from the Framingham Heart Study. Bmj 2012, 344, e1442. [Google Scholar] [CrossRef]
- Inzelberg, R.; Jankovic, J. Are Parkinson disease patients protected from some but not all cancers? Neurology 2007, 69, 1542–1550. [Google Scholar] [CrossRef]
- Tabarés-Seisdedos, R.; Dumont, N.; Baudot, A.; Valderas, J.M.; Climent, J.; Valencia, A.; Crespo-Facorro, B.; Vieta, E.; Gómez-Beneyto, M.; Martínez, S.; et al. No paradox, no progress: Inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011, 12, 604–608. [Google Scholar] [CrossRef]
- Shi, H.B.; Tang, B.; Liu, Y.W.; Wang, X.F.; Chen, G.J. Alzheimer disease and cancer risk: A meta-analysis. J. Cancer Res. Clin. Oncol. 2015, 141, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Baade, P.D.; Fritschi, L.; Freedman, D.M. Mortality due to amyotrophic lateral sclerosis and Parkinson’s disease among melanoma patients. Neuroepidemiology 2007, 28, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.M.; Curtis, R.E.; Daugherty, S.E.; Goedert, J.J.; Kuncl, R.W.; Tucker, M.A. The association between cancer and amyotrophic lateral sclerosis. Cancer Causes Control 2013, 24, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Frain, L.; Swanson, D.; Cho, K.; Gagnon, D.; Lu, K.P.; Betensky, R.A.; Driver, J. Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimer’s Dement. 2017, 13, 1364–1370. [Google Scholar] [CrossRef]
- Walter, U.; Heilmann, E.; Voss, J.; Riedel, K.; Zhivov, A.; Schäd, S.G.; Gross, G.E.; Benecke, R.; Trcka, J. Frequency and profile of Parkinson’s disease prodromi in patients with malignant melanoma. J. Neurol. Neurosurg. Psychiatry 2016, 87, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.L.; Goldacre, R.; Goldacre, M. Differential risks of cancer types in people with Parkinson’s disease: A national record-linkage study. Eur. J. Cancer 2014, 50, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Sugier, P.E.; Lucotte, E.A.; Domenighetti, C.; Law, M.H.; Iles, M.M.; Brown, K.; Amos, C.; McKay, J.D.; Hung, R.J.; Karimi, M.; et al. Investigation of Shared Genetic Risk Factors Between Parkinson’s Disease and Cancers. Mov. Disord. 2023, 38, 604–615. [Google Scholar] [CrossRef]
- Kesler, S.R.; Watson, C.L.; Blayney, D.W. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol. Aging 2015, 36, 2429–2442. [Google Scholar] [CrossRef]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef]
- Rozpędek-Kamińska, W.; Siwecka, N.; Wawrzynkiewicz, A.; Wojtczak, R.; Pytel, D.; Diehl, J.A.; Majsterek, I. The PERK-Dependent Molecular Mechanisms as a Novel Therapeutic Target for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 2108. [Google Scholar] [CrossRef]
- Liang, W.S.; Goetz, L.H.; Schork, N.J. Assessing brain and biological aging trajectories associated with Alzheimer’s disease. Front. Neurosci. 2022, 16, 1036102. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Mogavero, M.P.; Silvani, A.; DelRosso, L.M.; Salemi, M.; Ferri, R. Focus on the Complex Interconnection between Cancer, Narcolepsy and Other Neurodegenerative Diseases: A Possible Case of Orexin-Dependent Inverse Comorbidity. Cancers 2021, 13, 2612. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Meimaridou, E.; Tavassoli, M.; Melino, G.; Lovestone, S.; Killick, R. p53 is upregulated in Alzheimer’s disease and induces tau phosphorylation in HEK293a cells. Neurosci. Lett. 2007, 418, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Bretaud, S.; Allen, C.; Ingham, P.W.; Bandmann, O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson’s disease. J. Neurochem. 2007, 100, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.R.; Ghafouri, M.; Mukerjee, R.; Bagashev, A.; Chabrashvili, T.; Sawaya, B.E. Role of p53 in neurodegenerative diseases. Neurodegener. Dis. 2012, 9, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Meek, D.W. Regulation of the p53 response and its relationship to cancer. Biochem. J. 2015, 469, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.A.; Lu, K.P. Pin1: A new genetic link between Alzheimer’s disease, cancer and aging. Curr. Aging Sci. 2010, 3, 158–165. [Google Scholar] [CrossRef]
- Salemi, M.; Mogavero, M.P.; Lanza, G.; Mongioì, L.M.; Calogero, A.E.; Ferri, R. Examples of Inverse Comorbidity between Cancer and Neurodegenerative Diseases: A Possible Role for Noncoding RNA. Cells 2022, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Pandini, C.; Bordoni, M.; Pansarasa, O.; Rey, F.; Costa, A.; Minafra, B.; Diamanti, L.; Zucca, S.; Carelli, S.; et al. Alzheimer’s, Parkinson’s Disease and Amyotrophic Lateral Sclerosis Gene Expression Patterns Divergence Reveals Different Grade of RNA Metabolism Involvement. Int. J. Mol. Sci. 2020, 21, 9500. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.M.; Banerjee, A.; Ghosal, A.; Layek, B. Neuroinflammation in Neurodegenerative Disorders: Current Knowledge and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 3995. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.R.; O’Connell, K.M.S.; Kaczorowski, C.C. Gene-by-environment interactions in Alzheimer’s disease and Parkinson’s disease. Neurosci. Biobehav. Rev. 2019, 103, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 2024, 53, 47. [Google Scholar] [CrossRef]
- Villa, C.; Paudel, Y.N.; Piperi, C. New Insights into Molecular Mechanisms Underlying Neurodegenerative Disorders. Brain Sci. 2022, 12, 1190. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef]
- Duranti, E.; Villa, C. Molecular Investigations of Protein Aggregation in the Pathogenesis of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 24, 704. [Google Scholar] [CrossRef]
- Tsoi, P.S.; Quan, M.D.; Ferreon, J.C.; Ferreon, A.C.M. Aggregation of Disordered Proteins Associated with Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 3380. [Google Scholar] [CrossRef]
