Understanding the Role of Toll-Like Receptors 9 in Breast Cancer
Abstract
Simple Summary
Abstract
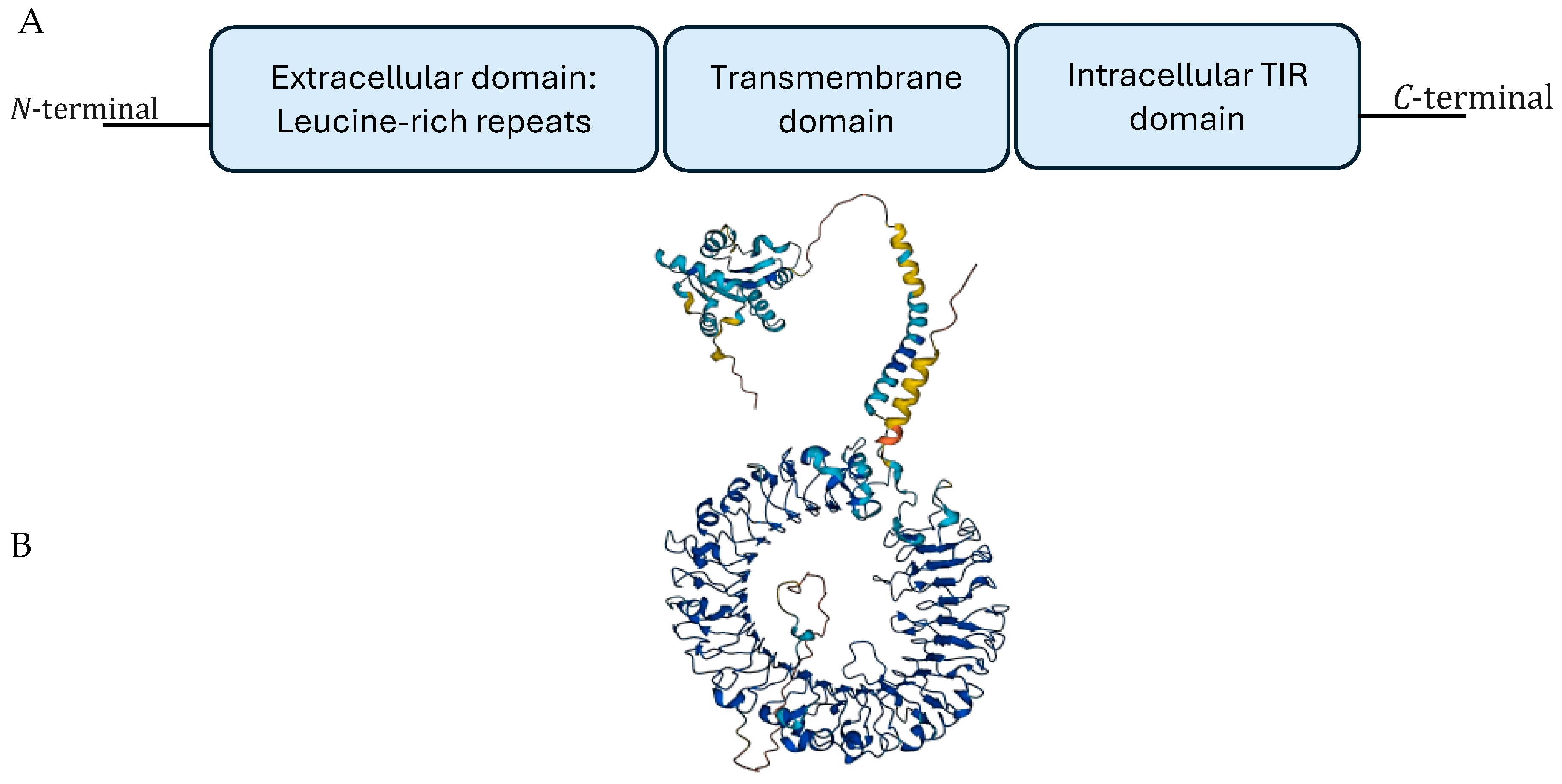
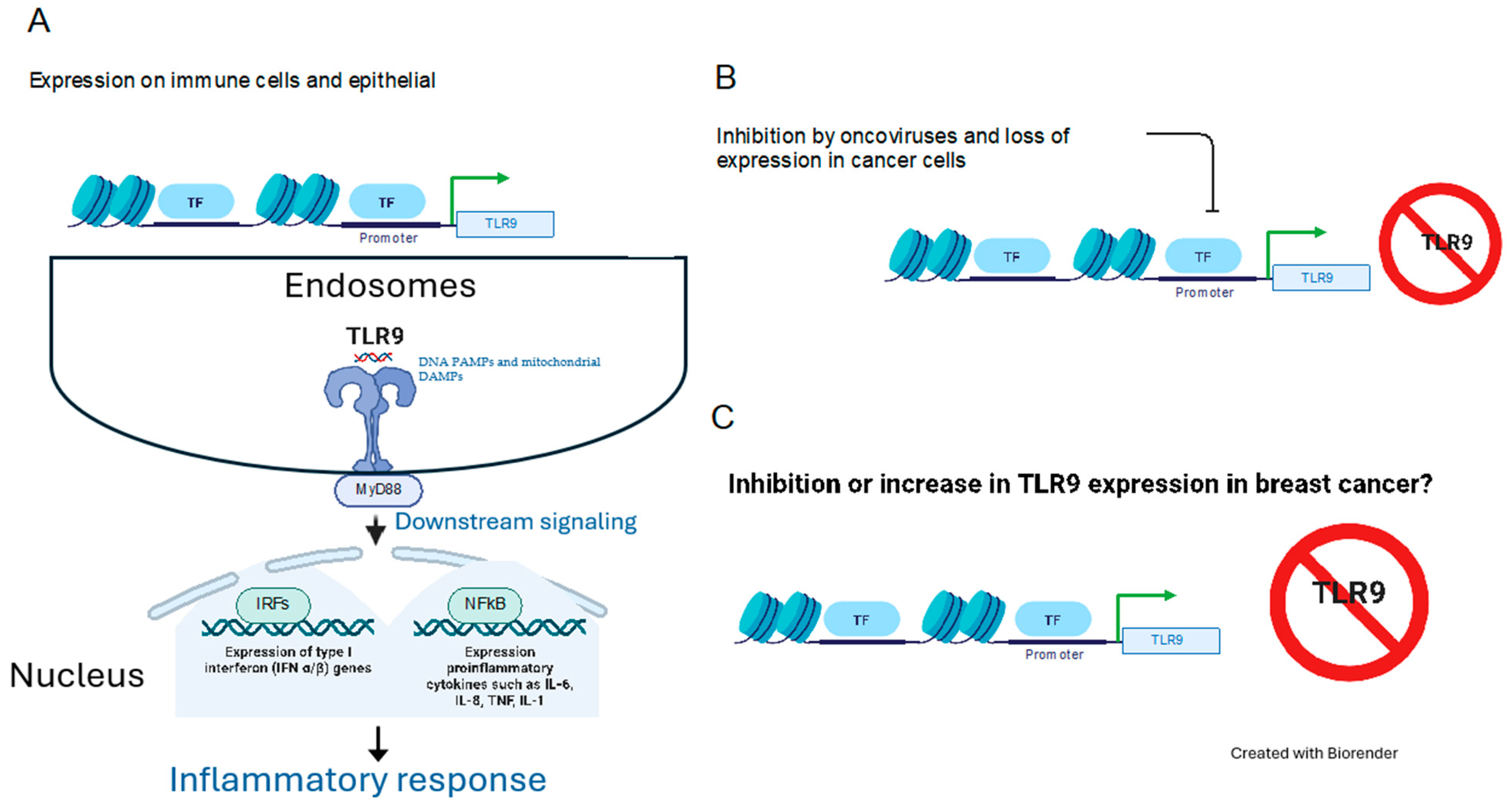
1. Introduction
2. Methods and Results
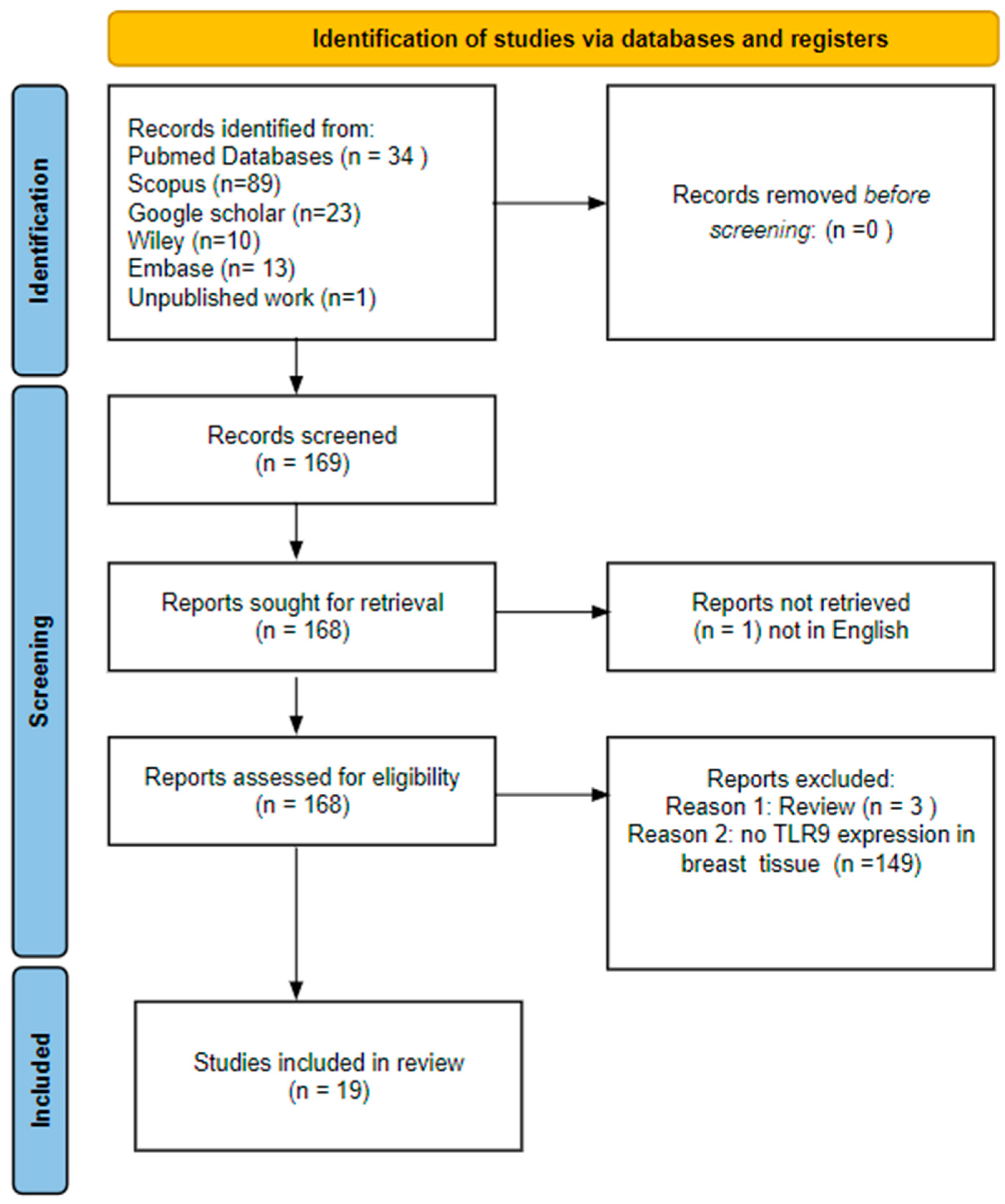
2.1. Literature Search and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
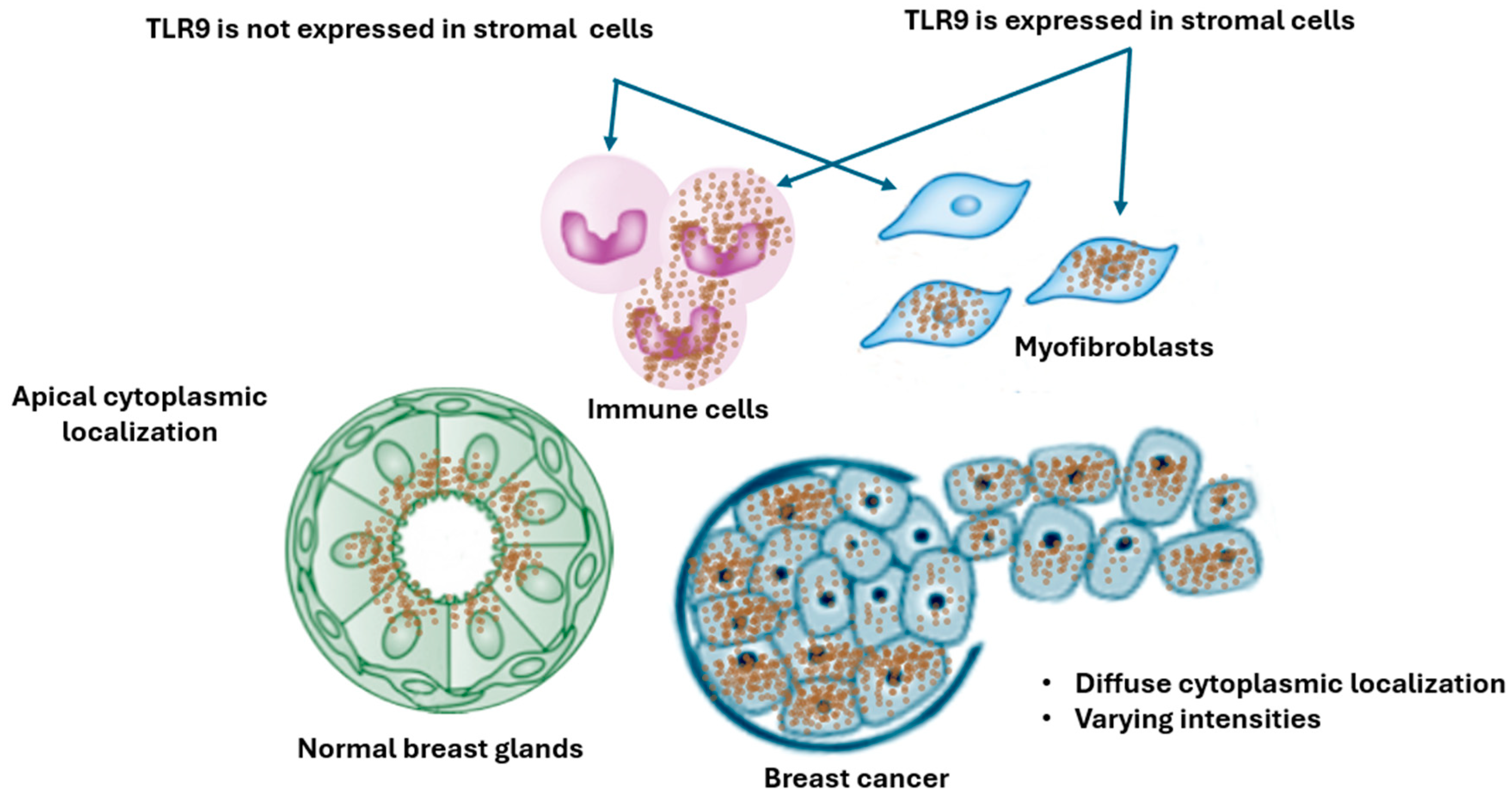
2.4. TLR9 Expression and Cellular Localization in Breast Tissue
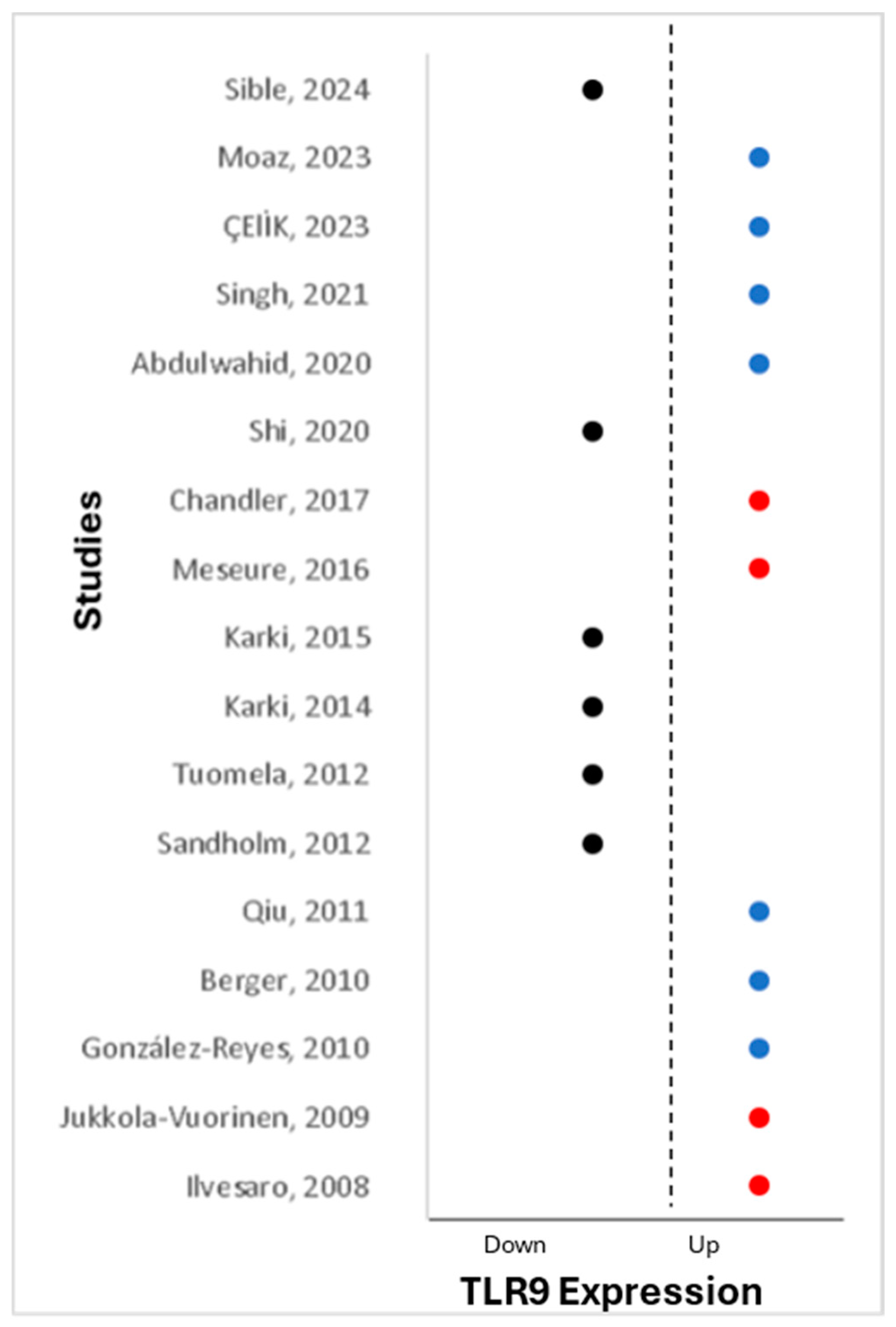
2.5. Upregulation of TLR9 and Association with Breast Clinicopathological Characteristics of Breast
2.6. Downregulation of TLR9 and Association with Breast Clinicopathological Characteristics
| Author | Breast Samples | Total Cases | TLR9 Detection Method | TLR9 Expression |
|---|---|---|---|---|