- Agnello, L.; Colletti, T.; Lo Sasso, B.; Vidali, M.; Spataro, R.; Gambino, C.M.; Giglio, R.V.; Piccoli, T.; Bivona, G.; La Bella, V.; et al. Tau protein as a diagnostic and prognostic biomarker in amyotrophic lateral sclerosis. Eur. J. Neurol. 2021, 28, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Scarafino, A.; D’Errico, E.; Introna, A.; Fraddosio, A.; Distaso, E.; Tempesta, I.; Morea, A.; Mastronardi, A.; Leante, R.; Ruggieri, M.; et al. Diagnostic and prognostic power of CSF Tau in amyotrophic lateral sclerosis. J. Neurol. 2018, 265, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cholerton, B.; Shi, M.; Ginghina, C.; Cain, K.C.; Auinger, P.; Zhang, J. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Caspell-Garcia, C.J.; Coffey, C.S.; Taylor, P.; Shaw, L.M.; Trojanowski, J.Q.; Singleton, A.; Frasier, M.; Marek, K.; Galasko, D. Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology 2017, 89, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Dolatshahi, M.; Pourmirbabaei, S.; Kamalian, A.; Ashraf-Ganjouei, A.; Yaseri, M.; Aarabi, M.H. Longitudinal Alterations of Alpha-Synuclein, Amyloid Beta, Total, and Phosphorylated Tau in Cerebrospinal Fluid and Correlations Between Their Changes in Parkinson’s Disease. Front. Neurol. 2018, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Twohig, D.; Nielsen, H.M. α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Villa, C.; Stoccoro, A. Epigenetic Peripheral Biomarkers for Early Diagnosis of Alzheimer’s Disease. Genes 2022, 13, 1308. [Google Scholar] [CrossRef]
- Villa, C. Biomarkers for Alzheimer’s Disease: Where Do We Stand and Where Are We Going? J. Pers. Med. 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Tamaoki, T.; Tanaka, K.; Motohashi, N.; Nakamura, M.; Hayashi, T.; Yamaguchi, H.; Shimohama, S.; Lee, H.G.; Zhu, X.; et al. Intraneuronal amyloid beta accumulation and oxidative damage to nucleic acids in Alzheimer disease. Neurobiol. Dis. 2010, 37, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Hedna, R.; Kovacic, H.; Pagano, A.; Peyrot, V.; Robin, M.; Devred, F.; Breuzard, G. Tau Protein as Therapeutic Target for Cancer? Focus on Glioblastoma. Cancers 2022, 14, 5386. [Google Scholar] [CrossRef] [PubMed]
- Caillet-Boudin, M.L.; Buée, L.; Sergeant, N.; Lefebvre, B. Regulation of human MAPT gene expression. Mol. Neurodegener. 2015, 10, 28. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Jakes, R.; Rutherford, D.; Crowther, R.A. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Orecchio, L.D.; Bakalis, S.; Neve, R.L. Developmentally regulated expression of specific tau sequences. Neuron 1989, 2, 1389–1397. [Google Scholar] [CrossRef]
- Buchholz, S.; Zempel, H. The six brain-specific TAU isoforms and their role in Alzheimer’s disease and related neurodegenerative dementia syndromes. Alzheimer’s Dement. 2024, 20, 3606–3628. [Google Scholar] [CrossRef] [PubMed]
- Ercan-Herbst, E.; Ehrig, J.; Schöndorf, D.C.; Behrendt, A.; Klaus, B.; Gomez Ramos, B.; Prat Oriol, N.; Weber, C.; Ehrnhoefer, D.E. A post-translational modification signature defines changes in soluble tau correlating with oligomerization in early stage Alzheimer’s disease brain. Acta Neuropathol. Commun. 2019, 7, 192. [Google Scholar] [CrossRef]
- Liu, F.; Li, B.; Tung, E.J.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur. J. Neurosci. 2007, 26, 3429–3436. [Google Scholar] [CrossRef]
- Gargini, R.; Segura-Collar, B.; Sánchez-Gómez, P. Novel Functions of the Neurodegenerative-Related Gene Tau in Cancer. Front. Aging Neurosci. 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, C.; Gurard-Levin, Z.A.; Andre, F.; Pusztai, L.; Rouzier, R. Predictive and Prognostic Value of the TauProtein in Breast Cancer. Anticancer Res. 2015, 35, 5179–5184. [Google Scholar] [PubMed]
- Spicakova, T.; O’Brien, M.M.; Duran, G.E.; Sweet-Cordero, A.; Sikic, B.I. Expression and silencing of the microtubule-associated protein Tau in breast cancer cells. Mol. Cancer Ther. 2010, 9, 2970–2981. [Google Scholar] [CrossRef][Green Version]
- Yang, H. Tau and stathmin proteins in breast cancer: A potential therapeutic target. Clin. Exp. Pharmacol. Physiol. 2022, 49, 445–452. [Google Scholar] [CrossRef]
- Barbolina, M.V. Targeting Microtubule-Associated Protein Tau in Chemotherapy-Resistant Models of High-Grade Serous Ovarian Carcinoma. Cancers 2022, 14, 4535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, N.; Shao, G.; Qian, J.; Shen, D.; Fei, Y.; Mao, W.; Wu, D. Relationship between gastric cancer tau protein expression and paclitaxel sensitivity. Pathol. Oncol. Res. 2013, 19, 429–435. [Google Scholar] [CrossRef]
- Zarin, B.; Eshraghi, A.; Zarifi, F.; Javanmard, S.H.; Laher, I.; Amin, B.; Vaseghi, G. A review on the role of tau and stathmin in gastric cancer metastasis. Eur. J. Pharmacol. 2021, 908, 174312. [Google Scholar] [CrossRef] [PubMed]
- Souter, S.; Lee, G. Microtubule-associated protein tau in human prostate cancer cells: Isoforms, phosphorylation, and interactions. J. Cell. Biochem. 2009, 108, 555–564. [Google Scholar] [CrossRef]
- Clementi, L.; Sabetta, S.; Zelli, V.; Compagnoni, C.; Tessitore, A.; Mattei, V.; Angelucci, A. Mitotic phosphorylation of Tau/MAPT modulates cell cycle progression in prostate cancer cells. J. Cancer Res. Clin. Oncol. 2023, 149, 7689–7701. [Google Scholar] [CrossRef]
- Cirak, Y.; Sarsik, B.; Cakar, B.; Sen, S.; Simsir, A.; Uslu, R. Predictive and prognostic values of Tau and BubR1 protein in prostate cancer and their relationship to the Gleason score. Med. Oncol. 2013, 30, 526. [Google Scholar] [CrossRef]
- Gargini, R.; Segura-Collar, B.; Herránz, B.; García-Escudero, V.; Romero-Bravo, A.; Núñez, F.J.; García-Pérez, D.; Gutiérrez-Guamán, J.; Ayuso-Sacido, A.; Seoane, J.; et al. The IDH-TAU-EGFR triad defines the neovascular landscape of diffuse gliomas. Sci. Transl. Med. 2020, 12, eaax1501. [Google Scholar] [CrossRef] [PubMed]
- Smoter, M.; Bodnar, L.; Grala, B.; Stec, R.; Zieniuk, K.; Kozlowski, W.; Szczylik, C. Tau protein as a potential predictive marker in epithelial ovarian cancer patients treated with paclitaxel/platinum first-line chemotherapy. J. Exp. Clin. Cancer Res. 2013, 32, 25. [Google Scholar] [CrossRef]
- Steffensen, K.D.; Smoter, M.; Waldstrøm, M.; Grala, B.; Bodnar, L.; Stec, R.; Szczylik, C.; Jakobsen, A. Resistance to first line platinum paclitaxel chemotherapy in serous epithelial ovarian cancer: The prediction value of ERCC1 and Tau expression. Int. J. Oncol. 2014, 44, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Jeong, J.H.; Gong, Y.; Ross, J.S.; Kim, C.; Paik, S.; Rouzier, R.; Andre, F.; Hortobagyi, G.N.; Wolmark, N.; et al. Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J. Clin. Oncol. 2009, 27, 4287–4292. [Google Scholar] [CrossRef]
- Moh, C.; Kubiak, J.Z.; Bajic, V.P.; Zhu, X.; Smith, M.A.; Lee, H.G. Cell cycle deregulation in the neurons of Alzheimer’s disease. In Cell Cycle in Development; Springer: Berlin/Heidelberg, Germany, 2011; Volume 53, pp. 565–576. [Google Scholar] [CrossRef]
- Cimini, S.; Giaccone, G.; Tagliavini, F.; Costantino, M.; Perego, P.; Rossi, G. P301L tau mutation leads to alterations of cell cycle, DNA damage response and apoptosis: Evidence for a role of tau in cancer. Biochem. Pharmacol. 2022, 200, 115043. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Matsumoto, M.; Kamura, T.; Murayama, M.; Chui, D.H.; Planel, E.; Takahashi, R.; Nakayama, K.I.; Takashima, A. U-box protein carboxyl terminus of Hsc70-interacting protein (CHIP) mediates poly-ubiquitylation preferentially on four-repeat Tau and is involved in neurodegeneration of tauopathy. J. Neurochem. 2004, 91, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, L.; Dickson, D.; Kehoe, K.; Taylor, J.; Snyder, H.; Grover, A.; De Lucia, M.; McGowan, E.; Lewis, J.; Prihar, G.; et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004, 13, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, H.L.; Xie, J.Z.; Meng, D.L.; Wang, X.C.; Ke, D.; Zeng, J.; Liu, R. Erratum to: Protein Phosphatase 2A as a Drug Target in the Treatment of Cancer and Alzheimer’s Disease. Curr. Med. Sci. 2020, 40, 389. [Google Scholar] [CrossRef]
- Ding, H.; Dolan, P.J.; Johnson, G.V. Histone deacetylase 6 interacts with the microtubule-associated protein tau. J. Neurochem. 2008, 106, 2119–2130. [Google Scholar] [CrossRef]
- Rouzier, R.; Rajan, R.; Wagner, P.; Hess, K.R.; Gold, D.L.; Stec, J.; Ayers, M.; Ross, J.S.; Zhang, P.; Buchholz, T.A.; et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 8315–8320. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Kuo, K.T.; Hu, F.C.; Lu, Y.S.; Huang, C.S.; Liau, J.Y.; Lee, W.C.; Hsu, C.; Kuo, W.H.; Chang, K.J.; et al. Predictive and prognostic values of tau and ERCC1 in advanced breast cancer patients treated with paclitaxel and cisplatin. Jpn. J. Clin. Oncol. 2010, 40, 286–293. [Google Scholar] [CrossRef]
- Frasor, J.; Stossi, F.; Danes, J.M.; Komm, B.; Lyttle, C.R.; Katzenellenbogen, B.S. Selective estrogen receptor modulators: Discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004, 64, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Taira, N.; Hara, F.; Fujita, T.; Yamamoto, H.; Soh, J.; Toyooka, S.; Nogami, T.; Shien, T.; Doihara, H.; et al. The estrogen receptor influences microtubule-associated protein tau (MAPT) expression and the selective estrogen receptor inhibitor fulvestrant downregulates MAPT and increases the sensitivity to taxane in breast cancer cells. Breast Cancer Res. 2010, 12, R43. [Google Scholar] [CrossRef] [PubMed]
- Matrone, M.A.; Whipple, R.A.; Thompson, K.; Cho, E.H.; Vitolo, M.I.; Balzer, E.M.; Yoon, J.R.; Ioffe, O.B.; Tuttle, K.C.; Tan, M.; et al. Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene 2010, 29, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qian, S.; Li, H.; He, W.; Tan, X.; Zhang, Q.; Han, G.; Chen, G.; Luo, R. Predictive value of microtubule-associated protein Tau in patients with recurrent and metastatic breast cancer treated with taxane-containing palliative chemotherapy. Tumour Biol. 2015, 36, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.H.; Lee, H.J.; Ahn, J.H.; Yoon, D.H.; Kim, S.B.; Gong, G.; Son, B.H.; Ahn, S.H.; Jung, K.H. Tau and PTEN status as predictive markers for response to trastuzumab and paclitaxel in patients with HER2-positive breast cancer. Tumour Biol. 2015, 36, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- García-Aranda, M.; Redondo, M. Protein Kinase Targets in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 2543. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Anand, U.; Pandey, S.K.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Dey, A. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist. Updat. 2021, 55, 100754. [Google Scholar] [CrossRef]
- Chambonnière, M.L.; Mosnier-Damet, M.; Mosnier, J.F. Expression of microtubule-associated protein tau by gastrointestinal stromal tumors. Hum. Pathol. 2001, 32, 1166–1173. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Guo, W.; Zhu, X.; Ahuja, N.; Fu, T. MAPT promoter CpG island hypermethylation is associated with poor prognosis in patients with stage II colorectal cancer. Cancer Manag. Res. 2019, 11, 7337–7343. [Google Scholar] [CrossRef]
- Huda, M.N.; Kim, D.H.; Erdene-Ochir, E.; Kim, Y.S.; Pan, C.-H. Expression, phosphorylation, localization, and microtubule binding of tau in colorectal cell lines. Appl. Biol. Chem. 2016, 59, 807–812. [Google Scholar] [CrossRef]
- Papin, S.; Paganetti, P. Emerging Evidences for an Implication of the Neurodegeneration-Associated Protein TAU in Cancer. Brain Sci. 2020, 10, 862. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Men’s Health 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. Mechanisms of Taxane Resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef]
- Vogt, A.S.; Jennings, G.T.; Mohsen, M.O.; Vogel, M.; Bachmann, M.F. Alzheimer’s Disease: A Brief History of Immunotherapies Targeting Amyloid β. Int. J. Mol. Sci. 2023, 24, 3895. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Penke, B.; Bogár, F.; Fülöp, L. β-Amyloid and the Pathomechanisms of Alzheimer’s Disease: A Comprehensive View. Molecules 2017, 22, 1692. [Google Scholar] [CrossRef]