| Merrell [37] | Lysate | 6 | WB: Img-431, Imgenex, Bhubaneswar, Odisha, India | Expressed in normal and tumor |
| Sandholm [35] | FFPE | 196 | mRNA | Downregulated in ER-positive |
| Singh A [40] | FFPE | 38 | IHC: PA5-20202, Thermo Fischer, Waltham, MA, USA | TLR9 in epithelial and stromal |
| Upregulation of TLR9 in breast cancer | ||||
| Ilvesaro [29] | FFPE | 35 | IHC: Img-305A, Imgenex | Upregulated in tumor |
| Jukkola-Vuorinen [38] | FFPE | 141 | IHC: Img-305A, Imgenex | Upregulated in aggressive tumor |
| Berger [42] | FFPE and Frozen | 240 | WB: sc13218, Santa Cruz, Santa Cruz, CA, USA | Upregulated in aggressive tumors |
| González-Reyes [43] | FFPE | 74 | IHC &WB: sc-25468, Santa Cruz | Upregulated in aggressive tumor |
| Qiu [27] | FFPE | 124 | IHC: 2254, Cell signaling, Danvers, MA, USA | Upregulated in aggressive tumor |
| Meseure [36] | FFPE and Frozen | 480 | IHC: Img-305A, Imgenex | Upregulated in tumor |
| Chandler [48] | FFPE | 51 | IHC: Img-305A; Imgenex | Upregulated in triple-negative |
| Abdulwahid [49] | Serum | 120 | ELISA (Elabscience, Houston, TX, USA) | Upregulated in tumor |
| Singh [39] | FFPE | 42 | IHC: PA5-20202, Thermo Fischer | Upregulated in tumor |
| ÇElİK [44] | FFPE | 139 | IHC: ab37154, Abcam, Cambridge, UK | Upregulated in aggressive tumor |
| Moaz [45] | Serum | 186 | ELISA: SG11478, Sino Gene Clone Biotech, Beijing, China | Upregulated in aggressive tumor |
| Downregulation of TLR9 in breast cancer | ||||
| Tuomela [47] | FFPE | 12 | WB: Img-431, Imgenex | Downregulated in triple-negative |
| Karki [50] | Serum | 180 | ELISA: USCN Life Science & Technology, Wuhan, China | Downregulated in tumor |