- Kummer, M.P.; Heneka, M.T. Truncated and modified amyloid-beta species. Alzheimer’s Res. Ther. 2014, 6, 28. [Google Scholar] [CrossRef]
- Tsatsanis, A.; Dickens, S.; Kwok, J.C.F.; Wong, B.X.; Duce, J.A. Post Translational Modulation of β-Amyloid Precursor Protein Trafficking to the Cell Surface Alters Neuronal Iron Homeostasis. Neurochem. Res. 2019, 44, 1367–1374. [Google Scholar] [CrossRef]
- Hansel, D.E.; Rahman, A.; Wehner, S.; Herzog, V.; Yeo, C.J.; Maitra, A. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res. 2003, 63, 7032–7037. [Google Scholar] [PubMed]
- Takayama, K.; Tsutsumi, S.; Suzuki, T.; Horie-Inoue, K.; Ikeda, K.; Kaneshiro, K.; Fujimura, T.; Kumagai, J.; Urano, T.; Sakaki, Y.; et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 2009, 69, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Ito, S.; Miyazaki, T.; Miki, Y.; Shibahara, Y.; Ishida, T.; Watanabe, M.; Inoue, S.; Sasano, H.; Suzuki, T. Amyloid precursor protein in human breast cancer: An androgen-induced gene associated with cell proliferation. Cancer Sci. 2013, 104, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.Y.; Kataoka, H.; Itoh, H.; Koono, M. Amyloid beta protein precursor is involved in the growth of human colon carcinoma cell in vitro and in vivo. Int. J. Cancer 2001, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zayas-Santiago, A.; Díaz-García, A.; Nuñez-Rodríguez, R.; Inyushin, M. Accumulation of amyloid beta in human glioblastomas. Clin. Exp. Immunol. 2020, 202, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavykh, L.Y.; Ortiz-Rivera, J.; Kucheryavykh, Y.V.; Zayas-Santiago, A.; Diaz-Garcia, A.; Inyushin, M.Y. Accumulation of Innate Amyloid Beta Peptide in Glioblastoma Tumors. Int. J. Mol. Sci. 2019, 20, 2482. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Joshi, V.; Upadhyay, A. Amyloids and brain cancer: Molecular linkages and crossovers. Biosci. Rep. 2023, 43, BSR20230489. [Google Scholar] [CrossRef]
- Pavliukeviciene, B.; Zentelyte, A.; Jankunec, M.; Valiuliene, G.; Talaikis, M.; Navakauskiene, R.; Niaura, G.; Valincius, G. Amyloid β oligomers inhibit growth of human cancer cells. PLoS ONE 2019, 14, e0221563. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Lu, C. Amyloid precursor protein promotes the migration and invasion of breast cancer cells by regulating the MAPK signaling pathway. Int. J. Mol. Med. 2020, 45, 162–174. [Google Scholar] [CrossRef]
- Castaneda, S.A.; Strasser, J. Updates in the Treatment of Breast Cancer with Radiotherapy. Surg. Oncol. Clin. N. Am. 2017, 26, 371–382. [Google Scholar] [CrossRef]
- Lim, S.; Yoo, B.K.; Kim, H.S.; Gilmore, H.L.; Lee, Y.; Lee, H.P.; Kim, S.J.; Letterio, J.; Lee, H.G. Amyloid-β precursor protein promotes cell proliferation and motility of advanced breast cancer. BMC Cancer 2014, 14, 928. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Jeong, M.S.; Jang, S.B. Molecular Characteristics of Amyloid Precursor Protein (APP) and Its Effects in Cancer. Int. J. Mol. Sci. 2021, 22, 4999. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, X.; Wang, J.; Long, D. Estrogen receptor α promotes non-amyloidogenic processing of platelet amyloid precursor protein via the MAPK/ERK pathway. J. Steroid. Biochem. Mol. Biol. 2014, 144 Pt B, 280–285. [Google Scholar] [CrossRef]
- Basile, D.; Cinausero, M.; Iacono, D.; Pelizzari, G.; Bonotto, M.; Vitale, M.G.; Gerratana, L.; Puglisi, F. Androgen receptor in estrogen receptor positive breast cancer: Beyond expression. Cancer Treat. Rev. 2017, 61, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Lee, M.A.; Chan, T.H.; Li, J.; Ni, Y.B.; Shao, Y.; Chan, S.K.; Cheungc, S.Y.; Lau, K.F.; Tse, G.M.K. Proteolytic cleavage of amyloid precursor protein by ADAM10 mediates proliferation and migration in breast cancer. EBioMedicine 2018, 38, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Culicchia, F.; Cui, J.G.; Li, Y.Y.; Lukiw, W.J. Upregulation of beta-amyloid precursor protein expression in glioblastoma multiforme. Neuroreport 2008, 19, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S. Glioblastoma and dementia may share a common cause. Med. Hypotheses 2010, 75, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Lanni, C.; Masi, M.; Racchi, M.; Govoni, S. Cancer and Alzheimer’s disease inverse relationship: An age-associated diverging derailment of shared pathways. Mol. Psychiatry 2021, 26, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Minogue, A.M.; Sala Frigerio, C.; Fadeeva, J.V.; Wasco, W.; Selkoe, D.J. The APP family of proteins: Similarities and differences. Biochem. Soc. Trans. 2007, 35, 416–420. [Google Scholar] [CrossRef]
- Pandey, P.; Rachagani, S.; Das, S.; Seshacharyulu, P.; Sheinin, Y.; Naslavsky, N.; Pan, Z.; Smith, B.L.; Peters, H.L.; Radhakrishnan, P.; et al. Amyloid precursor-like protein 2 (APLP2) affects the actin cytoskeleton and increases pancreatic cancer growth and metastasis. Oncotarget 2015, 6, 2064–2075. [Google Scholar] [CrossRef]
- Peters, H.L.; Tuli, A.; Wang, X.; Liu, C.; Pan, Z.; Ouellette, M.M.; Hollingsworth, M.A.; Macdonald, R.G.; Solheim, J.C. Relevance of amyloid precursor-like protein 2 C-terminal fragments in pancreatic cancer cells. Int. J. Oncol. 2012, 41, 1464–1474. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Amyloid precursor protein regulates migration and metalloproteinase gene expression in prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 452, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Beutler, A.S.; Li, S.; Nicol, R.; Walsh, M.J. Carbamazepine is an inhibitor of histone deacetylases. Life Sci. 2005, 76, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ying, Y.; Xiong, G.; Lai, L.; Wang, Q.; Yang, Y. Amyloid β precursor protein silencing attenuates epithelial-mesenchymal transition of nasopharyngeal carcinoma cells via inhibition of the MAPK pathway. Mol. Med. Rep. 2019, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; He, D.; Jiao, M.; Kong, L.; Shao, C.; Chen, J.; Fang, Z.; Ma, X.; Chen, H.; Li, L.; et al. Overexpression of Histone Deacetylase and Amyloid Precursor Protein in Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2017, 16, 586–594. [Google Scholar] [CrossRef]
- Ito, S.; Miki, Y.; Saito, R.; Inoue, C.; Okada, Y.; Sasano, H. Amyloid precursor protein and its phosphorylated form in non-small cell lung carcinoma. Pathol. Res. Pract. 2019, 215, 152463. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, M.; Hvid, L.G.; Thrue, C.; Johansson, S.; Franzén, E.; Dalgas, U.; Langeskov-Christensen, M. Muscle Strength and Power in People With Parkinson Disease: A Systematic Review and Meta-analysis. J. Neurol. Phys. Ther. 2023, 47, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Bougea, A.; Papageorgiou, S.G.; Villa, C. Psychosis in Parkinson’s Disease: A Lesson from Genetics. Genes 2022, 13, 1099. [Google Scholar] [CrossRef]
- Lee, J.Y.; Martin-Bastida, A.; Murueta-Goyena, A.; Gabilondo, I.; Cuenca, N.; Piccini, P.; Jeon, B. Multimodal brain and retinal imaging of dopaminergic degeneration in Parkinson disease. Nat. Rev. Neurol. 2022, 18, 203–220. [Google Scholar] [CrossRef]
- Nicastro, N.; Nencha, U.; Burkhard, P.R.; Garibotto, V. Dopaminergic imaging in degenerative parkinsonisms, an established clinical diagnostic tool. J. Neurochem. 2023, 164, 346–363. [Google Scholar] [CrossRef]
- Parkinson, J. An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 223–236; discussion 222. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Sekiya, H.; Kondru, N.; Ross, O.A.; Dickson, D.W. Neuropathology and molecular diagnosis of Synucleinopathies. Mol. Neurodegener. 2021, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Holton, J.L.; Revesz, T.; Dickson, D.W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011, 122, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kawashima, A.; Ruberu, N.N.; Fujiwara, H.; Koyama, S.; Sawabe, M.; Arai, T.; Nagura, H.; Yamanouchi, H.; Hasegawa, M.; et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. J. Neuropathol. Exp. Neurol. 2003, 62, 644–654. [Google Scholar] [CrossRef]
- Brás, I.C.; Outeiro, T.F. Alpha-Synuclein: Mechanisms of Release and Pathology Progression in Synucleinopathies. Cells 2021, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Li, J.D. The Roles of Post-translational Modifications on α-Synuclein in the Pathogenesis of Parkinson’s Diseases. Front. Neurosci. 2019, 13, 381. [Google Scholar] [CrossRef]
- Bianchini, M.; Giambelluca, M.; Scavuzzo, M.C.; Di Franco, G.; Guadagni, S.; Palmeri, M.; Furbetta, N.; Gianardi, D.; Costa, A.; Gentiluomo, M.; et al. In Pancreatic Adenocarcinoma Alpha-Synuclein Increases and Marks Peri-Neural Infiltration. Int. J. Mol. Sci. 2022, 23, 3775. [Google Scholar] [CrossRef]
- Dean, D.N.; Lee, J.C. Linking Parkinson’s Disease and Melanoma: Interplay Between α-Synuclein and Pmel17 Amyloid Formation. Mov. Disord. 2021, 36, 1489–1498. [Google Scholar] [CrossRef]
- Shekoohi, S.; Rajasekaran, S.; Patel, D.; Yang, S.; Liu, W.; Huang, S.; Yu, X.; Witt, S.N. Knocking out alpha-synuclein in melanoma cells dysregulates cellular iron metabolism and suppresses tumor growth. Sci. Rep. 2021, 11, 5267. [Google Scholar] [CrossRef]
- Fei, H.; Chen, X. Establishment and validation of an autophagy-related prognostic signature for survival predicting in cutaneous melanoma. Am. J. Cancer Res. 2021, 11, 5979–5991. [Google Scholar] [PubMed]
- Surguchov, A. Parkinson’s Disease: Assay of Phosphorylated α-Synuclein in Skin Biopsy for Early Diagnosis and Association with Melanoma. Brain Sci. 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Matsuo, Y.; Cashikar, A.G.; Kamitani, T. Role of Ser129 phosphorylation of α-synuclein in melanoma cells. J. Cell Sci. 2013, 126, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Xu, K. Alpha-synuclein contributes to malignant progression of human meningioma via the Akt/mTOR pathway. Cancer Cell Int. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Buneeva, O.; Medvedev, A. Ubiquitin Carboxyl-Terminal Hydrolase L1 and Its Role in Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, N.; Li, M.; Hong, T.; Meng, W.; Ouyang, T. Ubiquitin C-terminal hydrolase-L1: A new cancer marker and therapeutic target with dual effects (Review). Oncol. Lett. 2023, 25, 123. [Google Scholar] [CrossRef] [PubMed]
- Ho, X.D.; Phung, P.; Q Le, V.; H Nguyen, V.; Reimann, E.; Prans, E.; Kõks, G.; Maasalu, K.; Le, N.T.; H Trinh, L.; et al. Whole transcriptome analysis identifies differentially regulated networks between osteosarcoma and normal bone samples. Exp. Biol. Med. 2017, 242, 1802–1811. [Google Scholar] [CrossRef]
- Yu, J.; Tao, Q.; Cheung, K.F.; Jin, H.; Poon, F.F.; Wang, X.; Li, H.; Cheng, Y.Y.; Röcken, C.; Ebert, M.P.; et al. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology 2008, 48, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Li, L.; Yin, X.; Yuan, C.; Tan, C.; Su, X.; Xiong, L.; Putti, T.C.; Oberst, M.; Kelly, K.; et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS ONE 2012, 7, e29783. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yoshida, S.; Sugeno, N.; Kobayashi, J.; Aoki, M. DnaJ/Hsp40 Family and Parkinson’s Disease. Front. Neurosci. 2017, 11, 743. [Google Scholar] [CrossRef]
- Greene, M.K.; Maskos, K.; Landry, S.J. Role of the J-domain in the cooperation of Hsp40 with Hsp70. Proc. Natl. Acad. Sci. USA 1998, 95, 6108–6113. [Google Scholar] [CrossRef] [PubMed]
- Duranti, E.; Villa, C. Muscle Involvement in Amyotrophic Lateral Sclerosis: Understanding the Pathogenesis and Advancing Therapeutics. Biomolecules 2023, 13, 1582. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. The Spectrum of Cognitive Dysfunction in Amyotrophic Lateral Sclerosis: An Update. Int. J. Mol. Sci. 2023, 24, 4647. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem. 2011, 11, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.C.A.; Silva Magalhães, R.S.; de Araújo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Andersen, J.L. Mechanisms of SOD1 regulation by post-translational modifications. Redox Biol. 2019, 26, 101270. [Google Scholar] [CrossRef]