| Karki [30] | Serum | 210 | ELISA: USCN Life Science & Technology | Downregulated in tumor |
| Shi [51] | TCGA | 1215 | mRNA | Downregulated in tumor |
| Sible [46] | FFPE | 244 | IHC: Cell Signaling | Downregulated in tumor |
3. Discussion
3.1. Discrepancies Cellular and Subcellular Sublocalization of TLR9 in Breast Tissue
3.2. Dysregulation of TLR9 and Association with Breast Clinicopathological Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 13 May 2024).
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Place, A.E.; Jin Huh, S.; Polyak, K. The microenvironment in breast cancer progression: Biology and implications for treatment. Breast Cancer Res. 2011, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Guidroz, J.A.; Weigel, R.J. The Biology of Breast Cancer. In Breast Surgical Techniques and Interdisciplinary Management; Dirbas, F., Scott-Conner, C., Eds.; Springer: New York, NY, USA, 2011; pp. 83–96. [Google Scholar]
- American Cancer Society. Breast Cancer Facts & Stats 2024—Incidence, Age, Survival, & More. Available online: https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 29 June 2024).
- O’Neill, L.A.J.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.J.; O’Neill, L.A.J. Of Flies and Men-The Discovery of TLRs. Cells 2022, 11, 3127. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Gay, N.J.; Gangloff, M.; Weber, A.N. Toll-like receptors as molecular switches. Nat. Rev. Immunol. 2006, 6, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Genecards. TLR9 Gene—Toll Like Receptor 9. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TLR9#proteins-structures (accessed on 29 June 2024).