- Somwar, R.; Erdjument-Bromage, H.; Larsson, E.; Shum, D.; Lockwood, W.W.; Yang, G.; Sander, C.; Ouerfelli, O.; Tempst, P.J.; Djaballah, H.; et al. Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc. Natl. Acad. Sci. USA 2011, 108, 16375–16380. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Burley, S.K.; Zheng, X.F.S. Nuclear SOD1 in Growth Control, Oxidative Stress Response, Amyotrophic Lateral Sclerosis, and Cancer. Antioxidants 2022, 11, 427. [Google Scholar] [CrossRef]
- Glasauer, A.; Sena, L.A.; Diebold, L.P.; Mazar, A.P.; Chandel, N.S. Targeting SOD1 reduces experimental non–small-cell lung cancer. J. Clin. Investig. 2014, 124, 117–128. [Google Scholar] [CrossRef]
- Papa, L.; Hahn, M.; Marsh, E.L.; Evans, B.S.; Germain, D. SOD2 to SOD1 switch in breast cancer. J. Biol. Chem. 2014, 289, 5412–5416. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, T.; Chen, J.; Ni, H.; Li, W. Survivin in breast cancer-derived exosomes activates fibroblasts by up-regulating SOD1, whose feedback promotes cancer proliferation and metastasis. J. Biol. Chem. 2020, 295, 13737–13752. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.L.; Shah, N.; Kenny, T.C.; Jenkins, E.C., Jr.; Germain, D. SOD1 is essential for oncogene-driven mammary tumor formation but dispensable for normal development and proliferation. Oncogene 2019, 38, 5751–5765. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.K.; Chen, M.; Cheng, X.; Qi, Y.; Chen, Y.; Das, I.; Li, X.; Vallat, B.; Fu, L.W.; Qian, C.N.; et al. SOD1 Phosphorylation by mTORC1 Couples Nutrient Sensing and Redox Regulation. Mol. Cell 2018, 70, 502–515.e508. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zahurak, M.; Beer, T.M.; Ryan, C.J.; Wilding, G.; Mathew, P.; Morris, M.; Callahan, J.A.; Gordon, G.; Reich, S.D.; et al. A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naïve prostate cancer. Urol. Oncol. 2013, 31, 581–588. [Google Scholar] [CrossRef]
- Lowndes, S.A.; Adams, A.; Timms, A.; Fisher, N.; Smythe, J.; Watt, S.M.; Joel, S.; Donate, F.; Hayward, C.; Reich, S.; et al. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 7526–7534. [Google Scholar] [CrossRef]
- Babak, M.V.; Ahn, D. Modulation of Intracellular Copper Levels as the Mechanism of Action of Anticancer Copper Complexes: Clinical Relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef]
- Kawakami, I.; Arai, T.; Hasegawa, M. The basis of clinicopathological heterogeneity in TDP-43 proteinopathy. Acta Neuropathol. 2019, 138, 751–770. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Polymenidou, M.; Cleveland, D.W. TDP-43 and FUS/TLS: Emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 2010, 19, R46–R64. [Google Scholar] [CrossRef]
- Buratti, E. Functional Significance of TDP-43 Mutations in Disease. Adv. Genet. 2015, 91, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Cordts, I.; Wachinger, A.; Scialo, C.; Lingor, P.; Polymenidou, M.; Buratti, E.; Feneberg, E. TDP-43 Proteinopathy Specific Biomarker Development. Cells 2023, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Corbet, G.A.; Wheeler, J.R.; Parker, R.; Weskamp, K. TDP43 ribonucleoprotein granules: Physiologic function to pathologic aggregates. RNA Biol. 2021, 18, 128–138. [Google Scholar] [CrossRef]
- Buratti, E. TDP-43 post-translational modifications in health and disease. Expert Opin. Ther. Targets 2018, 22, 279–293. [Google Scholar] [CrossRef]
- Lin, T.W.; Chen, M.T.; Lin, L.T.; Huang, P.I.; Lo, W.L.; Yang, Y.P.; Lu, K.H.; Chen, Y.W.; Chiou, S.H.; Wu, C.W. TDP-43/HDAC6 axis promoted tumor progression and regulated nutrient deprivation-induced autophagy in glioblastoma. Oncotarget 2017, 8, 56612–56625. [Google Scholar] [CrossRef]
- Guo, F.; Wang, H.; Jiang, M.; Yang, Q.; Xiang, Q.; Zhou, H.; Hu, X.; Hao, K.; Yang, J.; Cao, H.; et al. TDP-43 induces EMT and promotes hepatocellular carcinoma metastasis via activating Wnt/β-catenin signaling pathway. Am. J. Cancer Res. 2020, 10, 3285–3301. [Google Scholar]
- Zeng, Q.; Cao, K.; Liu, R.; Huang, J.; Xia, K.; Tang, J.; Chen, X.; Zhou, M.; Xie, H.; Zhou, J. Identification of TDP-43 as an oncogene in melanoma and its function during melanoma pathogenesis. Cancer Biol. Ther. 2017, 18, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Zhao, L.; Zhang, H.; Feng, X.; Xu, H.; Hao, J.; Wang, S.; Yang, Q.; Zou, L.; Su, X.; et al. Loss of TDP43 inhibits progression of triple-negative breast cancer in coordination with SRSF3. Proc. Natl. Acad. Sci. USA 2018, 115, E3426–E3435. [Google Scholar] [CrossRef]
- Guo, L.; Ke, H.; Zhang, H.; Zou, L.; Yang, Q.; Lu, X.; Zhao, L.; Jiao, B. TDP43 promotes stemness of breast cancer stem cells through CD44 variant splicing isoforms. Cell Death Dis. 2022, 13, 428. [Google Scholar] [CrossRef]
- Kim, P.Y.; Tan, O.; Liu, B.; Trahair, T.; Liu, T.; Haber, M.; Norris, M.D.; Marshall, G.M.; Cheung, B.B. High TDP43 expression is required for TRIM16-induced inhibition of cancer cell growth and correlated with good prognosis of neuroblastoma and breast cancer patients. Cancer Lett. 2016, 374, 315–323. [Google Scholar] [CrossRef]
- Duranti, E.; Villa, C. Influence of DUX4 Expression in Facioscapulohumeral Muscular Dystrophy and Possible Treatments. Int. J. Mol. Sci. 2023, 24, 9503. [Google Scholar] [CrossRef] [PubMed]
- Tawil, R.; van der Maarel, S.M.; Tapscott, S.J. Facioscapulohumeral dystrophy: The path to consensus on pathophysiology. Skelet. Muscle 2014, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, S.; Salviati, L.; Desnuelle, C. Facioscapulohumeral muscular dystrophy. Biochim. Biophys. Acta 2015, 1852, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Zernov, N.; Skoblov, M. Genotype-phenotype correlations in FSHD. BMC Med. Genom. 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Snider, L.; Geng, L.N.; Lemmers, R.J.; Kyba, M.; Ware, C.B.; Nelson, A.M.; Tawil, R.; Filippova, G.N.; van der Maarel, S.M.; Tapscott, S.J.; et al. Facioscapulohumeral dystrophy: Incomplete suppression of a retrotransposed gene. PLoS Genet. 2010, 6, e1001181. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chadwick, B.P. Influence of Repressive Histone and DNA Methylation upon D4Z4 Transcription in Non-Myogenic Cells. PLoS ONE 2016, 11, e0160022. [Google Scholar] [CrossRef]
- Hamel, J.; Tawil, R. Facioscapulohumeral Muscular Dystrophy: Update on Pathogenesis and Future Treatments. Neurotherapeutics 2018, 15, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.N.; Eidahl, J.O.; Wallace, L.M.; Choudury, S.G.; Rashnonejad, A.; Daman, K.; Guggenbiller, M.J.; Saad, N.Y.; Hoover, M.E.; Zhang, L.; et al. Post-Translational Modifications of the DUX4 Protein Impact Toxic Function in FSHD Cell Models. Ann. Neurol. 2023, 94, 398–413. [Google Scholar] [CrossRef]
- Mocciaro, E.; Runfola, V.; Ghezzi, P.; Pannese, M.; Gabellini, D. DUX4 Role in Normal Physiology and in FSHD Muscular Dystrophy. Cells 2021, 10, 3322. [Google Scholar] [CrossRef]
- Dib, C.; Zakharova, V.; Popova, E.; Kiseleva, E.; Chernyak, B.; Lipinski, M.; Vassetzky, Y.S. DUX4 Pathological Expression: Causes and Consequences in Cancer. Trends Cancer 2019, 5, 268–271. [Google Scholar] [CrossRef]
- Lilljebjörn, H.; Henningsson, R.; Hyrenius-Wittsten, A.; Olsson, L.; Orsmark-Pietras, C.; von Palffy, S.; Askmyr, M.; Rissler, M.; Schrappe, M.; Cario, G.; et al. Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat. Commun. 2016, 7, 11790. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Tsuzuki, S.; Kawazu, M.; Hayakawa, F.; Kojima, S.; Ueno, T.; Imoto, N.; Kohsaka, S.; Kunita, A.; Doi, K.; et al. Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nat. Genet. 2016, 48, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; McCastlain, K.; Yoshihara, H.; Xu, B.; Chang, Y.; Churchman, M.L.; Wu, G.; Li, Y.; Wei, L.; Iacobucci, I.; et al. Deregulation of DUX4 and ERG in acute lymphoblastic leukemia. Nat. Genet. 2016, 48, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Kawamura-Saito, M.; Yamazaki, Y.; Kaneko, K.; Kawaguchi, N.; Kanda, H.; Mukai, H.; Gotoh, T.; Motoi, T.; Fukayama, M.; Aburatani, H.; et al. Fusion between CIC and DUX4 up-regulates PEA3 family genes in Ewing-like sarcomas with t(4;19)(q35;q13) translocation. Hum. Mol. Genet. 2006, 15, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Regulation and function of capicua in mammals. Exp. Mol. Med. 2020, 52, 531–537. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Tanaka, M.; Homme, M.; Yamazaki, Y.; Takazawa, Y.; Antonescu, C.R.; Nakamura, T. CIC-DUX4 Induces Small Round Cell Sarcomas Distinct from Ewing Sarcoma. Cancer Res. 2017, 77, 2927–2937. [Google Scholar] [CrossRef]
- Lin, Y.K.; Wu, W.; Ponce, R.K.; Kim, J.W.; Okimoto, R.A. Negative MAPK-ERK regulation sustains CIC-DUX4 oncoprotein expression in undifferentiated sarcoma. Proc. Natl. Acad. Sci. USA 2020, 117, 20776–20784. [Google Scholar] [CrossRef]
- DeSalvo, J.; Ban, Y.; Li, L.; Sun, X.; Jiang, Z.; Kerr, D.A.; Khanlari, M.; Boulina, M.; Capecchi, M.R.; Partanen, J.M.; et al. ETV4 and ETV5 drive synovial sarcoma through cell cycle and DUX4 embryonic pathway control. J. Clin. Investig. 2021, 131, e141908. [Google Scholar] [CrossRef]
- Bury, M.; Le Calvé, B.; Lessard, F.; Dal Maso, T.; Saliba, J.; Michiels, C.; Ferbeyre, G.; Blank, V. NFE2L3 Controls Colon Cancer Cell Growth through Regulation of DUX4, a CDK1 Inhibitor. Cell Rep. 2019, 29, 1469–1481.e9. [Google Scholar] [CrossRef]
- Barro, C.; Chitnis, T.; Weiner, H.L. Blood neurofilament light: A critical review of its application to neurologic disease. Ann. Clin. Transl. Neurol. 2020, 7, 2508–2523. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Otto, M.; Silani, V. Neurofilament Light Chain as Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2021, 15, 679199. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef]
- Lycke, J.N.; Karlsson, J.E.; Andersen, O.; Rosengren, L.E. Neurofilament protein in cerebrospinal fluid: A potential marker of activity in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1998, 64, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Varhaug, K.N.; Torkildsen, Ø.; Myhr, K.M.; Vedeler, C.A. Neurofilament Light Chain as a Biomarker in Multiple Sclerosis. Front. Neurol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Ramani, S.; Berard, J.A.; Walker, L.A.S. The relationship between neurofilament light chain and cognition in neurological disorders: A scoping review. J. Neurol. Sci. 2021, 420, 117229. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Sotirchos, E.S.; Smith, M.D.; Lord, H.N.; DuVal, A.; Mowry, E.M.; Calabresi, P.A. Contributors to Serum NfL Levels in People without Neurologic Disease. Ann. Neurol. 2022, 92, 688–698. [Google Scholar] [CrossRef]
- Yuan, A.; Nixon, R.A. Neurofilament Proteins as Biomarkers to Monitor Neurological Diseases and the Efficacy of Therapies. Front. Neurosci. 2021, 15, 689938. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, Z.; Zhang, L.; Pan, J.; Lei, Q.; Meng, Y.; Li, Z. Plasma Neurofilament Light Chain May Be a Biomarker for the Inverse Association Between Cancers and Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, J.; House, M.G.; Cullen, K.J.; Nephew, K.P.; Guo, Z. Role of neurofilament light polypeptide in head and neck cancer chemoresistance. Mol. Cancer Res. 2012, 10, 305–315. [Google Scholar] [CrossRef]