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Means, T.K.; Latz, E.; Hayashi, F.; Murali, M.R.; Golenbock, D.T.; Luster, A.D. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Investig. 2005, 115, 407–417. [Google Scholar] [CrossRef]
- Rybka, J.; Butrym, A.; Wróbel, T.; Jaźwiec, B.; Bogucka-Fedorczuk, A.; Poręba, R.; Kuliczkowski, K. The Expression of Toll-like Receptors in Patients with B-Cell Chronic Lymphocytic Leukemia. Arch. Immunol. Ther. Exp. 2016, 64, 147–150. [Google Scholar] [CrossRef]
- Cen, X.; Liu, S.; Cheng, K. The Role of Toll-Like Receptor in Inflammation and Tumor Immunity. Front. Pharmacol. 2018, 9, 878. [Google Scholar] [CrossRef]
- Nokhandani, N.; Naghavi-Alhosseini, M.; Davoodi, H. The role of toll-like receptors in breast cancer. J. Inflamm. Dis. 2019, 23, 262–277. [Google Scholar] [CrossRef]
- Davakis, S.; Kapelouzou, A.; Liakakos, T.; Mpoura, M.; Stergiou, D.; Sakellariou, S.; Charalabopoulos, A. The Role of Toll-like Receptors in Esophageal Cancer. Anticancer Res. 2022, 42, 2813–2818. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Redmond, H.P.; Wang, J.H. Links between Toll-like receptor 4 and breast cancer. Oncoimmunology 2013, 2, e22945. [Google Scholar] [CrossRef] [PubMed]
- Salaun, B.; Coste, I.; Rissoan, M.C.; Lebecque, S.J.; Renno, T. TLR3 can directly trigger apoptosis in human cancer cells. J. Immunol. 2006, 176, 4894–4901. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, M.; Yuan, H.; Ni, W.; Tai, G. Dual roles of myeloid-derived suppressor cells induced by Toll-like receptor signaling in cancer. Oncol. Lett. 2021, 21, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.J.; Highton, J.; Hessian, P.A. Cell-specific expression of TLR9 isoforms in inflammation. J. Autoimmun. 2011, 36, 76–86. [Google Scholar] [CrossRef]
- Kawai, T.; Sato, S.; Ishii, K.J.; Coban, C.; Hemmi, H.; Yamamoto, M.; Terai, K.; Matsuda, M.; Inoue, J.; Uematsu, S.; et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004, 5, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, F.; Suzuki, K.; Sasaki, S.; Ishii, N.; Klinman, D.M.; Ishii, K.J. Transcriptional regulation of the human TLR9 gene. J. Immunol. 2004, 173, 2552–2561. [Google Scholar] [CrossRef]
- Sandholm, J.; Selander, K.S. Toll-like receptor 9 in breast cancer. Front. Immunol. 2014, 5, 330. [Google Scholar] [CrossRef]
- Alzahrani, B. The Biology of Toll-Like Receptor 9 and Its Role in Cancer. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Jovasevic, V.; Wood, E.M.; Cicvaric, A.; Zhang, H.; Petrovic, Z.; Carboncino, A.; Parker, K.K.; Bassett, T.E.; Moltesen, M.; Yamawaki, N.; et al. Formation of memory assemblies through the DNA-sensing TLR9 pathway. Nature 2024, 628, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Shao, S.; Yang, G.; Shen, Z.; Zhang, Y. Association of Toll like receptor 9 expression with lymph node metastasis in human breast cancer. Neoplasma 2011, 58, 251–255. [Google Scholar] [CrossRef]
- Fehri, E.; Ennaifer, E.; Bel Haj Rhouma, R.; Ardhaoui, M.; Boubaker, S. TLR9 and Glioma: Friends or Foes? Cells 2022, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Ilvesaro, J.M.; Merrell, M.A.; Li, L.; Wakchoure, S.; Graves, D.; Brooks, S.; Rahko, E.; Jukkola-Vuorinen, A.; Vuopala, K.S.; Harris, K.W.; et al. Toll-like receptor 9 mediates CpG oligonucleotide-induced cellular invasion. Mol. Cancer Res. MCR 2008, 6, 1534–1543. [Google Scholar] [CrossRef]
- Karki, K.; Pande, D.; Negi, R.; Khanna, S.; Khanna, R.S.; Khanna, H.D. Correlation of serum toll like receptor 9 and trace elements with lipid peroxidation in the patients of breast diseases. J. Trace Elem. Med. Biol. 2015, 30, 11–16. [Google Scholar] [CrossRef]
- Hong, C.-P.; Yun, C.H.; Lee, G.-W.; Park, A.; Kim, Y.-M.; Jang, M.H. TLR9 regulates adipose tissue inflammation and obesity-related metabolic disorders. Obesity 2015, 23, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Fukuda, D.; Sata, M. Emerging roles of Toll-like receptor 9 in cardiometabolic disorders. Inflamm. Regen. 2020, 40, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Wang, C.; Liu, J.; Lu, W. Toll-Like Receptors Gene Polymorphisms in Autoimmune Disease. Front. Immunol. 2021, 12, 672346. [Google Scholar] [CrossRef]