- Mortensen, C.; Steffensen, K.D.; Simonsen, E.; Herskind, K.; Madsen, J.S.; Olsen, D.A.; Iversen, D.B.; Bergmann, T.K.; Pottegård, A.; Stage, T.B. Neurofilament light chain as a biomarker of axonal damage in sensory neurons and paclitaxel-induced peripheral neuropathy in patients with ovarian cancer. Pain 2023, 164, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Karteri, S.; Bruna, J.; Mariotto, S.; Simo, M.; Velissaris, D.; Kalofonou, F.; Cavaletti, G.; Ferrari, S.; Kalofonos, H.P. Serum neurofilament light chain levels as biomarker of paclitaxel-induced cognitive impairment in patients with breast cancer: A prospective study. Support. Care Cancer 2022, 30, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, T.; Deng, H.; Liu, C.; Yang, Y.; Chen, T.; Qin, Y.; Xie, X.; Xie, Z.; Liu, M.; et al. Serum neurofilament light chain or glial fibrillary acidic protein in the diagnosis and prognosis of brain metastases. J. Neurol. 2022, 269, 815–823. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Hviid, C.V.B.; Meldgaard, P.; Sorensen, B.S.; Sandfeld-Paulsen, B. Neurofilament Light Chain as A Biomarker for Brain Metastases. Cancers 2020, 12, 2852. [Google Scholar] [CrossRef]
- Shahim, P.; Zetterberg, H.; Tegner, Y.; Blennow, K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017, 88, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.H.; Hyun, J.W.; Kim, J.H.; Seo, S.S.; Kim, H.J.; Park, S.Y.; Lim, M.C. Blood neurofilament light chain as a biomarker for monitoring and predicting paclitaxel-induced peripheral neuropathy in patients with gynecological cancers. Front. Oncol. 2022, 12, 942960. [Google Scholar] [CrossRef]
- Karteri, S.; Bruna, J.; Argyriou, A.A.; Mariotto, S.; Velasco, R.; Alemany, M.; Kalofonou, F.; Alberti, P.; Dinoto, A.; Velissaris, D.; et al. Prospectively assessing serum neurofilament light chain levels as a biomarker of paclitaxel-induced peripheral neurotoxicity in breast cancer patients. J. Peripher. Nerv. Syst. 2022, 27, 166–174. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Franklin, W.A.; Gazdar, A.F.; Bunn, P.A., Jr. Early detection of lung cancer: Clinical perspectives of recent advances in biology and radiology. Clin. Cancer Res. 2001, 7, 5–22. [Google Scholar]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Brenner, D.J. Cancer risks from diagnostic radiology. Br. J. Radiol. 2008, 81, 362–378. [Google Scholar] [CrossRef]
- Srivastava, S.; Jayaswal, N.; Kumar, S.; Sharma, P.K.; Behl, T.; Khalid, A.; Mohan, S.; Najmi, A.; Zoghebi, K.; Alhazmi, H.A. Unveiling the potential of proteomic and genetic signatures for precision therapeutics in lung cancer management. Cell. Signal. 2024, 113, 110932. [Google Scholar] [CrossRef]
- Loke, S.Y.; Lee, A.S.G. The future of blood-based biomarkers for the early detection of breast cancer. Eur. J. Cancer 2018, 92, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Cree, I.A.; Uttley, L.; Buckley Woods, H.; Kikuchi, H.; Reiman, A.; Harnan, S.; Whiteman, B.L.; Philips, S.T.; Messenger, M.; Cox, A.; et al. The evidence base for circulating tumour DNA blood-based biomarkers for the early detection of cancer: A systematic mapping review. BMC Cancer 2017, 17, 697. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, M.A.; Oliveira-Costa, J.P.; Carter, B.S.; Stott, S.L.; Nahed, B.V. Blood-based biomarkers for the diagnosis and monitoring of gliomas. Neuro-Oncology 2018, 20, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.S.; Bu, X.L.; Liu, Y.H.; Shen, L.L.; Zhuang, Z.Q.; Jiao, S.S.; Zhu, C.; Wang, Q.H.; Zhou, H.D.; Zhang, T.; et al. Plasma Amyloid-Beta Levels in Patients with Different Types of Cancer. Neurotox. Res. 2017, 31, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Attoub, S.; Singh, M.N.; Martin, F.L.; El-Agnaf, O.M. Gamma-synuclein and the progression of cancer. FASEB J. 2007, 21, 3419–3430. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, T.F.; Peng, Y.F.; Xie, J.; Feng, B.; Qiu, M.Y.; Li, L.H.; Lu, A.G.; Liu, B.Y.; Zheng, M.H. Expression of alpha-, beta- and gamma-synuclein in colorectal cancer, and potential clinical significance in progression of the disease. Oncol. Rep. 2010, 23, 429–436. [Google Scholar] [PubMed]
- Skórska, K.B.; Płaczkowska, S.; Prescha, A.; Porębska, I.; Kosacka, M.; Pawełczyk, K.; Zabłocka-Słowińska, K. Serum Total SOD Activity and SOD1/2 Concentrations in Predicting All-Cause Mortality in Lung Cancer Patients. Pharmaceuticals 2021, 14, 1067. [Google Scholar] [CrossRef]
- Papa, L.; Manfredi, G.; Germain, D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer 2014, 5, 15–21. [Google Scholar] [CrossRef]
- Ma, X.; Ying, Y.; Xie, H.; Liu, X.; Wang, X.; Li, J. The Regulatory Role of RNA Metabolism Regulator TDP-43 in Human Cancer. Front. Oncol. 2021, 11, 755096. [Google Scholar] [CrossRef]
- Ford, E.S.; Bergmann, M.M.; Kröger, J.; Schienkiewitz, A.; Weikert, C.; Boeing, H. Healthy living is the best revenge: Findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch. Intern. Med. 2009, 169, 1355–1362. [Google Scholar] [CrossRef]
- Yaffe, K.; Barnes, D.; Nevitt, M.; Lui, L.Y.; Covinsky, K. A prospective study of physical activity and cognitive decline in elderly women: Women who walk. Arch. Intern. Med. 2001, 161, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, F.; Lanza, G.; Pennisi, M.; Vagli, C.; Cantone, M.; Falzone, L.; Pennisi, G.; Ferri, R.; Bella, R. Daily mocha coffee intake and psycho-cognitive status in non-demented non-smokers subjects with subcortical ischaemic vascular disease. Int. J. Food Sci. Nutr. 2022, 73, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wahlqvist, M.L.; Lee, M.S.; Tsai, H.N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimer’s Dis. 2011, 24, 485–493. [Google Scholar] [CrossRef]
- El-Mir, M.Y.; Detaille, D.; R-Villanueva, G.; Delgado-Esteban, M.; Guigas, B.; Attia, S.; Fontaine, E.; Almeida, A.; Leverve, X. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J. Mol. Neurosci. 2008, 34, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kickstein, E.; Krauss, S.; Thornhill, P.; Rutschow, D.; Zeller, R.; Sharkey, J.; Williamson, R.; Fuchs, M.; Köhler, A.; Glossmann, H.; et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 21830–21835. [Google Scholar] [CrossRef] [PubMed]
- Mielke, J.G.; Taghibiglou, C.; Wang, Y.T. Endogenous insulin signaling protects cultured neurons from oxygen-glucose deprivation-induced cell death. Neuroscience 2006, 143, 165–173. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).