- Miller, C.L.; Sagiv-Barfi, I.; Neuhöfer, P.; Czerwinski, D.K.; Bertozzi, C.R.; Cochran, J.R.; Levy, R. Targeted TLR9 Agonist Elicits Effective Antitumor Immunity against Spontaneously Arising Breast Tumors. J. Immunol. 2023, 211, 295–305. [Google Scholar] [CrossRef]
- Sandholm, J.; Kauppila, J.H.; Pressey, C.; Tuomela, J.; Jukkola-Vuorinen, A.; Vaarala, M.; Johnson, M.R.; Harris, K.W.; Selander, K.S. Estrogen receptor-α and sex steroid hormones regulate Toll-like receptor-9 expression and invasive function in human breast cancer cells. Breast Cancer Res. Treat. 2012, 132, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Meseure, D.; Vacher, S.; Drak Alsibai, K.; Trassard, M.; Nicolas, A.; Leclere, R.; Lerebours, F.; Guinebretiere, J.M.; Marangoni, E.; Lidereau, R.; et al. Biopathological Significance of TLR9 Expression in Cancer Cells and Tumor Microenvironment Across Invasive Breast Carcinomas Subtypes. Cancer Microenviron. 2016, 9, 107–118. [Google Scholar] [CrossRef]
- Merrell, M.A.; Ilvesaro, J.M.; Lehtonen, N.; Sorsa, T.; Gehrs, B.; Rosenthal, E.; Chen, D.; Shackley, B.; Harris, K.W.; Selander, K.S. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol. Cancer Res. 2006, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Jukkola-Vuorinen, A.; Rahko, E.; Vuopala, K.S.; Desmond, R.; Lehenkari, P.P.; Harris, K.W.; Selander, K.S. Toll-like receptor-9 expression is inversely correlated with estrogen receptor status in breast cancer. J. Innate Immun. 2009, 1, 59–68. [Google Scholar] [CrossRef]
- Singh, A.; Bandyopadhyay, A.; Mukherjee, N.; Basu, A. Toll-Like Receptor 9 Expression Levels in Breast Carcinoma Correlate with Improved Overall Survival in Patients Treated with Neoadjuvant Chemotherapy and Could Serve as a Prognostic Marker. Genet. Test. Mol. Biomark. 2021, 25, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bandyopadhyay, A.; Mukherjee, N.; Basu, A. α-Smooth Muscle Actin and TLR9 Expression and Correlation in Breast Cancer. Int. J. Pathol. Clin. Res. 2020, 6, 108. [Google Scholar] [CrossRef]
- Kou, M.; Wang, L. Surface toll-like receptor 9 on immune cells and its immunomodulatory effect. Front. Immunol. 2023, 14, 1259989. [Google Scholar] [CrossRef]
- Berger, R.; Fiegl, H.; Goebel, G.; Obexer, P.; Ausserlechner, M.; Doppler, W.; Hauser-Kronberger, C.; Reitsamer, R.; Egle, D.; Reimer, D.; et al. Toll-like receptor 9 expression in breast and ovarian cancer is associated with poorly differentiated tumors. Cancer Sci. 2010, 101, 1059–1066. [Google Scholar] [CrossRef]
- González-Reyes, S.; Marín, L.; González, L.; González, L.O.; del Casar, J.M.; Lamelas, M.L.; González-Quintana, J.M.; Vizoso, F.J. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer 2010, 10, 665. [Google Scholar] [CrossRef]
- ÇElİK, Z.E.; DemİR, F.; Yonar, H.; ÇElİK, M.; Eren, O.Ö. Association of Toll-Like Receptor 9 Expression With Prognosis In Breast Carcinoma. J. Contemp. Med. 2023, 13, 676–681. [Google Scholar] [CrossRef]
- Moaz, I.; Fouad, F.A.; Elmasry, H.; Tarek, G.; Elzoheiry, A.; Elgamal, M.; Ibrahim, R.; Hisham, Y.; Safwat, G.; Kamel, M.M.; et al. Associations Between Serum Soluble Toll-like Receptors 4 and 9 and Breast Cancer in Egyptian Patients. Cancer Control 2023, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sible, E.; Weissman, G.; Amoyal, S.; Roblot, G.; Marotel, M.; Ainouze, M.; Vermare, N.B.; Gillet, C.; Michallet, M.C.; Caux, C.; et al. Loss of TLR9 expression in breast cancer tumour cells and its role in the cell cycle. bioRxiv 2024. [Google Scholar] [CrossRef]
- Tuomela, J.; Sandholm, J.; Karihtala, P.; Ilvesaro, J.; Vuopala, K.S.; Kauppila, J.H.; Kauppila, S.; Chen, D.; Pressey, C.; Härkönen, P. Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res. Treat. 2012, 135, 481–493. [Google Scholar] [CrossRef]
- Chandler, M.R.; Keene, K.S.; Tuomela, J.M.; Forero-Torres, A.; Desmond, R.; Vuopala, K.S.; Harris, K.W.; Merner, N.D.; Selander, K.S. Lower frequency of TLR9 variant associated with protection from breast cancer among African Americans. PLoS ONE 2017, 12, e0183832. [Google Scholar] [CrossRef]
- Abdulwahid, A.G.; Abdullah, H.N. Expression of Serum IL-22, IL-23, and TLR9 as Tumor Markers in Untreated Breast Cancer Patients. Int. J. Drug Deliv. Sci. Technol. 2020, 10, 472–476. [Google Scholar]
- Karki, K.; Pande, D.; Negi, R.; Khanna, S.; Khanna, R.S.; Khanna, H.D. Expression of serum toll-like receptor 9 and oxidative damage markers in benign and malignant breast diseases. DNA Cell Biol. 2014, 33, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Xu, C.; Fang, X.; Zhang, Y.; Li, H.; Wen, W.; Yang, G. Expression profile of Toll-like receptors in human breast cancer. Mol. Med. Rep. 2020, 21, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Goto, Y.; Narita, N.; Hoon, D.S. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer Microenviron. 2009, 2 (Suppl. 1), 205–214. [Google Scholar] [CrossRef]
- Parroche, P.; Roblot, G.; Le Calvez-Kelm, F.; Tout, I.; Marotel, M.; Malfroy, M.; Durand, G.; McKay, J.; Ainouze, M.; Carreira, C.; et al. TLR9 re-expression in cancer cells extends the S-phase and stabilizes p16INK4a protein expression. Oncogenesis 2016, 5, e244. [Google Scholar] [CrossRef]
- Gao, C.; Qiao, T.; Zhang, B.; Yuan, S.; Zhuang, X.; Luo, Y. TLR9 signaling activation at different stages in colorectal cancer and NF-kappaB expression. OncoTargets Ther. 2018, 11, 5963–5971. [Google Scholar] [CrossRef]
- Resler, A.J.; Malone, K.E.; Johnson, L.G.; Malkki, M.; Petersdorf, E.W.; McKnight, B.; Madeleine, M.M. Genetic variation in TLR or NFkappaB pathways and the risk of breast cancer: A case-control study. BMC Cancer 2013, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, N.; Shuda, M.; Gheit, T.; Kwun, H.J.; Cornet, I.; Saidj, D.; Zannetti, C.; Hasan, U.; Chang, Y.; Moore, P.S.; et al. The T antigen locus of Merkel cell polyomavirus downregulates human Toll-like receptor 9 expression. J. Virol. 2013, 87, 13009–13019. [Google Scholar] [CrossRef] [PubMed]
- Hasan, U. Human papillomavirus (HPV) deregulation of Toll-like receptor 9. Oncoimmunology 2014, 3, e27257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pacini, L.; Savini, C.; Ghittoni, R.; Saidj, D.; Lamartine, J.; Hasan, U.A.; Accardi, R.; Tommasino, M. Downregulation of Toll-Like Receptor 9 Expression by Beta Human Papillomavirus 38 and Implications for Cell Cycle Control. J. Virol. 2015, 89, 11396–11405. [Google Scholar] [CrossRef]
- Tout, I.; Gomes, M.; Ainouze, M.; Marotel, M.; Pecoul, T.; Durantel, D.; Vaccarella, S.; Dubois, B.; Loustaud-Ratti, V.; Walzer, T.; et al. Hepatitis B Virus Blocks the CRE/CREB Complex and Prevents TLR9 Transcription and Function in Human B Cells. J. Immunol. 2018, 201, 2331–2344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-alem, U.; Al-Saruri, A.; Bamahros, H.; Mahmoud, A.M.; Sible, E.; Hasan, U.A. Understanding the Role of Toll-Like Receptors 9 in Breast Cancer. Cancers 2024, 16, 2679. https://doi.org/10.3390/cancers16152679
Al-alem U, Al-Saruri A, Bamahros H, Mahmoud AM, Sible E, Hasan UA. Understanding the Role of Toll-Like Receptors 9 in Breast Cancer. Cancers. 2024; 16(15):2679. https://doi.org/10.3390/cancers16152679
Chicago/Turabian StyleAl-alem, Umaima, Alaa Al-Saruri, Hasan Bamahros, Abeer M. Mahmoud, Emily Sible, and Uzma A. Hasan. 2024. "Understanding the Role of Toll-Like Receptors 9 in Breast Cancer" Cancers 16, no. 15: 2679. https://doi.org/10.3390/cancers16152679
APA StyleAl-alem, U., Al-Saruri, A., Bamahros, H., Mahmoud, A. M., Sible, E., & Hasan, U. A. (2024). Understanding the Role of Toll-Like Receptors 9 in Breast Cancer. Cancers, 16(15), 2679. https://doi.org/10.3390/cancers16152679







